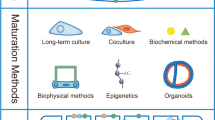
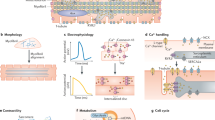
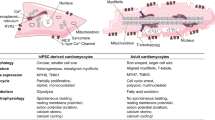
Abstract
Purpose of Review
Human induced pluripotent stem cells (hiPSC) have become one of the most promising cell biology tools over the past decade. With the potential to be differentiated into diverse human cell types, their use ranges from disease modeling and drug development, to future personalized cell therapies and regenerative medicine. One of the ongoing challenges of differentiating hiPSC is to obtain cells in vitro with a mature phenotype as close as possible to human adult cells. In this context, maturation of hiPSC-derived cardiomyocytes (hiPSC-CM) is a challenge where diverse regulatory factors must be considered to obtain functionally mature cells. This review provides an overview of the current protocols for hiPSC-CM maturation.
Recent Findings
The most promising maturation protocols include 3D tissue engineering, electrical stimulation, hormonal treatment, co-culture with non-myocyte cells, metabolic modifiers, and modification of the microenvironment, to better reproduce the native cardiac environment.
Summary
Each technique implements some improvements in hiPSC-CM maturation, leading to improved structure and function, and helping to unravel the puzzle of the heart maturation process. Future studies will need to combine different methods to further improve cardiomyocyte maturation to pave the way for heart regenerative medicine.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. https://doi.org/10.1016/j.cell.2007.11.019.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. https://doi.org/10.1126/science.1151526.
Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104(4):e30-41. https://doi.org/10.1161/CIRCRESAHA.108.192237.
Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27(4):806–11. https://doi.org/10.1002/stem.31.
Itoh M, Kiuru M, Cairo MS, Christiano AM. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2011;108(21):8797–802. https://doi.org/10.1073/pnas.1100332108.
Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19(11):1233–42. https://doi.org/10.1038/cr.2009.107.
Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122(Pt 17):3169–79. https://doi.org/10.1242/jcs.050393.
Parmar M, Grealish S, Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci. 2020;21(2):103–15. https://doi.org/10.1038/s41583-019-0257-7.
Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med. 2017;376(11):1038–46. https://doi.org/10.1056/NEJMoa1608368.
Guo Y, Pu WT. Cardiomyocyte maturation: new phase in development. Circ Res. 2020;126(8):1086–106. https://doi.org/10.1161/CIRCRESAHA.119.315862.
Ahmed RE, Anzai T, Chanthra N, Uosaki H. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front Cell Dev Biol. 2020;8:178. https://doi.org/10.3389/fcell.2020.00178.
van den Berg CW, Okawa S, Chuva de Sousa Lopes SM, van Iperen L, Passier R, Braam SR et al. Transcriptome of human foetal heart compared with cardiomyocytes from pluripotent stem cells. Development. 2015;142(18):3231–8. https://doi.org/10.1242/dev.123810.
Liu J, Laksman Z, Backx PH. The electrophysiological development of cardiomyocytes. Adv Drug Deliv Rev. 2016;96:253–73. https://doi.org/10.1016/j.addr.2015.12.023.
Maroli G, Braun T. The long and winding road of cardiomyocyte maturation. Cardiovasc Res. 2020. https://doi.org/10.1093/cvr/cvaa159.
Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol. 2020;17(6):341–59. https://doi.org/10.1038/s41569-019-0331-x.
Gilbert G, Demydenko K, Dries E, Puertas RD, Jin X, Sipido K et al. Calcium signaling in cardiomyocyte function. Cold Spring Harb Perspect Biol. 2020;12(3). https://doi.org/10.1101/cshperspect.a035428.
Stankovicova T, Szilard M, De Scheerder I, Sipido KR. M cells and transmural heterogeneity of action potential configuration in myocytes from the left ventricular wall of the pig heart. Cardiovasc Res. 2000;45(4):952–60. https://doi.org/10.1016/s0008-6363(99)00418-6.
Frisk M, Koivumaki JT, Norseng PA, Maleckar MM, Sejersted OM, Louch WE. Variable t-tubule organization and Ca2+ homeostasis across the atria. Am J Physiol Heart Circ Physiol. 2014;307(4):H609–20. https://doi.org/10.1152/ajpheart.00295.2014.
Johnson EK, Springer SJ, Wang W, Dranoff EJ, Zhang Y, Kanter EM, et al. Differential expression and remodeling of transient outward potassium currents in human left ventricles. Circ Arrhythm Electrophysiol. 2018;11(1): e005914. https://doi.org/10.1161/CIRCEP.117.005914.
Sanchez-Alonso JL, Loucks A, Schobesberger S, van Cromvoirt AM, Poulet C, Chowdhury RA, et al. Nanoscale regulation of L-type calcium channels differentiates between ischemic and dilated cardiomyopathies. EBioMedicine. 2020;57: 102845. https://doi.org/10.1016/j.ebiom.2020.102845.
Dries E, Santiago DJ, Gilbert G, Lenaerts I, Vandenberk B, Nagaraju CK, et al. Hyperactive ryanodine receptors in human heart failure and ischaemic cardiomyopathy reside outside of couplons. Cardiovasc Res. 2018;114(11):1512–24. https://doi.org/10.1093/cvr/cvy088.
Ohler A, Weisser-Thomas J, Piacentino V, Houser SR, Tomaselli GF, O’Rourke B. Two-photon laser scanning microscopy of the transverse-axial tubule system in ventricular cardiomyocytes from failing and non-failing human hearts. Cardiol Res Pract. 2009;2009: 802373. https://doi.org/10.4061/2009/802373.
Vermij SH, Abriel H, van Veen TA. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc Res. 2017;113(3):259–75. https://doi.org/10.1093/cvr/cvw259.
Jones PP, MacQuaide N, Louch WE. Dyadic plasticity in cardiomyocytes Front Physiol. 2018;9:1773. https://doi.org/10.3389/fphys.2018.01773.
Crossman DJ, Ruygrok PN, Soeller C, Cannell MB. Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS One. 2011;6(3): e17901. https://doi.org/10.1371/journal.pone.0017901.
Sanchez-Alonso JL, Bhargava A, O’Hara T, Glukhov AV, Schobesberger S, Bhogal N, et al. Microdomain-specific modulation of L-type calcium channels leads to triggered ventricular arrhythmia in heart failure. Circ Res. 2016;119(8):944–55. https://doi.org/10.1161/CIRCRESAHA.116.308698.
Lipsett DB, Frisk M, Aronsen JM, Norden ES, Buonarati OR, Cataliotti A, et al. Cardiomyocyte substructure reverts to an immature phenotype during heart failure. J Physiol. 2019;597(7):1833–53. https://doi.org/10.1113/JP277273.
Crocini C, Ferrantini C, Scardigli M, Coppini R, Mazzoni L, Lazzeri E, et al. Novel insights on the relationship between T-tubular defects and contractile dysfunction in a mouse model of hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2016;91:42–51. https://doi.org/10.1016/j.yjmcc.2015.12.013.
Crossman DJ, Young AA, Ruygrok PN, Nason GP, Baddelely D, Soeller C, et al. T-tubule disease: Relationship between t-tubule organization and regional contractile performance in human dilated cardiomyopathy. J Mol Cell Cardiol. 2015;84:170–8. https://doi.org/10.1016/j.yjmcc.2015.04.022.
Hou Y, Jayasinghe I, Crossman DJ, Baddeley D, Soeller C. Nanoscale analysis of ryanodine receptor clusters in dyadic couplings of rat cardiac myocytes. J Mol Cell Cardiol. 2015;80:45–55. https://doi.org/10.1016/j.yjmcc.2014.12.013.
Wong J, Baddeley D, Bushong EA, Yu Z, Ellisman MH, Hoshijima M, et al. Nanoscale distribution of ryanodine receptors and caveolin-3 in mouse ventricular myocytes: dilation of t-tubules near junctions. Biophys J. 2013;104(11):L22–4. https://doi.org/10.1016/j.bpj.2013.02.059.
Jayasinghe I, Crossman D, Soeller C, Cannell M. Comparison of the organization of T-tubules, sarcoplasmic reticulum and ryanodine receptors in rat and human ventricular myocardium. Clin Exp Pharmacol Physiol. 2012;39(5):469–76. https://doi.org/10.1111/j.1440-1681.2011.05578.x.
Seidel T, Navankasattusas S, Ahmad A, Diakos NA, Xu WD, Tristani-Firouzi M, et al. Sheet-like remodeling of the transverse tubular system in human heart failure impairs excitation-contraction coupling and functional recovery by mechanical unloading. Circulation. 2017;135(17):1632–45. https://doi.org/10.1161/CIRCULATIONAHA.116.024470.
Kaprielian RR, Stevenson S, Rothery SM, Cullen MJ, Severs NJ. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation. 2000;101(22):2586–94. https://doi.org/10.1161/01.cir.101.22.2586.
Rog-Zielinska EA, Kong CHT, Zgierski-Johnston CM, Verkade P, Mantell J, Cannell MB et al. Species differences in the morphology of transverse tubule openings in cardiomyocytes. Europace. 2018;20(suppl_3):iii120-iii4. https://doi.org/10.1093/europace/euy245.
Glukhov AV, Balycheva M, Sanchez-Alonso JL, Ilkan Z, Alvarez-Laviada A, Bhogal N, et al. Direct evidence for microdomain-specific localization and remodeling of functional L-Type calcium channels in rat and human atrial myocytes. Circulation. 2015;132(25):2372–84. https://doi.org/10.1161/CIRCULATIONAHA.115.018131.
Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. 2003;92(11):1182–92. https://doi.org/10.1161/01.RES.0000074908.17214.FD.
Richards MA, Clarke JD, Saravanan P, Voigt N, Dobrev D, Eisner DA, et al. Transverse tubules are a common feature in large mammalian atrial myocytes including human. Am J Physiol Heart Circ Physiol. 2011;301(5):H1996-2005. https://doi.org/10.1152/ajpheart.00284.2011.
Munro ML, Soeller C. Early transverse tubule development begins in utero in the sheep heart. J Muscle Res Cell Motil. 2016;37(6):195–202. https://doi.org/10.1007/s10974-016-9462-4.
Ziman AP, Gomez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol. 2010;48(2):379–86. https://doi.org/10.1016/j.yjmcc.2009.09.016.
Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, et al. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8(2): e1000312. https://doi.org/10.1371/journal.pbio.1000312.
Reynolds JO, Chiang DY, Wang W, Beavers DL, Dixit SS, Skapura DG, et al. Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc Res. 2013;100(1):44–53. https://doi.org/10.1093/cvr/cvt133.
Chen B, Guo A, Zhang C, Chen R, Zhu Y, Hong J, et al. Critical roles of junctophilin-2 in T-tubule and excitation-contraction coupling maturation during postnatal development. Cardiovasc Res. 2013;100(1):54–62. https://doi.org/10.1093/cvr/cvt180.
Di Maio A, Karko K, Snopko RM, Mejia-Alvarez R, Franzini-Armstrong C. T-tubule formation in cardiacmyocytes: two possible mechanisms? J Muscle Res Cell Motil. 2007;28(4–5):231–41. https://doi.org/10.1007/s10974-007-9121-x.
Dai H, Much AA, Maor E, Asher E, Younis A, Xu Y, et al. Global, regional, and national burden of ischemic heart disease and its attributable risk factors, 1990–2017: results from the global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2020. https://doi.org/10.1093/ehjqcco/qcaa076.
Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–45. https://doi.org/10.1093/eurheartj/ehw334.
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics–2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–743. https://doi.org/10.1161/CIR.0000000000000950.
Dries E, Amoni M, Vandenberk B, Johnson DM, Gilbert G, Nagaraju CK, et al. Altered adrenergic response in myocytes bordering a chronic myocardial infarction underlies in vivo triggered activity and repolarization instability. J Physiol. 2020;598(14):2875–95. https://doi.org/10.1113/JP278839.
Weinberger F, Eschenhagen T. Cardiac regeneration: new hope for an old dream. Annu Rev Physiol. 2021;83:59–81. https://doi.org/10.1146/annurev-physiol-031120-103629.
• Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. 2018;71(4):429-38. https://doi.org/10.1016/j.jacc.2017.11.047. Proof of concept to use a cardiac patch of stem cells in a diseased human heart.
Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med. 2016;8(363):363ra148. https://doi.org/10.1126/scitranslmed.aaf8781.
Castro L, Geertz B, Reinsch M, Aksehirlioglu B, Hansen A, Eschenhagen T et al. Implantation of hiPSC-derived cardiac-muscle patches after myocardial injury in a guinea pig model. J Vis Exp. 2019(145). https://doi.org/10.3791/58810.
Querdel E, Reinsch M, Castro L, Kose D, Bahr A, Reich S, et al. Human engineered heart tissue patches remuscularize the injured heart in a dose-dependent manner. Circulation. 2021. https://doi.org/10.1161/CIRCULATIONAHA.120.047904.
Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun. 2017;8(1):1825. https://doi.org/10.1038/s41467-017-01946-x.
Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun. 2019;10(1):3123. https://doi.org/10.1038/s41467-019-11091-2.
Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538(7625):388–91. https://doi.org/10.1038/nature19815.
Fan C, Fast VG, Tang Y, Zhao M, Turner JF, Krishnamurthy P, et al. Cardiomyocytes from CCND2-overexpressing human induced-pluripotent stem cells repopulate the myocardial scar in mice: A 6-month study. J Mol Cell Cardiol. 2019;137:25–33. https://doi.org/10.1016/j.yjmcc.2019.09.011.
Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489(7415):322–5. https://doi.org/10.1038/nature11317.
Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc. 2013;8(1):162–75. https://doi.org/10.1038/nprot.2012.150.
Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109(27):E1848–57. https://doi.org/10.1073/pnas.1200250109.
Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11(8):855–60. https://doi.org/10.1038/nmeth.2999.
Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111(3):344–58. https://doi.org/10.1161/CIRCRESAHA.110.227512.
Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A. 2017;114(40):E8372–81. https://doi.org/10.1073/pnas.1707316114.
Abilez OJ, Tzatzalos E, Yang H, Zhao MT, Jung G, Zollner AM, et al. Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells. 2018;36(2):265–77. https://doi.org/10.1002/stem.2732.
Goldfracht I, Protze S, Shiti A, Setter N, Gruber A, Shaheen N, et al. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat Commun. 2020;11(1):75. https://doi.org/10.1038/s41467-019-13868-x.
Meijer van Putten RM, Mengarelli I, Guan K, Zegers JG, van Ginneken AC, Verkerk AO et al. Ion channelopathies in human induced pluripotent stem cell derived cardiomyocytes: a dynamic clamp study with virtual IK1. Front Physiol. 2015;6:7. https://doi.org/10.3389/fphys.2015.00007.
Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114(3):511–23. https://doi.org/10.1161/CIRCRESAHA.114.300558.
Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight. 2018;3(12). https://doi.org/10.1172/jci.insight.99941.
Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. 2019;176(4):913–27 e18. https://doi.org/10.1016/j.cell.2018.11.042.
Lemme M, Ulmer BM, Lemoine MD, Zech ATL, Flenner F, Ravens U, et al. Atrial-like engineered heart tissue: an in vitro model of the human atrium. Stem Cell Reports. 2018;11(6):1378–90. https://doi.org/10.1016/j.stemcr.2018.10.008.
Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, et al. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat Biotechnol. 2017;35(1):56–68. https://doi.org/10.1038/nbt.3745.
Moss JB, Xavier-Neto J, Shapiro MD, Nayeem SM, McCaffery P, Drager UC, et al. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev Biol. 1998;199(1):55–71. https://doi.org/10.1006/dbio.1998.8911.
Xavier-Neto J, Shapiro MD, Houghton L, Rosenthal N. Sequential programs of retinoic acid synthesis in the myocardial and epicardial layers of the developing avian heart. Dev Biol. 2000;219(1):129–41. https://doi.org/10.1006/dbio.1999.9588.
Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100(2):263–72. https://doi.org/10.1161/01.RES.0000257776.05673.ff.
Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109(1):47–59. https://doi.org/10.1161/CIRCRESAHA.110.237206.
Schaaf S, Shibamiya A, Mewe M, Eder A, Stohr A, Hirt MN, et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6(10): e26397. https://doi.org/10.1371/journal.pone.0026397.
Soong PL, Tiburcy M, Zimmermann WH. Cardiac differentiation of human embryonic stem cells and their assembly into engineered heart muscle. Curr Protoc Cell Biol. 2012;Chapter 23:Unit23 8. https://doi.org/10.1002/0471143030.cb2308s55.
Leonard A, Bertero A, Powers JD, Beussman KM, Bhandari S, Regnier M, et al. Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. J Mol Cell Cardiol. 2018;118:147–58. https://doi.org/10.1016/j.yjmcc.2018.03.016.
•• Huang CY, Peres Moreno Maia-Joca R, Ong CS, Wilson I, DiSilvestre D, Tomaselli GF et al. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. J Mol Cell Cardiol. 2020;138:1–11. https://doi.org/10.1016/j.yjmcc.2019.10.001. First combination of co-culture, chemical treatment and 3D to mature hiPSC-CM.
Breckwoldt K, Letuffe-Breniere D, Mannhardt I, Schulze T, Ulmer B, Werner T, et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat Protoc. 2017;12(6):1177–97. https://doi.org/10.1038/nprot.2017.033.
Balistreri M, Davis JA, Campbell KF, Da Rocha AM, Treadwell MC, Herron TJ. Effect of glucose on 3D cardiac microtissues derived from human induced pluripotent stem cells. Pediatr Cardiol. 2017;38(8):1575–82. https://doi.org/10.1007/s00246-017-1698-2.
Jackman CP, Carlson AL, Bursac N. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials. 2016;111:66–79. https://doi.org/10.1016/j.biomaterials.2016.09.024.
Zimmermann WH. Engineered Heart Muscle Models in Phenotypic Drug Screens. Handb Exp Pharmacol. 2020. https://doi.org/10.1007/164_2020_385.
Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao ML, et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation. 2017;135(19):1832–47. https://doi.org/10.1161/CIRCULATIONAHA.116.024145.
Mannhardt I, Saleem U, Mosqueira D, Loos MF, Ulmer BM, Lemoine MD, Larsson C, Améen C, de Korte T, Vlaming ML, Harris K. Comparison of 10 control hPSC lines for drug screening in an engineered heart tissue format. Stem cell reports. 2020 Oc MAt 13;15(4):983-98. https://doi.org/10.1016/j.stemcr.2020.09.002.
Mannhardt I, Breckwoldt K, Letuffe-Breniere D, Schaaf S, Schulz H, Neuber C, et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Reports. 2016;7(1):29–42. https://doi.org/10.1016/j.stemcr.2016.04.011.
Lemoine MD, Mannhardt I, Breckwoldt K, Prondzynski M, Flenner F, Ulmer B, et al. Human iPSC-derived cardiomyocytes cultured in 3D engineered heart tissue show physiological upstroke velocity and sodium current density. Sci Rep. 2017;7(1):5464. https://doi.org/10.1038/s41598-017-05600-w.
Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, et al. Contractile work contributes to maturation of energy metabolism in hiPSC-derived cardiomyocytes. Stem Cell Reports. 2018;10(3):834–47. https://doi.org/10.1016/j.stemcr.2018.01.039.
Lemoine MD, Krause T, Koivumaki JT, Prondzynski M, Schulze ML, Girdauskas E, et al. Human induced pluripotent stem cell-derived engineered heart tissue as a sensitive test system for QT prolongation and arrhythmic triggers. Circ Arrhythm Electrophysiol. 2018;11(7): e006035. https://doi.org/10.1161/CIRCEP.117.006035.
Uzun AU, Mannhardt I, Breckwoldt K, Horvath A, Johannsen SS, Hansen A, et al. Ca(2+)-Currents in human induced pluripotent stem cell-derived cardiomyocytes effects of two different culture conditions. Front Pharmacol. 2016;7:300. https://doi.org/10.3389/fphar.2016.00300.
Horvath A, Lemoine MD, Loser A, Mannhardt I, Flenner F, Uzun AU et al. Low resting membrane potential and low inward rectifier potassium currents are not inherent features of hiPSC-derived cardiomyocytes. stem cell reports. 2018;10(3):822–33. https://doi.org/10.1016/j.stemcr.2018.01.012.
Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556(7700):239–43. https://doi.org/10.1038/s41586-018-0016-3.
Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10(8):781–7. https://doi.org/10.1038/nmeth.2524.
Wang EY, Rafatian N, Zhao Y, Lee A, Lai BFL, Lu RX, et al. Biowire model of interstitial and focal cardiac fibrosis. ACS Cent Sci. 2019;5(7):1146–58. https://doi.org/10.1021/acscentsci.9b00052.
Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Bornchen C, Muller C, et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol. 2014;74:151–61. https://doi.org/10.1016/j.yjmcc.2014.05.009.
Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, et al. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation. 2016;134(20):1557–67. https://doi.org/10.1161/CIRCULATIONAHA.114.014998.
Geng L, Wang Z, Cui C, Zhu Y, Shi J, Wang J, et al. Rapid electrical stimulation increased cardiac apoptosis through disturbance of calcium homeostasis and mitochondrial dysfunction in human induced pluripotent stem cell-derived cardiomyocytes. Cell Physiol Biochem. 2018;47(3):1167–80. https://doi.org/10.1159/000490213.
Cui C, Geng L, Shi J, Zhu Y, Yang G, Wang Z, et al. Structural and electrophysiological dysfunctions due to increased endoplasmic reticulum stress in a long-term pacing model using human induced pluripotent stem cell-derived ventricular cardiomyocytes. Stem Cell Res Ther. 2017;8(1):109. https://doi.org/10.1186/s13287-017-0566-6.
Litvinukova M, Talavera-Lopez C, Maatz H, Reichart D, Worth CL, Lindberg EL, et al. Cells of the adult human heart. Nature. 2020;588(7838):466–72. https://doi.org/10.1038/s41586-020-2797-4.
Rubart M, Tao W, Lu XL, Conway SJ, Reuter SP, Lin SF, et al. Electrical coupling between ventricular myocytes and myofibroblasts in the infarcted mouse heart. Cardiovasc Res. 2018;114(3):389–400. https://doi.org/10.1093/cvr/cvx163.
Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X, van Helden RWJ et al. Human-iPSC- derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell. 2020;26(6):862–79 e11. https://doi.org/10.1016/j.stem.2020.05.004.
Beauchamp P, Jackson CB, Ozhathil LC, Agarkova I, Galindo CL, Sawyer DB, et al. 3D Co-culture of hiPSC-derived cardiomyocytes with cardiac fibroblasts improves tissue-like features of cardiac spheroids. Front Mol Biosci. 2020;7:14. https://doi.org/10.3389/fmolb.2020.00014.
Giacomelli E, Bellin M, Sala L, van Meer BJ, Tertoolen LG, Orlova VV, et al. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development. 2017;144(6):1008–17. https://doi.org/10.1242/dev.143438.
Dunn KK, Reichardt IM, Simmons AD, Jin G, Floy ME, Hoon KM, et al. Coculture of endothelial cells with human pluripotent stem cell-derived cardiac progenitors reveals a differentiation stage-specific enhancement of cardiomyocyte maturation. Biotechnol J. 2019;14(8): e1800725. https://doi.org/10.1002/biot.201800725.
Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370(6514):364–8. https://doi.org/10.1126/science.abc8861.
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207–58. https://doi.org/10.1152/physrev.00015.2009.
Yang X, Rodriguez ML, Leonard A, Sun L, Fischer KA, Wang Y, et al. Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Reports. 2019;13(4):657–68. https://doi.org/10.1016/j.stemcr.2019.08.013.
Feyen DAM, McKeithan WL, Bruyneel AAN, Spiering S, Hormann L, Ulmer B, et al. Metabolic maturation media improve physiological function of human iPSC-derived cardiomyocytes. Cell Rep. 2020;32(3): 107925. https://doi.org/10.1016/j.celrep.2020.107925.
Li M, Iismaa SE, Naqvi N, Nicks A, Husain A, Graham RM. Thyroid hormone action in postnatal heart development. Stem Cell Res. 2014;13(3 Pt B):582–91. https://doi.org/10.1016/j.scr.2014.07.001.
Rog-Zielinska EA, Craig MA, Manning JR, Richardson RV, Gowans GJ, Dunbar DR, et al. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1alpha. Cell Death Differ. 2015;22(7):1106–16. https://doi.org/10.1038/cdd.2014.181.
Yang X, Rodriguez M, Pabon L, Fischer KA, Reinecke H, Regnier M, et al. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol. 2014;72:296–304. https://doi.org/10.1016/j.yjmcc.2014.04.005.
Birket MJ, Ribeiro MC, Kosmidis G, Ward D, Leitoguinho AR, van de Pol V, et al. Contractile defect caused by mutation in MYBPC3 revealed under conditions optimized for human PSC-cardiomyocyte function. Cell Rep. 2015;13(4):733–45. https://doi.org/10.1016/j.celrep.2015.09.025.
Jackman C, Li H, Bursac N. Long-term contractile activity and thyroid hormone supplementation produce engineered rat myocardium with adult-like structure and function. Acta Biomater. 2018;78:98–110. https://doi.org/10.1016/j.actbio.2018.08.003.
•• Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K et al. Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res. 2017;121(12):1323-30. https://doi.org/10.1161/CIRCRESAHA.117.311920. Improved functional and structural maturation of hiPSC-CM with the presence of a tubule network and homogeneous calcium transiants.
Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, et al. Matrigel mattress: a method for the generation of single contracting human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res. 2015;117(12):995–1000. https://doi.org/10.1161/CIRCRESAHA.115.307580.
Rao C, Prodromakis T, Kolker L, Chaudhry UA, Trantidou T, Sridhar A, et al. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials. 2013;34(10):2399–411. https://doi.org/10.1016/j.biomaterials.2012.11.055.
Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci USA. 2015;112(41):12705–10. https://doi.org/10.1073/pnas.1508073112.
Silbernagel N, Korner A, Balitzki J, Jaggy M, Bertels S, Richter B, et al. Shaping the heart: structural and functional maturation of iPSC-cardiomyocytes in 3D-micro-scaffolds. Biomaterials. 2020;227: 119551. https://doi.org/10.1016/j.biomaterials.2019.119551.
Cho GS, Lee DI, Tampakakis E, Murphy S, Andersen P, Uosaki H, et al. Neonatal transplantation confers maturation of PSC-derived cardiomyocytes conducive to modeling cardiomyopathy. Cell Rep. 2017;18(2):571–82. https://doi.org/10.1016/j.celrep.2016.12.040.
Cho GS, Tampakakis E, Andersen P, Kwon C. Use of a neonatal rat system as a bioincubator to generate adult-like mature cardiomyocytes from human and mouse pluripotent stem cells. Nat Protoc. 2017;12(10):2097–109. https://doi.org/10.1038/nprot.2017.089.
Kadota S, Pabon L, Reinecke H, Murry CE. In vivo maturation of human induced pluripotent stem cell-derived cardiomyocytes in neonatal and adult rat hearts. Stem Cell Reports. 2017;8(2):278–89. https://doi.org/10.1016/j.stemcr.2016.10.009.
Guyette JP, Charest JM, Mills RW, Jank BJ, Moser PT, Gilpin SE, et al. Bioengineering human myocardium on native extracellular matrix. Circ Res. 2016;118(1):56–72. https://doi.org/10.1161/CIRCRESAHA.115.306874.
Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun. 2013;4:2307. https://doi.org/10.1038/ncomms3307.
De La Mata A, Tajada S, O’Dwyer S, Matsumoto C, Dixon RE, Hariharan N, et al. BIN1 induces the formation of T-tubules and adult-like Ca(2+) release units in developing cardiomyocytes. Stem Cells. 2019;37(1):54–64. https://doi.org/10.1002/stem.2927.
Funding
Dr Guillaume Gilbert received funding from a FWO postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Gilbert has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gilbert, G. Approaches to Optimize Stem Cell-Derived Cardiomyocyte Maturation and Function. Curr Stem Cell Rep 7, 140–160 (2021). https://doi.org/10.1007/s40778-021-00197-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-021-00197-3