Abstract
Purpose of Review
Describe the rationale for preconditioning MSCs prior to use as therapy and the state-of-the-art of using preconditioning of MSCs in clinical settings.
Recent Findings
Mounting preclinical data supports preconditioning of mesenchymal stromal cells (MSCs) to enhance their therapeutic efficacy. Most research has focused on cytokine priming and hypoxic preconditioning, while other approaches, such as glycoengineering, remain relatively understudied. Despite strong preclinical data, clinical evidence supporting preconditioning strategies are limited to six Phase I clinical trials (most of them in progress).
Summary
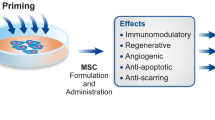
Here, we succinctly discuss the rationale for preconditioning using cytokines, hypoxia, and glycoengineering, while elaborating on the respective clinical experiences. Overall, we note that preconditioning is highly dependent on the desired application, and therefore requires elucidating the mechanism of action of the MSCs used for therapy. Preconditioning may also help mitigate heterogeneity of MSC lots. Based on the remarkable safety profile of MSCs, even when used in allogeneic settings, the role of preconditioning prior to their final formulation might be the key to reach expected therapeutic outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over one thousand clinical trials have demonstrated that administration of mesenchymal stem cells/multipotent stromal cells (MSCs) can be safe, but only a few trials have reached the expected therapeutic efficacy [1,2,3]. Factors likely limiting clinical efficacy include insufficient cell potency (primarily paracrine activity), not fully elucidated mechanisms of action of the cells, low efficiency to reach target tissues, low retention due to poor cell survival, and inadequate patient selection [1, 4]. In this review, we briefly discuss strategies that may help mitigate some of these limitations while not risking the good safety profile of the cells.
Important decisions for clinical success include, cell source, infusion of fresh vs. cryopreserved cells [5,6,7], clinical dose and dosage, route of administration, and final formulation. This review will focus on preconditioning strategies, referring to treatments on the MSCs performed within a few days or hours prior to final product formulation. Therefore, this review will not cover approaches such as genetic engineering, combination products of MSCs with other cell types, devices, or biomaterials, bioprinting, or long-term culture of MSCs in spheroids or special bioreactors. Importantly, the optimal preconditioning strategy depends on the intended application. All preconditioning strategies discussed here are transient. Also, most of the preconditioning strategies listed below can be used individually or in combination, a notion that needs to be evaluated on a case-by-case basis.
Cytokine Priming
One of the first avenues explored to modulate MSC activity was cytokine priming, which is primarily used to enhance the immunomodulatory capacity of MSCs [7, 9]. Through the introduction of pro-inflammatory cytokines (IFNγ, TNF-α, IL-17, IL-18, IL-1b, MCP-1) in vitro, MSCs can be activated to exhibit a stronger response after infusion into patients [8, 9]. Cytokine priming aims to mimic microenvironmental stimuli in vivo, where a single or cocktail of cytokines induces the expression of immunomodulatory signals such as the secretion of IDO, PGE2, TGF-b, MCP-1, and HGF [9]. Extensive studies have been conducted utilizing cytokine priming on MSCs to assess the immunomodulatory effects and involved mechanisms (see reviews [11,12,13,14]). These results suggest that cytokine priming have therapeutic potential by enhancing the immunomodulatory properties of MSCs, although the number of in vitro studies far outweighs in vivo studies in animal models. Cytokine priming strategies that have been utilized to test efficacy within in vivo models are summarized in Table 1.
The most common cytokine priming tested to enhance the immunomodulatory capabilities of MSCs is interferon gamma (IFN-γ). Human MSCs primed with IFN-γ significantly improved the survival of mice modeling graft-versus-host disease (GVHD) [15, 16]. Preconditioning with IFN-γ has also been reported to improve microvascular hemodynamics within a murine model of sepsis, by reducing the adhesion of white blood cells to venules [17]. Various groups have tested the safety of preconditioning human MSCs with IFN-γ [18, 19]. Such safety studies are pending for other cytokine-priming strategies. Of note, IFN-γ may cause upregulation of class I and class II HLA expression [20], therefore increasing the immunogenicity of MSCs and subsequently a faster clearing of the cells.
Summary of preconditioning strategies for MSCs in both preclinical and clinical trials. The clinical efficacy of MSCs can be enhanced through various preconditioning strategies that alter its biological properties. Cytokine priming can enhance the immunosuppressive capabilities of MSCs, which may be useful for immunomodulation and graft survival. Hypoxia preconditioning reduces glucose consumption and promotes retention and secretion of angiogenic factors. Glycoengineering can promote selectin binding and increase trafficking and migration of MSCs to the bone and/or inflamed tissues
When developing a potential MSC based therapy, it is important to consider the heterogeneity of MSCs, and differences among lots due to donor-to-donor variations. Interestingly, when stimulated with either IFN-γ or TNF-α, MSCs derived from different donors exhibit a more similar immune suppressive potential both in vitro and in vivo [21], suggesting that cytokine priming may also be useful to reduce variations among lots of MSCs.
Tumor necrosis factor alpha (TNF-α) is another common pro-inflammatory cytokine used to prime MSCs. However, the intended increase in immunomodulatory function may depend on the tissue source (e.g., umbilical cord vs. bone marrow) of MSCs [22]. Rat bone marrow-derived MSCs (BM-MSCs) preconditioned with TNF-α implanted into rat Achilles tendon segmental defects depicted modest regenerative potential [23]. However, it was noted that MSCs primed with TNF-α showed a reduction in IL-12 and M1 macrophages and an increase in IL-4 and M2 macrophages, suggesting that priming of MSCs with TNF-α may enhance the ability to modulate macrophage polarization.
Priming of MSCs with TNF-α has also been combined with Interleukin 1 beta (IL-1β) to prevent immune-mediated rejection seen with corneal transplantation (keratoplasty) [24]. Using a rat model of orthotopic corneal transplantation followed by intravenously administered MSCs, Murphy et al. showed that the corneal allograft had better survival when using the TNF-a/IL-1b-preconditioned MSCs, which was attributed to an increase of regulatory T cells and a decrease of inflammatory cytokines within draining lymph nodes. Surprisingly, corneal immune rejection after keratoplasty has also been improved by preconditioning MSCs with transforming growth factor beta 1 (TGF-β1) [25], a cytokine that primarily inhibits inflammation. Mice treated with TGF-β1-primed MSCs showed less corneal neovascularization and superior opacity score, suggesting that priming MSCs with TGF-β1 may also prevent immune-mediated rejection of corneal allografts.
Because pneumonia causes a strong increase of the proinflammatory cytokine IL-18, Liao et al. tested if priming umbilical cord-derived MSCs (UC-MSCs) with IL-18 would reduce acute lung injury in a murine model of H1N1 influenza virus-induced severe pneumonia [10]. As compared to controls, UC-MSCs primed with IL-18 showed enhanced immunosuppressive properties and significantly reduced systemic IFN-g and IL-1b levels. Monocyte Chemoattractant Protein 1 (MCP-1) is a chemokine involved in inflammation that attracts monocytes and basophils. MCP-1 is highly upregulated in a mouse model of contact hypersensitivity [26]. In this model, Liu et al. demonstrated that injecting human MSCs primed with MCP-1 intravenously reduced ear swelling in part by decreasing proinflammatory cytokines (IFN-g, TNF-a, IL-6), while increasing the anti-inflammatory cytokine IL-10. Mechanistically, it was suggested that priming with MCP-1 activates STAT3 signaling, inducing expression of cyclooxygenase-2 (COX2), leading to increased PGE2. These two studies serve as examples for the value of understanding the molecular signature of a disease to educate the optimal preconditioning strategy for MSCs.
Altogether, a large body of preclinical work supports cytokine priming as a preconditioning strategy for MSCs (Table 1) aiming to increase the immunomodulatory function of the cells. However, clinical use of such preconditioning remains limited to only a few trials (Table 2).
Glycoengineering
Glycoengineering is the process by which glycosylation, especially of proteins, is modulated to alter the biological properties of cells. By taking advantage of the natural glycosylation pathway, this preconditioning approach can be safe and reversible with the distinct advantage of avoiding genetic manipulation. Glycoengineering strategies in other fields have been previously reviewed [27,28,29]. In pioneering work, Sackstein et al. showed that specific glycoengineering of hMSCs enhances its homing to the bone [30]. Hematopoietic stem/progenitor cells home efficiently to bone marrow in part by expressing a unique glycoform of CD44, which contains a terminal tetrasaccharide sialyl Lewis X (sLeX) motif [31]. MSCs do not express this unique glycoform. Rather, MSCs express high levels of sialylated CD44 without the characteristic antennary fucosylations of sLeX motifs. To induce such fucosylations, MSCs in a confluent layer or in suspension can be incubated for 40 min with fucosyltransferase 6 (FUT6) and GDP-fucose. These glycoengineered MSCs show enhanced E-selectin binding and rolling behavior under shear stress conditions. Most importantly, when injected intravenously into mice, glycoengineered MSCs show enhanced homing to the calvarium (and possibly other bones), although the total number of homed cells remains low. Noteworthy, the injected cells colocalized with human osteocalcin staining, suggesting that the injected MSCs were contributing to new bone formation through direct differentiation into osteoblasts. This glycoengineering approach may greatly improve the therapeutic outcome of MSCs used to promote bone repair.
In a small clinical trial (Table 2), 10 female patients with advanced osteoporosis were treated with autologous exo-fucosylated MSCs (2–6 × 106 cells/kg body weight). After a median follow-up of 3 months, patients reported no new osteoporotic fractures and an overall decrease in pain score [32]. We have shown that FGF2 increases the motility of MSCs in part by upregulating fucosyltransferase 8 (FUT8), which transfers core fucosylations to N-glycans. In turn, silencing FUT8 impairs the recruitment of MSCs into the bone callus during fracture repair [33]. Therefore, both antennary and core fucosylations are likely critical to the osteotropism of MSCs.
Sarkar et al. showed that conjugating a sLeX-polyacrylamide-biotin to the surface of MSCs increases the recruitment of MSCs to sites of inflammation [34]. Zheng et al. recently demonstrated that the sLeX motif can be glycoengineered onto CD63, a common biomarker for extracellular vesicles (EV). These CD63 + MSC-EVs showed increased uptake by endothelial cells both in vitro and in vivo [35]. Therefore, glycoengineering may not only improve the delivery of MSCs to target sites but also improve the delivery of MSC-derived EVs.
Kifunensine, a small molecule that inhibits Mannosidase I, causes a strong enrichment of high-mannose N-glycans [36]. We have shown that preconditioning MSCs with Kifunensine promotes cell motility in vitro and in vivo towards a bone fracture, when injected intramuscularly into immune deficient mice [37]. Importantly, glycoengineering with either small molecules or incubation with enzymes and sugars are transient effect that last for 4–6 days, depending on protein turnover.
Overall, glycoengineering is a promising approach to enhance MSCs’ efficacy, especially by improving the delivery of cells to specific sites.
Hypoxic Preconditioning
Preconditioning of MSCs in hypoxia has been extensively reviewed [9, 14, 38, 39]. The in vivo counterpart to MSCs (pericytes, adventitial stromal cells, etc.) resides in low-oxygen environments. For example, bone marrow has levels of 1 to 7% oxygen, while the umbilical cord has oxygen levels around 5%. However, MSCs are typically cultured under “normoxic” conditions (20.9%). This high oxygen level may damage DNA and cause cellular senescence due to oxidative stress [40, 41]. Conversely, hypoxia-preconditioned MSCs show increased differentiation potential, reduced telomeric shortening, and decreased cellular senescence. Hypoxic preconditioning inhibits the expression of p16 which in turn reduces ROS-associated stress of MSCs, decreasing cellular senescence.
Hypoxic preconditioning also increases immunomodulatory factors such as, HLA-G, PGE- 2, and IDO [9]. Huang et al. showed that hypoxic preconditioning promotes anti-inflammatory and immunomodulatory properties that are retained after injected in a mouse model [42]. They showed that hypoxic preconditioning of MSCs reduces the accumulation of host natural killer (NK) cells in ischemic tissue. MSCs cultured in normoxia would be lysed by NK cells but cells preconditioned in hypoxia were able to evade NK cell lysis. Hypoxia also promotes secretion of IL-6, a regulator of dendritic cell differentiation and function [41]. Hypoxia increases p21 which in turn reduces tumor potential in ischemic tissues. A safety assessment was conducted by Tsai et al. who showed that MSCs preconditioned in hypoxia keep their genetic integrity and develop no tumors in a mouse model [43].
We and others have shown that hypoxic preconditioning of MSCs also increases proangiogenic signals and enhances cell retention after transplantation into immune-deficient mice [44,45,46,47]. The increased survival is most likely driven by reducing the metabolic requirements of the cells and therefore adapting better to the injection site. This improved retention has likely therapeutic implications, since it has been shown that hypoxic preconditioning of MSCs show increased viability and enhanced angiogenic potential in animal models of critical limb ischemia/peripheral artery disease [48, 49].
A clinical trial conducted in Indonesia showed that conditioned media derived from hypoxic-preconditioned MSCs improved pulmonary function after damage from COVID-19 [50] by normalizing levels of neutrophils, monocytes and lymphocytes. Of note, the secretome of hypoxic-preconditioned MSCs showed high expression of angiogenic growth factors (VEGF and PDGF) and anti-inflammatory cytokines (IL-10 and TGF-b).
Various clinical trials have used hypoxic-preconditioned MSCs (Table 1), suggesting that pretreatment of MSCs in hypoxia does not jeopardize the good safety profile of the cells. However, to the best of our knowledge, these studies did not include an arm of MSCs without hypoxic preconditioning, hence challenging our understanding of the clinical benefit of this type of preconditioning.
Conclusion
There is a large body of literature supporting preconditioning strategies for MSCs. They are expected to not alter the good safety profile of the cells but enhance their therapeutic efficacy by transiently exacerbating specific cellular functions. However, clinical uses of preconditioned MSCs are still in the very early phases. The results of such clinical trials will be instrumental to further support these pre-formulation approaches for MSC-based therapies.
Availability of Data and Materials
As a review, all information shared is available from literature available online.
References
Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22.
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22.
Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringdén O, Volk HD, Geissler S, Reinke P. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149–63.
Galipeau J, Krampera M, Leblanc K, Nolta JA, Phinney DG, Shi Y, Tarte K, Viswanathan S, Martin I. Mesenchymal stromal cell variables influencing clinical potency: the impact of viability, fitness, route of administration and host predisposition. Cytotherapy. 2021. This article by the International Society for Cell & Gene Therapy MSC committee outlines bottlenecks to reach clinical efficacy using MSCs.
Chinnadurai R, Garcia MA, Sakurai Y, Lam WA, Kirk AD, Galipeau J, Copland IB. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Rep. 2014;3(1):60–72.
Dave C, Mei SH, McRae A, Hum C, Sullivan KJ, Champagne J, Ramsay T, McIntyre L. Comparison of freshly cultured versus cryopreserved mesenchymal stem cells in animal models of inflammation: a pre-clinical systematic review. eLife. 2022;11. This systematic review summarizes 257 studies, where MSCs were differently prepared prior to use in preclinical studies.
Cottle C, Porter AP, Lipat A, Turner-Lyles C, Nguyen J, Moll G, Chinnadurai R. Impact of cryopreservation and freeze-thawing on therapeutic properties of mesenchymal stromal/stem cells and other common cellular therapeutics. Curr Stem Cell Rep. 2022;8(2):72–92.
Najar M, Krayem M, Merimi M, Burny A, Meuleman N, Bron D, Raicevic G, Lagneaux L. Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts. Inflamm Res. 2018;67(6):467–77.
Noronha NC, Mizukami A, Caliari-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10(1):131.
Liao Y, Fu Z, Huang Y, Wu S, Wang Z, Ye S, Zeng W, Zeng G, Li D, Yang Y, Pei K, Yang J, Hu Z, Liang X, Hu J, Liu M, Jin J, Cai C. Interleukin-18-primed human umbilical cord-mesenchymal stem cells achieve superior therapeutic efficacy for severe viral pneumonia via enhancing T-cell immunosuppression. Cell Death Dis. 2023;14(1):66.
Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402.
Zhou Y, Tsai TL, Li WJ. Strategies to retain properties of bone marrow-derived mesenchymal stem cells ex vivo. Ann N Y Acad Sci. 2017;1409(1):3–17.
Petrenko Y, Sykova E, Kubinova S. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res Ther. 2017;8(1):94.
Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22(3):1428–42.
Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW, Sung KW, Koo HH, Yoo KH. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-gamma. EBioMedicine. 2018;28:261–73.
Corbett JM, Hawthorne I, Dunbar H, Coulter I, Chonghaile MN, Flynn CM, English K. Cyclosporine A and IFNgamma licencing enhances human mesenchymal stromal cell potency in a humanised mouse model of acute graft versus host disease. Stem Cell Res Ther. 2021;12(1):238.
Baudry N, Starck J, Aussel C, Lund K, Aletti M, Duranteau J, Banzet S, Lataillade JJ, Vicaut E, Peltzer J. Effect of preconditioned mesenchymal stromal cells on early microvascular disturbance in a mouse sepsis model. Stem Cells Dev. 2019;28(24):1595–606.
Park SJ, Kim DS, Choi M, Han KH, Han JS, Yoo KH, Moon KS. Preclinical evaluation of interferon-gamma primed human Wharton’s jelly-derived mesenchymal stem cells. Hum Exp Toxicol. 2023;42:9603271231171650.
Guess AJ, Daneault B, Wang R, Bradbury H, La Perle KMD, Fitch J, Hedrick SL, Hamelberg E, Astbury C, White P, Overolt K, Rangarajan H, Abu-Arja R, Devine SM, Otsuru S, Dominici M, O’Donnell L, Horwitz EM. Safety profile of good manufacturing practice manufactured interferon gamma-primed mesenchymal stem/stromal cells for clinical trials. Stem Cells Transl Med. 2017;6(10):1868–79.
Galipeau J. Reply: “Function of cryopreserved mesenchymal stromal cells with and without interferon-gamma prelicensing is context dependent.” Stem cells. 2017;35(5):1440–1.
Szabo E, Fajka-Boja R, Kriston-Pal E, Hornung A, Makra I, Kudlik G, Uher F, Katona RL, Monostori E, Czibula A. Licensing by inflammatory cytokines abolishes heterogeneity of immunosuppressive function of mesenchymal stem cell population. Stem Cells Dev. 2015;24(18):2171–80.
Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS ONE. 2010;5(2): e9016.
Aktas E, Chamberlain CS, Saether EE, Duenwald-Kuehl SE, Kondratko-Mittnacht J, Stitgen M, Lee JS, Clements AE, Murphy WL, Vanderby R. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J Orthop Res. 2017;35(2):269–80.
Murphy N, Treacy O, Lynch K, Morcos M, Lohan P, Howard L, Fahy G, Griffin MD, Ryan AE, Ritter T. TNF-alpha/IL-1beta-licensed mesenchymal stromal cells promote corneal allograft survival via myeloid cell-mediated induction of Foxp3(+) regulatory T cells in the lung. FASEB J. 2019;33(8):9404–21.
Lynch K, Treacy O, Chen X, Murphy N, Lohan P, Islam MN, Donohoe E, Griffin MD, Watson L, McLoughlin S, O’Malley G, Ryan AE, Ritter T. TGF-beta1-licensed murine MSCs show superior therapeutic efficacy in modulating corneal allograft immune rejection in vivo. Mol Ther. 2020;28(9):2023–43.
Liu Q, Ji S, Xia T, Liu J, Liu Z, Chen X, Zang ZJ. MCP-1 priming enhanced the therapeutic effects of human mesenchymal stromal cells on contact hypersensitivity mice by activating the COX2-PGE2/STAT3 pathway. Stem Cells Dev. 2020;29(16):1073–83.
Li Y, Zhang Y, Tao Y, Huang X, Yu C, Xu H, Chen J, Xia K, Shi K, Zhang Y, Wang J, Shu J, Cheng F, Wang S, Liang C, Li F, Zhou X, Chen Q. metabolic glycoengineering: a promising strategy to remodel microenvironments for regenerative therapy. Stem Cells Int. 2023;2023:1655750.
Rocamora F, Peralta AG, Shin S, Sorrentino J, Wu MYM, Toth EA, Fuerst TR, Lewis NE. Glycosylation shapes the efficacy and safety of diverse protein, gene and cell therapies. Biotechnol Adv. 2023;67:108206.
Kufleitner M, Haiber LM, Wittmann V. Metabolic glycoengineering - exploring glycosylation with bioorthogonal chemistry. Chem Soc Rev. 2023;52(2):510–35.
Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14(2):181–7.
Dimitroff CJ, Lee JY, Fuhlbrigge RC, Sackstein R. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc Natl Acad Sci U S A. 2000;97(25):13841–6.
Linares LF, Lozano-Rivas N, Marras-Fernandez-Cid C, Garcia-Hernandez AM, Algueró MDC, Iniesta F, Sanchez-Salinas D, López-Lucas MD, Rodriguez-Valiente M, Cabañas V, García-Bernal D, Molina MDM, Lopez S, Ramirez-Tovar F, Ruiz Sará JE, García B, Blanquer M, Olmo Fernandez-Delgado JA, Espinosa M, Zamarro J, Becerra-Ratia J, Peris JL, López-Exposito I, Bafalliu JA, Ruiz-Espejo F, Domenech E, Morales-Cano MD, Arrabal PM, Soler G, Vera A, Guzman-Aroca F, Moraleda JM, Sackstein R. AB1011 Clinical trial of intravenous infusion of fucosylated bone marrow mesenchymal stem cells in patients with osteoporosis. Ann Rheum Dis. 2018;77(2):1.
Awan B, Turkov D, Schumacher C, Jacobo A, McEnerney A, Ramsey A, Xu G, Park D, Kalomoiris S, Yao W, Jao LE, Allende ML, Lebrilla CB, Fierro FA. FGF2 induces migration of human bone marrow stromal cells by increasing core fucosylations on N-glycans of integrins. Stem Cell Rep. 2018;11(2):325–33.
Sarkar D, Spencer JA, Phillips JA, Zhao W, Schafer S, Spelke DP, Mortensen LJ, Ruiz JP, Vemula PK, Sridharan R, Kumar S, Karnik R, Lin CP, Karp JM. Engineered cell homing. Blood. 2011;118(25):e184–91.
Zheng W, He R, Liang X, Roudi S, Bost J, Coly PM, van Niel G, Andaloussi SEL. Cell-specific targeting of extracellular vesicles though engineering the glycocalyx. J Extracell Vesicles. 2022;11(12):e12290.
Elbein AD, Tropea JE, Mitchell M, Kaushal GP. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem. 1990;265(26):15599–605.
Alonso-Garcia V, Chaboya C, Li Q, Le B, Congleton TJ, Florez J, Tran V, Liu GY, Yao W, Lebrilla CB, Fierro FA. High mannose N-glycans promote migration of bone-marrow-derived mesenchymal stromal cells. Int J Mol Sci. 2020;21(19).
Yang Y, Lee EH, Yang Z. Hypoxia-conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng Part B Rev. 2022;28(5):966–77.
Tsai CC, Yew TL, Yang DC, Huang WH, Hung SC. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am J Blood Res. 2012;2(3):148–59.
Han YS, Lee JH, Yoon YM, Yun CW, Noh H, Lee SH. Hypoxia-induced expression of cellular prion protein improves the therapeutic potential of mesenchymal stem cells. Cell Death Dis. 2016;7(10):e2395.
Mendoza SV, Genetos DC, Yellowley CE. Hypoxia-Inducible Factor-2alpha Signaling in the Skeletal System. JBMR Plus. 2023;7(4):e10733.
Huang WH, Chen HL, Huang PH, Yew TL, Lin MW, Lin SJ, Hung SC. Hypoxic mesenchymal stem cells engraft and ameliorate limb ischaemia in allogeneic recipients. Cardiovasc Res. 2014;101(2):266–76.
Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A–p21 by HIF-TWIST. Blood. 2011;117(2):459–69.
Zhu H, Sun A, Zou Y, Ge J. Inducible metabolic adaptation promotes mesenchymal stem cell therapy for ischemia: a hypoxia-induced and glycogen-based energy prestorage strategy. Arterioscler Thromb Vasc Biol. 2014;34(4):870–6.
Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299(6):C1562–70.
Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, Fierro FA. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival and promotes cell retention in vivo. Stem cells. 2015.
Lakatos K, Kalomoiris S, Merkely B, Nolta JA, Fierro FA. Mesenchymal stem cells respond to hypoxia by increasing diacylglycerols. J Cell Biochem. 2015.
Peng X, Liang B, Wang H, Hou J, Yuan Q. Hypoxia pretreatment improves the therapeutic potential of bone marrow mesenchymal stem cells in hindlimb ischemia via upregulation of NRG-1. Cell Tissue Res. 2022;388(1):105–16.
Liu J, Hao H, Xia L, Ti D, Huang H, Dong L, Tong C, Hou Q, Zhao Y, Liu H, Fu X, Han W. Hypoxia pretreatment of bone marrow mesenchymal stem cells facilitates angiogenesis by improving the function of endothelial cells in diabetic rats with lower ischemia. PLoS ONE. 2015;10(5):e0126715.
Putra A, Widyatmoko A, Ibrahim S, Amansyah F, Amansyah F, Berlian MA, Retnaningsih R, Pasongka Z, Sari FE, Rachmad B. Case series of the first three severe COVID-19 patients treated with the secretome of hypoxia-mesenchymal stem cells in Indonesia. F1000Res. 2021;10:228.
Funding
B.L. is a T32 fellow of the Vision Science Training Program at UC Davis. A.C. is a T32 fellow of the MusculoSkeletal Clinical Learning Experience Program at UC Davis. F.A.F. is funded by NIAMS 1R01AR081336.
Author information
Authors and Affiliations
Contributions
All authors contributed discussing the topics of this review, searching the literature, and writing the manuscript. All authors have reviewed the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Le, B., Cressman, A., Morales, D. et al. First Clinical Experiences Using Preconditioning Approaches to Improve MSC-Based Therapies. Curr Stem Cell Rep 10, 1–7 (2024). https://doi.org/10.1007/s40778-023-00232-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-023-00232-5