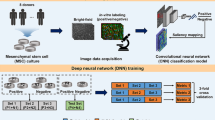
Abstract
Purpose of Review
Machine learning (ML) enables high-throughput analysis of multimodal data generated from stem cell experiments such as gene expression data, images of cells, or proteomic data. In this review, we analyse the progression of ML adaptation in advancing the field of stem cell research.
Recent Findings
On the one hand, the field of stem cell phenotypic characterisation is experiencing a significant growth, largely due to the successful implementation of deep networks in domains with similar problem characteristics (i.e., rapid advances of the image recognition field). On the other hand, genotypic characterisation is gradually gaining traction as researchers are beginning to apply ML to understand the genetic and molecular mechanisms behind stem cell behaviour.
Summary
The use of advanced machine learning techniques, such as deep networks, is demonstrating promising results in phenotypic stem cell characterisation, although it is still lagging slightly in genotypic characterisation. Despite this progress, significant challenges persist, including ensuring the interpretability of ML models, limited availability of annotated datasets, improving the accuracy and quality of training data, and navigating ethical considerations.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Sarker IH. Machine learning: algorithms, real-world applications and research directions. SN computer science. 2021;2(3):160.
LeCun Y, Bengio Y, Hinton G. Deep learning nature. 2015;521(7553):436–44.
Silver D, Huang A, Maddison CJ, Guez A, Sifre L, Van Den Driessche G, et al. Mastering the game of Go with deep neural networks and tree search. Nature. 2016;529(7587):484–9.
Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, et al. Attention is all you need. Adv Neural Info Process Sys. 2017;30.
Silver D, Schrittwieser J, Simonyan K, Antonoglou I, Huang A, Guez A, et al. Mastering the game of Go without human knowledge. Nature. 2017;550(7676):354–9.
• Rajpurkar P, Chen E, Banerjee O, Topol EJ. AI in health and medicine. Nat Med. 2022;28(1):31–8. The study discusses the potential impact of artificial intelligence (AI) on medicine and healthcare. It covers the key findings from a 2-year effort to track and share developments in medical AI, including advances in medical image analysis, potential uses of non-image data sources and unconventional problem formulations, and human–AI collaboration.
Subbiah V. The next generation of evidence-based medicine. Nat Med. 2023;16:1.
Shortliffe EH, Sepúlveda MJ. Clinical decision support in the era of artificial intelligence. JAMA. 2018;320(21):2199–200.
Yang X, Wang Y, Byrne R, Schneider G, Yang S. Concepts of artificial intelligence for computer-assisted drug discovery. Chem Rev. 2019;119(18):10520–94.
Meskó B, Drobni Z, Bényei É, Gergely B, Győrffy Z. Digital health is a cultural transformation of traditional healthcare. Mhealth. 2017;3.
Briganti G, Le Moine O. Artificial intelligence in medicine: today and tomorrow. Front Med. 2020;5(7):27.
He J, Baxter SL, Xu J, et al. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25:30–6. https://doi.org/10.1038/s41591-018-0307-0.
Joshi G, Jain A, Adhikari S, Garg H, Bhandari M. FDA approved artificial intelligence and machine learning (AI/ML)-enabled medical devices: an updated 2022 landscape. medRxiv. 2022;2022–12.
Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ digital medicine. 2020;3(1):118.
Grand View Research Homepage. Stem cells market size, share & trends analysis report by product (adult stem cells, human embryonic stem cells), by application, by technology, by therapy, by end use, by region, and segment forecasts, 2022–2030 [Internet]. Grand View Research; 2023 [cited 2022 Nov 15]. Available from: https://www.grandviewresearch.com/industry-analysis/stem-cells-market.
• Zaman WS, Karman SB, Ramlan EI, Tukimin SN, Ahmad MY. Machine learning in stem cells research: application for biosafety and bioefficacy assessment. IEEE Access. 2021;2(9):25926–45. This study focuses on the potential for machine learning–based analysis in assessing the biosafety and bio-efficacy of stem cells for clinical application, particularly in addressing the major concern of tumorigenicity.
TechCrunch Disrupt 2021. Cellino is using AI and machine learning to scale production of stem cell therapies [Internet]. TechCrunch; 2021 [cited 2022 Nov 25]. Available from: https://techcrunch.com/2021/09/22/cellino-is-using-ai-and-machine-learning-to-scale-production-of-stem-cell-therapies/
Libby AR, Briers D, Haghighi I, Joy DA, Conklin BR, Belta C, et al. Automated design of pluripotent stem cell self-organization. Cell Syst. 2019;9(5):483–95.
Wan Kamarul Zaman WS, Nurul AA, Nordin F. Stem cells and cancer stem cells: the Jekyll and Hyde Scenario and their implications in stem cell therapy. Biomedicines. 2021;9(9):1245.
• Ouyang JF, Chothani S, Rackham OJ. Deep learning models will shape the future of stem cell research. Stem Cell Reports. 2023;18(1):6–12. The study reviews the current state of deep-learning implementation in stem cell research and highlights future challenges for a successful adoption of the technology.
Ren E, Kim S, Mohamad S, Huguet SF, Shi Y, Cohen AR, et al. Deep learning-enhanced morphological profiling predicts cell fate dynamics in real-time in hPSCs. bioRxiv. 2021;2021–07.
Guo J, Wang P, Sozen B, Qiu H, Zhu Y, Zhang X, et al. Machine learning-assisted high-content analysis of pluripotent stem cell-derived embryos in vitro. Stem cell reports. 2021;16(5):1331–46.
Stumpf PS, MacArthur BD. Machine learning of stem cell identities from single-cell expression data via regulatory network archetypes. Front Genet. 2019;22(10):2.
Miotto R, Wang F, Wang S, Jiang X, Dudley JT. Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform. 2018;19(6):1236–46.
Shen D, Wu G, Suk HI. Deep learning in medical image analysis. Annu Rev Biomed Eng. 2017;21(19):221–48.
Ker J, Wang L, Rao J, Lim T. Deep learning applications in medical image analysis. IEEE Access. 2017;29(6):9375–89.
Chen X, Wang X, Zhang K, Fung KM, Thai TC, Moore K, et al. Recent advances and clinical applications of deep learning in medical image analysis. Med Image Anal. 2022;4: 102444.
Buggenthin F, Buettner F, Hoppe PS, Endele M, Kroiss M, Strasser M, et al. Prospective identification of hematopoietic lineage choice by deep learning. Nat Methods. 2017;14(4):403–6.
Su YT, Lu Y, Chen M, Liu AA. Spatiotemporal joint mitosis detection using CNN-LSTM network in time-lapse phase contrast microscopy images. IEEE Access. 2017;29(5):18033–41.
Kusumoto D, Lachmann M, Kunihiro T, Yuasa S, Kishino Y, Kimura M, et al. Automated deep learning-based system to identify endothelial cells derived from induced pluripotent stem cells. Stem cell reports. 2018;10(6):1687–95.
Waisman A, La Greca A, Möbbs AM, Scarafía MA, Velazque NL, Neiman G, et al. Deep learning neural networks highly predict very early onset of pluripotent stem cell differentiation. Stem cell reports. 2019;12(4):845–59.
Kavitha MS, Kurita T, Park SY, Chien SI, Bae JS, Ahn BC. Deep vector-based convolutional neural network approach for automatic recognition of colonies of induced pluripotent stem cells. PLoS ONE. 2017;12(12): e0189974.
Orita K, Sawada K, Matsumoto N, Ikegaya Y. Machine-learning-based quality control of contractility of cultured human-induced pluripotent stem-cell-derived cardiomyocytes. Biochem Biophys Res Commun. 2020;526(3):751–5.
Zhu Y, Huang R, Wu Z, Song S, Cheng L, Zhu R. Deep learning-based predictive identification of neural stem cell differentiation. Nat Commun. 2021;12(1):2614.
Pan G, Jiang L, Tang J, Guo F. A novel computational method for detecting DNA methylation sites with DNA sequence information and physicochemical properties. Int J Mol Sci. 2018;19(2):511.
Angermueller C, Lee HJ, Reik W, Stegle O. DeepCpG: accurate prediction of single-cell DNA methylation states using deep learning. Genome Biol. 2017;18(1):1–3.
Zhang W, Spector TD, Deloukas P, Bell JT, Engelhardt BE. Predicting genome-wide DNA methylation using methylation marks, genomic position, and DNA regulatory elements. Genome Biol. 2015;16:1–20.
Nguyen QH, Lukowski SW, Chiu HS, Senabouth A, Bruxner TJ, Christ AN, et al. Single-cell RNA-seq of human induced pluripotent stem cells reveals cellular heterogeneity and cell state transitions between subpopulations. Genome Res. 2018;28(7):1053–66.
Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells: a review. Cancer Cell Int. 2007;7(1):1–1.
Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 2018;173(2):338–54.
Lian H, Han YP, Zhang YC, Zhao Y, Yan S, Li QF, et al. Integrative analysis of gene expression and DNA methylation through one-class logistic regression machine learning identifies stemness features in medulloblastoma. Mol Oncol. 2019;13(10):2227–45.
Pan S, Zhan Y, Chen X, Wu B, Liu B. Identification of biomarkers for controlling cancer stem cell characteristics in bladder cancer by network analysis of transcriptome data stemness indices. Front Oncol. 2019;4(9):613.
Chen W, Hong Z, Kang S, Lv X, Song C. Analysis of Stemness and Prognosis of Subtypes in Breast Cancer Using the Transcriptome Sequencing Data. Journal of Oncology. 2022;9:2022.
Duddu S, Chakrabarti R, Ghosh A, Shukla PC. Hematopoietic stem cell transcription factors in cardiovascular pathology. Front Genet. 2020;16(11): 588602.
Rauch A, Haakonsson AK, Madsen JG, Larsen M, Forss I, Madsen MR, et al. Osteogenesis depends on commissioning of a network of stem cell transcription factors that act as repressors of adipogenesis. Nat Genet. 2019;51(4):716–27.
Hamey FK, Göttgens B. Machine learning predicts putative hematopoietic stem cells within large single-cell transcriptomics data sets. Exp Hematol. 2019;1(78):11–20.
Fidanza A, Stumpf PS, Ramachandran P, Tamagno S, Babtie A, Lopez-Yrigoyen M, et al. Single-cell analyses and machine learning define hematopoietic progenitor and HSC-like cells derived from human PSCs. Blood. 2020;136(25):2893–904.
Laurila E, Ahola A, Hyttinen J, Aalto-Setälä K. Methods for in vitro functional analysis of iPSC derived cardiomyocytes—Special focus on analyzing the mechanical beating behavior. Biochi Biophys Acta (BBA)-Mol Cell Res. 2016;1863(7):1864–72.
Juhola M, Joutsijoki H, Penttinen K, Aalto-Setälä K. Detection of genetic cardiac diseases by Ca2+ transient profiles using machine learning methods. Sci Rep. 2018;8(1):9355.
Hwang H, Liu R, Maxwell JT, Yang J, Xu C. Machine learning identifies abnormal Ca 2+ transients in human induced pluripotent stem cell-derived cardiomyocytes. Sci Rep. 2020;10(1):16977.
Franks JM, Martyanov V, Wang Y, Wood TA, Pinckney A, Crofford LJ, et al. Machine learning predicts stem cell transplant response in severe scleroderma. Ann Rheum Dis. 2020;79(12):1608–15.
Zhang Y, Tseng JT, Lien IC, Li F, Wu W, Li H. mRNAsi index: machine learning in mining lung adenocarcinoma stem cell biomarkers. Genes. 2020;11(3):257.
Li J, Lu L, Zhang YH, Xu Y, Liu M, Feng K, et al. Identification of leukemia stem cell expression signatures through Monte Carlo feature selection strategy and support vector machine. Cancer Gene Ther. 2020;27(1–2):56–69.
Aida S, Okugawa J, Fujisaka S, Kasai T, Kameda H, Sugiyama T. Deep learning of cancer stem cell morphology using conditional generative adversarial networks. Biomolecules. 2020;10(6):931.
Xu Y, Liu X, Cao X, Huang C, Liu E, Qian S, et al. Artificial intelligence: A powerful paradigm for scientific research. The Innovation. 2021;2(4).
Orita K, Sawada K, Koyama R, Ikegaya Y. Deep learning-based quality control of cultured human-induced pluripotent stem cell-derived cardiomyocytes. J Pharmacol Sci. 2019;140(4):313–6.
Ziaei D, Chapman D, Yesha Y, Halem M. Segmentation of stem cell colonies in fluorescence microscopy images with transfer learning. In Medical Imaging 2020: Image Processing 2020;11313:580–588. SPIE.
Park K, Lee JY, Lee SY, Jeong I, Park SY, Kim JW, et al. Deep learning predicts the differentiation of kidney organoids derived from human induced pluripotent stem cells. Kidney Research and Clinical Practice. 2023;42(1):75.
Adnan N, Umer F, Malik S. Implementation of transfer learning for the segmentation of human mesenchymal stem cells—A validation study. Tissue Cell. 2023;1(83): 102149.
Goodfellow I. Nips 2016 tutorial: Generative adversarial networks. arXiv preprint arXiv:1701.00160. 2016.
Krizhevsky A, Sutskever I, Hinton GE. Imagenet classification with deep convolutional neural networks. Commun ACM. 2017;60(6):84–90.
Kusumoto D, Yuasa S. The application of convolutional neural network to stem cell biology. Inflammation and regeneration. 2019;39(1):1–7.
Ramakrishna RR, Abd Hamid Z, Zaki WM, Huddin AB, Mathialagan R. Stem cell imaging through convolutional neural networks: current issues and future directions in artificial intelligence technology. PeerJ. 2020;18(8): e10346.
Azuri I, Rosenhek-Goldian I, Regev-Rudzki N, Fantner G, Cohen SR. The role of convolutional neural networks in scanning probe microscopy: a review. Beilstein J Nanotechnol. 2021;12(1):878–901.
Ottoboni L, von Wunster B, Martino G. Therapeutic plasticity of neural stem cells. Front Neurol. 2020;20(11):148.
Li Y, Nowak CM, Pham U, Nguyen K, Bleris L. Cell morphology-based machine learning models for human cell state classification. NPJ systems biology and applications. 2021;7(1):23.
Bredenoord AL, Mostert M, Isasi R, Knoppers BM. Data sharing in stem cell translational science: policy statement by the international stem cell forum ethics working party. Regen Med. 2015;10(7):857–61.
Schwessinger R, Gosden M, Downes D, Brown RC, Oudelaar AM, Telenius J, et al. DeepC: predicting 3D genome folding using megabase-scale transfer learning. Nat Methods. 2020;17(11):1118–24.
Schwab JD, Ikonomi N, Werle SD, Weidner FM, Geiger H, Kestler HA. Reconstructing Boolean network ensembles from single-cell data for unraveling dynamics in the aging of human hematopoietic stem cells. Comput Struct Biotechnol J. 2021;1(19):5321–32.
Nanni L, Paci M, Caetano dos Santos FL, Skottman H, Juuti-Uusitalo K, Hyttinen J. Texture descriptors ensembles enable image-based classification of maturation of human stem cell-derived retinal pigmented epithelium. PLoS One. 2016;11(2):e0149399.
Guan BX, Bhanu B, Theagarajan R, Liu H, Talbot P, Weng N. Human embryonic stem cell classification: Random network with autoencoded feature extractor. J Biomed Optics. 2021;26(5):052913-.
Kim G, Jeon JH, Park K, Kim SW, Kim DH, Lee S. High throughput screening of mesenchymal stem cell lines using deep learning. Sci Rep. 2022;12(1):17507.
Taylor L, Nitschke G. Improving deep learning with generic data augmentation. In2018 IEEE symposium series on computational intelligence (SSCI) 2018;1542–1547. IEEE.
Kingma DP, Welling M. Auto-encoding variational bayes. arXiv preprint arXiv:1312.6114. 2013.
Xu Y, Zhang Z, You L, Liu J, Fan Z, Zhou X. scIGANs: single-cell RNA-seq imputation using generative adversarial networks. Nucleic Acids Res. 2020;48(15):e85-e85.
Marouf M, Machart P, Bansal V, Kilian C, Magruder DS, Krebs CF, et al. Realistic in silico generation and augmentation of single-cell RNA-seq data using generative adversarial networks. Nat Commun. 2020;11(1):166.
Fan K, Zhang S, Zhang Y, Lu J, Holcombe M, Zhang X. A machine learning assisted, label-free, non-invasive approach for somatic reprogramming in induced pluripotent stem cell colony formation detection and prediction. Sci Rep. 2017;7(1):13496.
Novakovsky G, Dexter N, Libbrecht MW, Wasserman WW, Mostafavi S. Obtaining genetics insights from deep learning via explainable artificial intelligence. Nat Rev Genet. 2022;3:1–3.
Meena J, Hasija Y. Application of explainable artificial intelligence in the identification of Squamous Cell Carcinoma biomarkers. Comput Biol Med. 2022;1(146): 105505.
Keyl P, Bischoff P, Dernbach G, Bockmayr M, Fritz R, Horst D, et al. Single-cell gene regulatory network prediction by explainable AI. Nucleic Acids Res. 2023.
Yang G, Ye Q, Xia J. Unbox the black-box for the medical explainable AI via multi-modal and multi-centre data fusion: A mini-review, two showcases and beyond. Information Fusion. 2022;1(77):29–52.
Ghassemi M, Oakden-Rayner L, Beam AL. The false hope of current approaches to explainable artificial intelligence in health care. The Lancet Digital Health. 2021;3(11):e745–50.
Ashraf M, Khalilitousi M, Laksman Z. Applying machine learning to stem cell culture and differentiation. Current Protocols. 2021;1(9): e261.
Augustine S, Cheng W, Avey MT, Chan ML, Lingappa SM, Hutton B, et al. Are all stem cells equal? Systematic review, evidence map, and meta-analyses of preclinical stem cell-based therapies for bronchopulmonary dysplasia. Stem Cells Transl Med. 2020;9(2):158–68.
Kucinski I, Gottgens B. Advancing Stem Cell Research through Multimodal Single-Cell Analysis. Cold Spring Harb Perspect Biol. 2020;12(7): a035725.
van den Berg PR, Bérenger-Currias NM, Budnik B, Slavov N, Semrau S. Integration of a multi-omics stem cell differentiation dataset using a dynamical model. PLoS Genet. 2023;19(5): e1010744.
Vandereyken K, Sifrim A, Thienpont B, Voet T. Methods and applications for single-cell and spatial multi-omics. Nat Rev Genet. 2023;2:1–22.
Del Sol A, Jung S. The importance of computational modeling in stem cell research. Trends Biotechnol. 2021;39(2):126–36.
Baltrušaitis T, Ahuja C, Morency LP. Multimodal machine learning: A survey and taxonomy. IEEE Trans Pattern Anal Mach Intell. 2018;41(2):423–43.
Ramachandram D, Taylor GW. Deep multimodal learning: A survey on recent advances and trends. IEEE Signal Process Mag. 2017;34(6):96–108.
Van Den Hurk M, Bardy C. Single-cell multimodal transcriptomics to study neuronal diversity in human stem cell-derived brain tissue and organoid models. J Neurosci Methods. 2019;1(325): 108350.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mazalan, M., Do, TD., Zaman, W.S.W.K. et al. Machine Learning Approaches for Stem Cells. Curr Stem Cell Rep 9, 43–56 (2023). https://doi.org/10.1007/s40778-023-00228-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-023-00228-1