Abstract
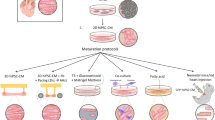
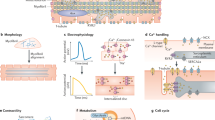
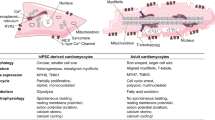
Human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) represent one of the most promising ways to treat cardiovascular diseases. High-purity cardiomyocytes (CM) from different cell sources could be obtained at present. However, the immature nature of these cardiomyocytes hinders its further clinical application. From immature to mature state, it involves structural, functional, and metabolic changes in cardiomyocytes. Generally, two types of culturing (2D and 3D) systems have been reported to induce cardiomyocyte maturation. 2D culture mainly achieves the maturation of cardiomyocytes through long-term culture, co-culture, supplementation of small molecule compounds, and the application of biophysical cues. The combined use of biomaterial's surface topography and biophysical cues also facilitates the maturation of cardiomyocytes. Cardiomyocyte maturation is a complex process involving many signaling pathways, and current methods fail to fully reproduce this process. Therefore, analyzing the signaling pathway network related to the maturation and producing hPSC-CMs with adult-like phenotype is a challenge. In this review, we summarized the structural and functional differences between hPSC-CMs and mature cardiomyocytes, and introduced various methods to induce cardiomyocyte maturation.
Graphical Abstract



Similar content being viewed by others
Data Availability
Not applicable
References
Xiang, Y., Tanaka, Y., Patterson, B., Kang, Y.-J., Govindaiah, G., Roselaar, N., et al. (2017). Fusion of regionally specified hPSC-derived Organoids models human brain development and interneuron migration. Cell Stem Cell, 21(3). https://doi.org/10.1016/j.stem.2017.07.007
Zhu, Z., Li, Q. V., Lee, K., Rosen, B. P., González, F., Soh, C.-L., & Huangfu, D. (2016). Genome editing of lineage determinants in human pluripotent stem cells reveals mechanisms of pancreatic development and diabetes. Cell Stem Cell, 18(6), 755–768. https://doi.org/10.1016/j.stem.2016.03.015
Jiang, X., Chen, Y., Liu, X., Ye, L., Yu, M., Shen, Z., Lei, W., & Hu, S. (2021). Uncovering inherited cardiomyopathy with human induced pluripotent stem cells. Frontiers in Cell and Development Biology, 9, 672039. https://doi.org/10.3389/fcell.2021.672039
Ye, L., Ni, X., Zhao, Z. A., Lei, W., & Hu, S. (2018). The application of induced pluripotent stem cells in cardiac disease modeling and drug testing. Journal of Cardiovascular Translational Research, 11(5), 366–374. https://doi.org/10.1007/s12265-018-9811-3
Garg, P., Garg, V., Shrestha, R., Sanguinetti, M. C., Kamp, T. J., & Wu, J. C. (2018). Human induced pluripotent stem cell-derived Cardiomyocytes as models for cardiac Channelopathies: A primer for non-Electrophysiologists. Circulation Research, 123(2), 224–243. https://doi.org/10.1161/CIRCRESAHA.118.311209
Matsa, E., Ahrens, J. H., & Wu, J. C. (2016). Human induced pluripotent stem cells as a platform for personalized and precision cardiovascular medicine. Physiological Reviews, 96(3), 1093–1126. https://doi.org/10.1152/physrev.00036.2015
Ye, L., Yu, Y., Zhao, Z. A., Zhao, D., Ni, X., Wang, Y., Fang, X., Yu, M., Wang, Y., Tang, J. M., Chen, Y., Shen, Z., Lei, W., & Hu, S. (2021). Patient-specific iPSC-derived cardiomyocytes reveal abnormal regulation of FGF16 in a familial atrial septal defect. Cardiovascular Research. https://doi.org/10.1093/cvr/cvab154
Lam, C. K., & Wu, J. C. (2021). Clinical trial in a dish: Using patient-derived induced pluripotent stem cells to identify risks of drug-induced Cardiotoxicity. Arteriosclerosis, Thrombosis, and Vascular Biology, 41(3), 1019–1031. https://doi.org/10.1161/ATVBAHA.120.314695
Paik, D. T., Chandy, M., & Wu, J. C. (2020). Patient and disease-specific induced pluripotent stem cells for discovery of personalized cardiovascular drugs and therapeutics. Pharmacological Reviews, 72(1), 320–342. https://doi.org/10.1124/pr.116.013003
Ni, X., Yang, Z. Z., Ye, L. Q., Han, X. L., Zhao, D. D., Ding, F. Y., Ding, N., Wu, H. C., Yu, M., Xu, G. Y., Zhao, Z. A., Lei, W., & Hu, S. J. (2021). Establishment of an in vitro safety assessment model for lipid-lowering drugs using same-origin human pluripotent stem cell-derived cardiomyocytes and endothelial cells. Acta Pharmacologica Sinica. https://doi.org/10.1038/s41401-021-00621-8
Yanamandala, M., Zhu, W., Garry, D. J., Kamp, T. J., Hare, J. M., Jun, H. W., Yoon, Y. S., Bursac, N., Prabhu, S. D., Dorn 2nd, G. W., Bolli, R., Kitsis, R. N., & Zhang, J. (2017). Overcoming the roadblocks to cardiac cell therapy using tissue engineering. Journal of the American College of Cardiology, 70(6), 766–775. https://doi.org/10.1016/j.jacc.2017.06.012
Meyer, T., Tiburcy, M., & Zimmermann, W. H. (2019). Cardiac macrotissues-on-a-plate models for phenotypic drug screens. Advanced Drug Delivery Reviews, 140, 93–100. https://doi.org/10.1016/j.addr.2019.03.002
Liu, Y. W., Chen, B., Yang, X., Fugate, J. A., Kalucki, F. A., Futakuchi-Tsuchida, A., Couture, L., Vogel, K. W., Astley, C. A., Baldessari, A., Ogle, J., Don, C. W., Steinberg, Z. L., Seslar, S. P., Tuck, S. A., Tsuchida, H., Naumova, A. V., Dupras, S. K., Lyu, M. S., et al. (2018). Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nature Biotechnology, 36(7), 597–605. https://doi.org/10.1038/nbt.4162
Gerdes, A. M., & Capasso, J. M. (1995). Structural remodeling and mechanical dysfunction of cardiac myocytes in heart failure. Journal of Molecular and Cellular Cardiology, 27(3), 849–856. https://doi.org/10.1016/0022-2828(95)90000-4
Lundy, S. D., Zhu, W. Z., Regnier, M., & Laflamme, M. A. (2013). Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells and Development, 22(14), 1991–2002. https://doi.org/10.1089/scd.2012.0490
Ribeiro, M. C., Tertoolen, L. G., Guadix, J. A., Bellin, M., Kosmidis, G., D'Aniello, C., Monshouwer-Kloots, J., Goumans, M. J., Wang, Y. L., Feinberg, A. W., Mummery, C. L., & Passier, R. (2015). Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro--correlation between contraction force and electrophysiology. Biomaterials, 51, 138–150. https://doi.org/10.1016/j.biomaterials.2015.01.067
Ronaldson-Bouchard, K., Ma, S. P., Yeager, K., Chen, T., Song, L., Sirabella, D., Morikawa, K., Teles, D., Yazawa, M., & Vunjak-Novakovic, G. (2018). Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature, 556(7700), 239–243. https://doi.org/10.1038/s41586-018-0016-3
Feric, N. T., & Radisic, M. (2016). Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Advanced Drug Delivery Reviews, 96, 110–134. https://doi.org/10.1016/j.addr.2015.04.019
Feinberg, A. W., Alford, P. W., Jin, H., Ripplinger, C. M., Werdich, A. A., Sheehy, S. P., Grosberg, A., & Parker, K. K. (2012). Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials, 33(23), 5732–5741. https://doi.org/10.1016/j.biomaterials.2012.04.043
Kamakura, T., Makiyama, T., Sasaki, K., Yoshida, Y., Wuriyanghai, Y., Chen, J., Hattori, T., Ohno, S., Kita, T., Horie, M., Yamanaka, S., & Kimura, T. (2013). Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circulation Journal, 77(5), 1307–1314. https://doi.org/10.1253/circj.cj-12-0987
Dai, D. F., Danoviz, M. E., Wiczer, B., Laflamme, M. A., & Tian, R. (2017). Mitochondrial maturation in human pluripotent stem cell derived Cardiomyocytes. Stem Cells International, 2017, 5153625. https://doi.org/10.1155/2017/5153625
Scuderi, G. J., & Butcher, J. (2017). Naturally engineered maturation of Cardiomyocytes. Frontiers in Cell and Development Biology, 5, 50. https://doi.org/10.3389/fcell.2017.00050
Broughton, K. M., & Sussman, M. A. (2019). Adult Cardiomyocyte cell cycle detour: Off-ramp to quiescent destinations. Trends in Endocrinology and Metabolism, 30(8), 557–567. https://doi.org/10.1016/j.tem.2019.05.006
Liu, S. J. (2013). Characterization of functional capacity of adult ventricular myocytes in long-term culture. International Journal of Cardiology, 168(3), 1923–1936. https://doi.org/10.1016/j.ijcard.2012.12.100
Gan, P., Patterson, M., & Sucov, H. M. (2020). Cardiomyocyte polyploidy and implications for heart regeneration. Annual Review of Physiology, 82, 45–61. https://doi.org/10.1146/annurev-physiol-021119-034618
Guo, Y., & Pu, W. T. (2020). Cardiomyocyte maturation: New phase in development. Circulation Research, 126(8), 1086–1106. https://doi.org/10.1161/CIRCRESAHA.119.315862
Maroli, G., & Braun, T. (2021). The long and winding road of cardiomyocyte maturation. Cardiovascular Research, 117(3), 712–726. https://doi.org/10.1093/cvr/cvaa159
Piquereau, J., & Ventura-Clapier, R. (2018). Maturation of cardiac energy metabolism during perinatal development. Frontiers in Physiology, 9, 959. https://doi.org/10.3389/fphys.2018.00959
Malandraki-Miller, S., Lopez, C. A., Al-Siddiqi, H., & Carr, C. A. (2018). Changing metabolism in differentiating cardiac progenitor cells-can stem cells become metabolically flexible Cardiomyocytes? Frontiers in Cardiovascular Medicine, 5, 119. https://doi.org/10.3389/fcvm.2018.00119
Stincone, A., Prigione, A., Cramer, T., Wamelink, M. M., Campbell, K., Cheung, E., Olin-Sandoval, V., Gruning, N. M., Kruger, A., Tauqeer Alam, M., Keller, M. A., Breitenbach, M., Brindle, K. M., Rabinowitz, J. D., & Ralser, M. (2015). The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biological Reviews of the Cambridge Philosophical Society, 90(3), 927–963. https://doi.org/10.1111/brv.12140
Varro, A., Tomek, J., Nagy, N., Virag, L., Passini, E., Rodriguez, B., & Baczko, I. (2021). Cardiac transmembrane ion channels and action potentials: Cellular physiology and arrhythmogenic behavior. Physiological Reviews, 101(3), 1083–1176. https://doi.org/10.1152/physrev.00024.2019
Veerman, C. C., Mengarelli, I., Lodder, E. M., Kosmidis, G., Bellin, M., Zhang, M., Dittmann, S., Guan, K., Wilde, A. A. M., Schulze-Bahr, E., Greber, B., Bezzina, C. R., & Verkerk, A. O. (2017). Switch from fetal to adult SCN5A isoform in human induced pluripotent stem cell-derived Cardiomyocytes unmasks the cellular phenotype of a conduction disease-causing mutation. Journal of the American Heart Association, 6(7). https://doi.org/10.1161/JAHA.116.005135
Wang, Y., Zhu, R., & Tung, L. (2019). Contribution of potassium channels to action potential repolarization of human embryonic stem cell-derived cardiomyocytes. British Journal of Pharmacology, 176(15), 2780–2794. https://doi.org/10.1111/bph.14704
Dewenter, M., von der Lieth, A., Katus, H. A., & Backs, J. (2017). Calcium signaling and transcriptional regulation in Cardiomyocytes. Circulation Research, 121(8), 1000–1020. https://doi.org/10.1161/CIRCRESAHA.117.310355
Eisner, D. A., Caldwell, J. L., Kistamas, K., & Trafford, A. W. (2017). Calcium and excitation-contraction coupling in the heart. Circulation Research, 121(2), 181–195. https://doi.org/10.1161/CIRCRESAHA.117.310230
Shattock, M. J., Ottolia, M., Bers, D. M., Blaustein, M. P., Boguslavskyi, A., Bossuyt, J., Bridge, J. H., Chen-Izu, Y., Clancy, C. E., Edwards, A., Goldhaber, J., Kaplan, J., Lingrel, J. B., Pavlovic, D., Philipson, K., Sipido, K. R., & Xie, Z. J. (2015). Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. The Journal of Physiology, 593(6), 1361–1382. https://doi.org/10.1113/jphysiol.2014.282319
Li, S., Chopra, A., Keung, W., Chan, C. W. Y., Costa, K. D., Kong, C. W., Hajjar, R. J., Chen, C. S., & Li, R. A. (2019). Sarco/endoplasmic reticulum Ca(2+)-ATPase is a more effective calcium remover than sodium-calcium exchanger in human embryonic stem cell-derived cardiomyocytes. American Journal of Physiology Heart and Circulatory Physiology, 317(5), H1105–H1115. https://doi.org/10.1152/ajpheart.00540.2018
Lieu, D. K., Liu, J., Siu, C. W., McNerney, G. P., Tse, H. F., Abu-Khalil, A., Huser, T., & Li, R. A. (2009). Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells and Development, 18(10), 1493–1500. https://doi.org/10.1089/scd.2009.0052
Yanagi, K., Takano, M., Narazaki, G., Uosaki, H., Hoshino, T., Ishii, T., Misaki, T., & Yamashita, J. K. (2007). Hyperpolarization-activated cyclic nucleotide-gated channels and T-type calcium channels confer automaticity of embryonic stem cell-derived cardiomyocytes. Stem Cells, 25(11), 2712–2719. https://doi.org/10.1634/stemcells.2006-0388
Lindsey, S. E., Butcher, J. T., & Yalcin, H. C. (2014). Mechanical regulation of cardiac development. Frontiers in Physiology, 5, 318. https://doi.org/10.3389/fphys.2014.00318
Marchiano, S., Bertero, A., & Murry, C. E. (2019). Learn from your elders: Developmental biology lessons to guide maturation of stem cell-derived Cardiomyocytes. Pediatric Cardiology, 40(7), 1367–1387. https://doi.org/10.1007/s00246-019-02165-5
Litvinukova, M., Talavera-Lopez, C., Maatz, H., Reichart, D., Worth, C. L., Lindberg, E. L., Kanda, M., Polanski, K., Heinig, M., Lee, M., Nadelmann, E. R., Roberts, K., Tuck, L., Fasouli, E. S., DeLaughter, D. M., McDonough, B., Wakimoto, H., Gorham, J. M., Samari, S., et al. (2020). Cells of the adult human heart. Nature, 588(7838), 466–472. https://doi.org/10.1038/s41586-020-2797-4
Deb, A., & Ubil, E. (2014). Cardiac fibroblast in development and wound healing. Journal of Molecular and Cellular Cardiology, 70, 47–55. https://doi.org/10.1016/j.yjmcc.2014.02.017
Hu, D., Linders, A., Yamak, A., Correia, C., Kijlstra, J. D., Garakani, A., Xiao, L., Milan, D. J., van der Meer, P., Serra, M., Alves, P. M., & Domian, I. J. (2018). Metabolic maturation of human pluripotent stem cell-derived Cardiomyocytes by inhibition of HIF1alpha and LDHA. Circulation Research, 123(9), 1066–1079. https://doi.org/10.1161/CIRCRESAHA.118.313249
Abuid, J., Stinson, D. A., & Larsen, P. R. (1973). Serum triiodothyronine and thyroxine in the neonate and the acute increases in these hormones following delivery. The Journal of Clinical Investigation, 52(5), 1195–1199. https://doi.org/10.1172/JCI107286
Hirose, K., Payumo, A. Y., Cutie, S., Hoang, A., Zhang, H., Guyot, R., Lunn, D., Bigley, R. B., Yu, H., Wang, J., Smith, M., Gillett, E., Muroy, S. E., Schmid, T., Wilson, E., Field, K. A., Reeder, D. M., Maden, M., Yartsev, M. M., et al. (2019). Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science, 364(6436), 184–188. https://doi.org/10.1126/science.aar2038
Fleischer, S., Jahnke, H. G., Fritsche, E., Girard, M., & Robitzki, A. A. (2019). Comprehensive human stem cell differentiation in a 2D and 3D mode to cardiomyocytes for long-term cultivation and multiparametric monitoring on a multimodal microelectrode array setup. Biosensors & Bioelectronics, 126, 624–631. https://doi.org/10.1016/j.bios.2018.10.061
Ebert, A., Joshi, A. U., Andorf, S., Dai, Y., Sampathkumar, S., Chen, H., Li, Y., Garg, P., Toischer, K., Hasenfuss, G., Mochly-Rosen, D., & Wu, J. C. (2019). Proteasome-dependent regulation of distinct metabolic states during long-term culture of human iPSC-derived Cardiomyocytes. Circulation Research, 125(1), 90–103. https://doi.org/10.1161/CIRCRESAHA.118.313973
Pasquier, J., Gupta, R., Rioult, D., Hoarau-Vechot, J., Courjaret, R., Machaca, K., Al Suwaidi, J., Stanley, E. G., Rafii, S., Elliott, D. A., Abi Khalil, C., & Rafii, A. (2017). Coculturing with endothelial cells promotes in vitro maturation and electrical coupling of human embryonic stem cell-derived cardiomyocytes. The Journal of Heart and Lung Transplantation, 36(6), 684–693. https://doi.org/10.1016/j.healun.2017.01.001
Lee, D. S., Chen, J. H., Lundy, D. J., Liu, C. H., Hwang, S. M., Pabon, L., Shieh, R. C., Chen, C. C., Wu, S. N., Yan, Y. T., Lee, S. T., Chiang, P. M., Chien, S., Murry, C. E., & Hsieh, P. C. (2015). Defined MicroRNAs induce aspects of maturation in mouse and human embryonic-stem-cell-derived Cardiomyocytes. Cell Reports, 12(12), 1960–1967. https://doi.org/10.1016/j.celrep.2015.08.042
Dunn, K. K., Reichardt, I. M., Simmons, A. D., Jin, G., Floy, M. E., Hoon, K. M., & Palecek, S. P. (2019). Coculture of endothelial cells with human pluripotent stem cell-derived cardiac progenitors reveals a differentiation stage-specific enhancement of Cardiomyocyte maturation. Biotechnology Journal, 14(8), e1800725. https://doi.org/10.1002/biot.201800725
Biendarra-Tiegs, S. M., Clemens, D. J., Secreto, F. J., & Nelson, T. J. (2020). Human induced pluripotent stem cell-derived non-Cardiomyocytes modulate cardiac electrophysiological maturation through Connexin 43-mediated cell-cell interactions. Stem Cells and Development, 29(2), 75–89. https://doi.org/10.1089/scd.2019.0098
Giacomelli, E., Meraviglia, V., Campostrini, G., Cochrane, A., Cao, X., van Helden, R. W. J., Krotenberg Garcia, A., Mircea, M., Kostidis, S., Davis, R. P., van Meer, B. J., Jost, C. R., Koster, A. J., Mei, H., Miguez, D. G., Mulder, A. A., Ledesma-Terron, M., Pompilio, G., Sala, L., et al. (2020). Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell, 26(6), 862–879.e811. https://doi.org/10.1016/j.stem.2020.05.004
Varzideh, F., Mahmoudi, E., & Pahlavan, S. (2019). Coculture with noncardiac cells promoted maturation of human stem cell-derived cardiomyocyte microtissues. Journal of Cellular Biochemistry, 120(10), 16681–16691. https://doi.org/10.1002/jcb.28926
Winbo, A., Ramanan, S., Eugster, E., Jovinge, S., Skinner, J. R., & Montgomery, J. M. (2020). Functional coculture of sympathetic neurons and cardiomyocytes derived from human-induced pluripotent stem cells. American Journal of Physiology Heart and Circulatory Physiology, 319(5), H927–H937. https://doi.org/10.1152/ajpheart.00546.2020
Yoshida, S., Miyagawa, S., Fukushima, S., Kawamura, T., Kashiyama, N., Ohashi, F., Toyofuku, T., Toda, K., & Sawa, Y. (2018). Maturation of human induced pluripotent stem cell-derived Cardiomyocytes by soluble factors from human Mesenchymal stem cells. Molecular Therapy, 26(11), 2681–2695. https://doi.org/10.1016/j.ymthe.2018.08.012
Tallawi, M., Rai, R., Boccaccini, A. R., & Aifantis, K. E. (2015). Effect of substrate mechanics on cardiomyocyte maturation and growth. Tissue Engineering. Part B, Reviews, 21(1), 157–165. https://doi.org/10.1089/ten.TEB.2014.0383
Weber, N., Schwanke, K., Greten, S., Wendland, M., Iorga, B., Fischer, M., Geers-Knorr, C., Hegermann, J., Wrede, C., Fiedler, J., Kempf, H., Franke, A., Piep, B., Pfanne, A., Thum, T., Martin, U., Brenner, B., Zweigerdt, R., & Kraft, T. (2016). Stiff matrix induces switch to pure beta-cardiac myosin heavy chain expression in human ESC-derived cardiomyocytes. Basic Research in Cardiology, 111(6), 68. https://doi.org/10.1007/s00395-016-0587-9
Martewicz, S., Serena, E., Zatti, S., Keller, G., & Elvassore, N. (2017). Substrate and mechanotransduction influence SERCA2a localization in human pluripotent stem cell-derived cardiomyocytes affecting functional performance. Stem Cell Research, 25, 107–114. https://doi.org/10.1016/j.scr.2017.10.011
Feaster, T. K., Cadar, A. G., Wang, L., Williams, C. H., Chun, Y. W., Hempel, J. E., Bloodworth, N., Merryman, W. D., Lim, C. C., Wu, J. C., Knollmann, B. C., & Hong, C. C. (2015). Matrigel mattress: A method for the generation of single contracting human-induced pluripotent stem cell-derived Cardiomyocytes. Circulation Research, 117(12), 995–1000. https://doi.org/10.1161/CIRCRESAHA.115.307580
Happe, C. L., & Engler, A. J. (2016). Mechanical forces reshape differentiation cues that guide cardiomyogenesis. Circulation Research, 118(2), 296–310. https://doi.org/10.1161/CIRCRESAHA.115.305139
Stoppel, W. L., Kaplan, D. L., & Black 3rd., L. D. (2016). Electrical and mechanical stimulation of cardiac cells and tissue constructs. Advanced Drug Delivery Reviews, 96, 135–155. https://doi.org/10.1016/j.addr.2015.07.009
Hirt, M. N., Boeddinghaus, J., Mitchell, A., Schaaf, S., Bornchen, C., Muller, C., Schulz, H., Hubner, N., Stenzig, J., Stoehr, A., Neuber, C., Eder, A., Luther, P. K., Hansen, A., & Eschenhagen, T. (2014). Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. Journal of Molecular and Cellular Cardiology, 74, 151–161. https://doi.org/10.1016/j.yjmcc.2014.05.009
Crestani, T., Steichen, C., Neri, E., Rodrigues, M., Fonseca-Alaniz, M. H., Ormrod, B., Holt, M. R., Pandey, P., Harding, S., Ehler, E., & Krieger, J. E. (2020). Electrical stimulation applied during differentiation drives the hiPSC-CMs towards a mature cardiac conduction-like cells. Biochemical and Biophysical Research Communications, 533(3), 376–382. https://doi.org/10.1016/j.bbrc.2020.09.021
Ma, R., Liang, J., Huang, W., Guo, L., Cai, W., Wang, L., Paul, C., Yang, H. T., Kim, H. W., & Wang, Y. (2018). Electrical stimulation enhances cardiac differentiation of human induced pluripotent stem cells for myocardial infarction therapy. Antioxidants & Redox Signaling, 28(5), 371–384. https://doi.org/10.1089/ars.2016.6766
Ruan, J. L., Tulloch, N. L., Saiget, M., Paige, S. L., Razumova, M. V., Regnier, M., Tung, K. C., Keller, G., Pabon, L., Reinecke, H., & Murry, C. E. (2015). Mechanical stress promotes maturation of human myocardium from pluripotent stem cell-derived progenitors. Stem Cells, 33(7), 2148–2157. https://doi.org/10.1002/stem.2036
Abilez, O. J., Tzatzalos, E., Yang, H., Zhao, M. T., Jung, G., Zollner, A. M., Tiburcy, M., Riegler, J., Matsa, E., Shukla, P., Zhuge, Y., Chour, T., Chen, V. C., Burridge, P. W., Karakikes, I., Kuhl, E., Bernstein, D., Couture, L. A., Gold, J. D., et al. (2018). Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells, 36(2), 265–277. https://doi.org/10.1002/stem.2732
Leonard, A., Bertero, A., Powers, J. D., Beussman, K. M., Bhandari, S., Regnier, M., Murry, C. E., & Sniadecki, N. J. (2018). Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. Journal of Molecular and Cellular Cardiology, 118, 147–158. https://doi.org/10.1016/j.yjmcc.2018.03.016
Hirt, M. N., Sorensen, N. A., Bartholdt, L. M., Boeddinghaus, J., Schaaf, S., Eder, A., Vollert, I., Stohr, A., Schulze, T., Witten, A., Stoll, M., Hansen, A., & Eschenhagen, T. (2012). Increased afterload induces pathological cardiac hypertrophy: A new in vitro model. Basic Research in Cardiology, 107(6), 307. https://doi.org/10.1007/s00395-012-0307-z
Kit-Anan, W., Mazo, M. M., Wang, B. X., Leonardo, V., Pence, I. J., Gopal, S., Gelmi, A., Nagelkerke, A., Becce, M., Chiappini, C., Harding, S. E., Terracciano, C. M., & Stevens, M. M. (2021). Multiplexing physical stimulation on single human induced pluripotent stem cell-derived cardiomyocytes for phenotype modulation. Biofabrication, 13(2), 025004. https://doi.org/10.1088/1758-5090/abce0a
Ruan, J. L., Tulloch, N. L., Razumova, M. V., Saiget, M., Muskheli, V., Pabon, L., Reinecke, H., Regnier, M., & Murry, C. E. (2016). Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation, 134(20), 1557–1567. https://doi.org/10.1161/CIRCULATIONAHA.114.014998
Ulmer, B. M., Stoehr, A., Schulze, M. L., Patel, S., Gucek, M., Mannhardt, I., Funcke, S., Murphy, E., Eschenhagen, T., & Hansen, A. (2018). Contractile work contributes to maturation of energy metabolism in hiPSC-derived Cardiomyocytes. Stem Cell Reports, 10(3), 834–847. https://doi.org/10.1016/j.stemcr.2018.01.039
Rog-Zielinska, E. A., Thomson, A., Kenyon, C. J., Brownstein, D. G., Moran, C. M., Szumska, D., Michailidou, Z., Richardson, J., Owen, E., Watt, A., Morrison, H., Forrester, L. M., Bhattacharya, S., Holmes, M. C., & Chapman, K. E. (2013). Glucocorticoid receptor is required for foetal heart maturation. Human Molecular Genetics, 22(16), 3269–3282. https://doi.org/10.1093/hmg/ddt182
Parikh, S. S., Blackwell, D. J., Gomez-Hurtado, N., Frisk, M., Wang, L., Kim, K., Dahl, C. P., Fiane, A., Tonnessen, T., Kryshtal, D. O., Louch, W. E., & Knollmann, B. C. (2017). Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived Cardiomyocytes. Circulation Research, 121(12), 1323–1330. https://doi.org/10.1161/CIRCRESAHA.117.311920
Thorpe-Beeston, J. G., Nicolaides, K. H., Felton, C. V., Butler, J., & McGregor, A. M. (1991). Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. The New England Journal of Medicine, 324(8), 532–536. https://doi.org/10.1056/NEJM199102213240805
Yang, X., Rodriguez, M., Pabon, L., Fischer, K. A., Reinecke, H., Regnier, M., Sniadecki, N. J., Ruohola-Baker, H., & Murry, C. E. (2014). Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. Journal of Molecular and Cellular Cardiology, 72, 296–304. https://doi.org/10.1016/j.yjmcc.2014.04.005
Garbern, J. C., & Lee, R. T. (2021). Mitochondria and metabolic transitions in cardiomyocytes: Lessons from development for stem cell-derived cardiomyocytes. Stem Cell Research & Therapy, 12(1), 177. https://doi.org/10.1186/s13287-021-02252-6
Correia, C., Koshkin, A., Duarte, P., Hu, D., Teixeira, A., Domian, I., Serra, M., & Alves, P. M. (2017). Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Scientific Reports, 7(1), 8590. https://doi.org/10.1038/s41598-017-08713-4
Horikoshi, Y., Yan, Y., Terashvili, M., Wells, C., Horikoshi, H., Fujita, S., Bosnjak, Z. J., & Bai, X. (2019). Fatty acid-treated induced pluripotent stem cell-derived human Cardiomyocytes exhibit adult Cardiomyocyte-like energy metabolism phenotypes. Cells, 8(9). https://doi.org/10.3390/cells8091095
Yang, X., Rodriguez, M. L., Leonard, A., Sun, L., Fischer, K. A., Wang, Y., Ritterhoff, J., Zhao, L., Kolwicz Jr., S. C., Pabon, L., Reinecke, H., Sniadecki, N. J., Tian, R., Ruohola-Baker, H., Xu, H., & Murry, C. E. (2019). Fatty acids enhance the maturation of Cardiomyocytes derived from human pluripotent stem cells. Stem Cell Reports, 13(4), 657–668. https://doi.org/10.1016/j.stemcr.2019.08.013
Puente, B. N., Kimura, W., Muralidhar, S. A., Moon, J., Amatruda, J. F., Phelps, K. L., Grinsfelder, D., Rothermel, B. A., Chen, R., Garcia, J. A., Santos, C. X., Thet, S., Mori, E., Kinter, M. T., Rindler, P. M., Zacchigna, S., Mukherjee, S., Chen, D. J., Mahmoud, A. I., et al. (2014). The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell, 157(3), 565–579. https://doi.org/10.1016/j.cell.2014.03.032
Lim, G. B. (2020). Inhibiting fatty acid oxidation promotes cardiomyocyte proliferation. Nature Reviews Cardiology, 17(5), 266–267. https://doi.org/10.1038/s41569-020-0361-4
Nakano, H., Minami, I., Braas, D., Pappoe, H., Wu, X., Sagadevan, A., Vergnes, L., Fu, K., Morselli, M., Dunham, C., Ding, X., Stieg, A. Z., Gimzewski, J. K., Pellegrini, M., Clark, P. M., Reue, K., Lusis, A. J., Ribalet, B., Kurdistani, S. K., et al. (2017). Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. eLife, 6. https://doi.org/10.7554/eLife.29330
Gentillon, C., Li, D., Duan, M., Yu, W. M., Preininger, M. K., Jha, R., Rampoldi, A., Saraf, A., Gibson, G. C., Qu, C. K., Brown, L. A., & Xu, C. (2019). Targeting HIF-1alpha in combination with PPARalpha activation and postnatal factors promotes the metabolic maturation of human induced pluripotent stem cell-derived cardiomyocytes. Journal of Molecular and Cellular Cardiology, 132, 120–135. https://doi.org/10.1016/j.yjmcc.2019.05.003
Semenza, G. L. (2014). Hypoxia-inducible factor 1 and cardiovascular disease. Annual Review of Physiology, 76, 39–56. https://doi.org/10.1146/annurev-physiol-021113-170322
Menendez-Montes, I., Escobar, B., Palacios, B., Gomez, M. J., Izquierdo-Garcia, J. L., Flores, L., Jimenez-Borreguero, L. J., Aragones, J., Ruiz-Cabello, J., Torres, M., & Martin-Puig, S. (2016). Myocardial VHL-HIF signaling controls an embryonic metabolic switch essential for cardiac maturation. Developmental Cell, 39(6), 724–739. https://doi.org/10.1016/j.devcel.2016.11.012
Semenza, G. L. (2001). Hypoxia-inducible factor 1: Control of oxygen homeostasis in health and disease. Pediatric Research, 49(5), 614–617. https://doi.org/10.1203/00006450-200105000-00002
Rey, S., Luo, W., Shimoda, L. A., & Semenza, G. L. (2011). Metabolic reprogramming by HIF-1 promotes the survival of bone marrow-derived angiogenic cells in ischemic tissue. Blood, 117(18), 4988–4998. https://doi.org/10.1182/blood-2010-11-321190
Rowe, G. C., Jiang, A., & Arany, Z. (2010). PGC-1 coactivators in cardiac development and disease. Circulation Research, 107(7), 825–838. https://doi.org/10.1161/CIRCRESAHA.110.223818
Liu, Y., Bai, H., Guo, F., Thai, P. N., Luo, X., Zhang, P., Yang, C., Feng, X., Zhu, D., Guo, J., Liang, P., Xu, Z., Yang, H., & Lu, X. (2020). PGC-1alpha activator ZLN005 promotes maturation of cardiomyocytes derived from human embryonic stem cells. Aging (Albany NY), 12(8), 7411–7430. https://doi.org/10.18632/aging.103088
Murphy, S. A., Miyamoto, M., Kervadec, A., Kannan, S., Tampakakis, E., Kambhampati, S., Lin, B. L., Paek, S., Andersen, P., Lee, D. I., Zhu, R., An, S. S., Kass, D. A., Uosaki, H., Colas, A. R., & Kwon, C. (2021). PGC1/PPAR drive cardiomyocyte maturation at single cell level via YAP1 and SF3B2. Nature Communications, 12(1), 1648. https://doi.org/10.1038/s41467-021-21957-z
Rupert, C. E., & Coulombe, K. L. K. (2017). IGF1 and NRG1 enhance proliferation, metabolic maturity, and the force-frequency response in hESC-derived engineered cardiac tissues. Stem Cells International, 2017, 7648409. https://doi.org/10.1155/2017/7648409
McDevitt, T. C., Laflamme, M. A., & Murry, C. E. (2005). Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. Journal of Molecular and Cellular Cardiology, 39(6), 865–873. https://doi.org/10.1016/j.yjmcc.2005.09.007
Chong, Z. Z., Shang, Y. C., & Maiese, K. (2011). Cardiovascular disease and mTOR signaling. Trends in Cardiovascular Medicine, 21(5), 151–155. https://doi.org/10.1016/j.tcm.2012.04.005
Garbern, J. C., Helman, A., Sereda, R., Sarikhani, M., Ahmed, A., Escalante, G. O., Ogurlu, R., Kim, S. L., Zimmerman, J. F., Cho, A., MacQueen, L., Bezzerides, V. J., Parker, K. K., Melton, D. A., & Lee, R. T. (2020). Inhibition of mTOR signaling enhances maturation of Cardiomyocytes derived from human-induced pluripotent stem cells via p53-induced quiescence. Circulation, 141(4), 285–300. https://doi.org/10.1161/circulationaha.119.044205
Jackman, C. P., Carlson, A. L., & Bursac, N. (2016). Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials, 111, 66–79. https://doi.org/10.1016/j.biomaterials.2016.09.024
Sakamoto, T., Matsuura, T. R., Wan, S., Ryba, D. M., Kim, J. U., Won, K. J., Lai, L., Petucci, C., Petrenko, N., Musunuru, K., Vega, R. B., & Kelly, D. P. (2020). A critical role for estrogen-related receptor signaling in cardiac maturation. Circulation Research, 126(12), 1685–1702. https://doi.org/10.1161/CIRCRESAHA.119.316100
Miao, S., Zhao, D., Wang, X., Ni, X., Fang, X., Yu, M., Ye, L., Yang, J., Wu, H., Han, X., Qu, L., Li, L., Lan, F., Shen, Z., Lei, W., Zhao, Z. A., & Hu, S. (2020). Retinoic acid promotes metabolic maturation of human embryonic stem cell-derived Cardiomyocytes. Theranostics, 10(21), 9686–9701. https://doi.org/10.7150/thno.44146
Yang, J., Ding, N., Zhao, D., Yu, Y., Shao, C., Ni, X., Zhao, Z. A., Li, Z., Chen, J., Ying, Z., Yu, M., Lei, W., & Hu, S. (2021). Intermittent starvation promotes maturation of human embryonic stem cell-derived Cardiomyocytes. Frontiers in Cell and Development Biology, 9, 687769. https://doi.org/10.3389/fcell.2021.687769
Abbas, N., Perbellini, F., & Thum, T. (2020). Non-coding RNAs: Emerging players in cardiomyocyte proliferation and cardiac regeneration. Basic Research in Cardiology, 115(5), 52. https://doi.org/10.1007/s00395-020-0816-0
Fang, X., Miao, S., Yu, Y., Ding, F., Han, X., Wu, H., Zhao, Z. A., Wang, Y., Hu, S., & Lei, W. (2019). MIR148A family regulates cardiomyocyte differentiation of human embryonic stem cells by inhibiting the DLL1-mediated NOTCH signaling pathway. Journal of Molecular and Cellular Cardiology, 134, 1–12. https://doi.org/10.1016/j.yjmcc.2019.06.014
Devaux, Y., Zangrando, J., Schroen, B., Creemers, E. E., Pedrazzini, T., Chang, C. P., Dorn 2nd, G. W., Thum, T., Heymans, S., & Cardiolinc, n. (2015). Long noncoding RNAs in cardiac development and ageing. Nature Reviews Cardiology, 12(7), 415–425. https://doi.org/10.1038/nrcardio.2015.55
Kay, M., & Soltani, B. M. (2021). LncRNAs in Cardiomyocyte maturation: New window for cardiac regenerative medicine. Noncoding RNA, 7(1). https://doi.org/10.3390/ncrna7010020
Kumar, N., Dougherty, J. A., Manring, H. R., Elmadbouh, I., Mergaye, M., Czirok, A., Greta Isai, D., Belevych, A. E., Yu, L., Janssen, P. M. L., Fadda, P., Gyorke, S., Ackermann, M. A., Angelos, M. G., & Khan, M. (2019). Assessment of temporal functional changes and miRNA profiling of human iPSC-derived cardiomyocytes. Scientific Reports, 9(1), 13188. https://doi.org/10.1038/s41598-019-49653-5
Poon, E. N., Hao, B., Guan, D., Jun Li, M., Lu, J., Yang, Y., Wu, B., Wu, S. C., Webb, S. E., Liang, Y., Miller, A. L., Yao, X., Wang, J., Yan, B., & Boheler, K. R. (2018). Integrated transcriptomic and regulatory network analyses identify microRNA-200c as a novel repressor of human pluripotent stem cell-derived cardiomyocyte differentiation and maturation. Cardiovascular Research, 114(6), 894–906. https://doi.org/10.1093/cvr/cvy019
Kuppusamy, K. T., Jones, D. C., Sperber, H., Madan, A., Fischer, K. A., Rodriguez, M. L., Pabon, L., Zhu, W. Z., Tulloch, N. L., Yang, X., Sniadecki, N. J., Laflamme, M. A., Ruzzo, W. L., Murry, C. E., & Ruohola-Baker, H. (2015). Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proceedings of the National Academy of Sciences of the United States of America, 112(21), E2785–E2794. https://doi.org/10.1073/pnas.1424042112
Miklas, J. W., Clark, E., Levy, S., Detraux, D., Leonard, A., Beussman, K., Showalter, M. R., Smith, A. T., Hofsteen, P., Yang, X., Macadangdang, J., Manninen, T., Raftery, D., Madan, A., Suomalainen, A., Kim, D. H., Murry, C. E., Fiehn, O., Sniadecki, N. J., et al. (2019). TFPa/HADHA is required for fatty acid beta-oxidation and cardiolipin re-modeling in human cardiomyocytes. Nature Communications, 10(1), 4671. https://doi.org/10.1038/s41467-019-12482-1
Zhang, Q., Cheng, Z., Yu, Z., Zhu, C., & Qian, L. (2019). Role of lncRNA uc.457 in the differentiation and maturation of cardiomyocytes. Molecular Medicine Reports, 19(6), 4927–4934. https://doi.org/10.3892/mmr.2019.10132
Huang, S., Li, X., Zheng, H., Si, X., Li, B., Wei, G., Li, C., Chen, Y., Chen, Y., Liao, W., Liao, Y., & Bin, J. (2019). Loss of super-enhancer-regulated circRNA Nfix induces cardiac regeneration after myocardial infarction in adult mice. Circulation, 139(25), 2857–2876. https://doi.org/10.1161/CIRCULATIONAHA.118.038361
Gilsbach, R., Schwaderer, M., Preissl, S., Gruning, B. A., Kranzhofer, D., Schneider, P., Nuhrenberg, T. G., Mulero-Navarro, S., Weichenhan, D., Braun, C., Dressen, M., Jacobs, A. R., Lahm, H., Doenst, T., Backofen, R., Krane, M., Gelb, B. D., & Hein, L. (2018). Distinct epigenetic programs regulate cardiac myocyte development and disease in the human heart in vivo. Nature Communications, 9(1), 391. https://doi.org/10.1038/s41467-017-02762-z
Saheli, M., Pirhajati Mahabadi, V., Mesbah-Namin, S. A., Seifalian, A., & Bagheri-Hosseinabadi, Z. (2020). DNA methyltransferase inhibitor 5-azacytidine in high dose promotes ultrastructural maturation of cardiomyocyte. Stem Cell Investig, 7, 22. https://doi.org/10.21037/sci-2020-007
Biermann, M., Cai, W., Lang, D., Hermsen, J., Profio, L., Zhou, Y., Czirok, A., Isai, D. G., Napiwocki, B. N., Rodriguez, A. M., Brown, M. E., Woon, M. T., Shao, A., Han, T., Park, D., Hacker, T. A., Crone, W. C., Burlingham, W. J., Glukhov, A. V., et al. (2019). Epigenetic priming of human pluripotent stem cell-derived cardiac progenitor cells accelerates Cardiomyocyte maturation. Stem Cells, 37(7), 910–923. https://doi.org/10.1002/stem.3021
Lee, J., Sutani, A., Kaneko, R., Takeuchi, J., Sasano, T., Kohda, T., Ihara, K., Takahashi, K., Yamazoe, M., Morio, T., Furukawa, T., & Ishino, F. (2020). In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nature Communications, 11(1), 4283. https://doi.org/10.1038/s41467-020-18031-5
Hofbauer, P., Jahnel, S. M., Papai, N., Giesshammer, M., Deyett, A., Schmidt, C., Penc, M., Tavernini, K., Grdseloff, N., Meledeth, C., Ginistrelli, L. C., Ctortecka, C., Salic, S., Novatchkova, M., & Mendjan, S. (2021). Cardioids reveal self-organizing principles of human cardiogenesis. Cell. https://doi.org/10.1016/j.cell.2021.04.034
Nugraha, B., Buono, M. F., & Emmert, M. Y. (2018). Modelling human cardiac diseases with 3D organoid. European Heart Journal, 39(48), 4234–4237. https://doi.org/10.1093/eurheartj/ehy765
Richards, D. J., Li, Y., Kerr, C. M., Yao, J., Beeson, G. C., Coyle, R. C., Chen, X., Jia, J., Damon, B., Wilson, R., Starr Hazard, E., Hardiman, G., Menick, D. R., Beeson, C. C., Yao, H., Ye, T., & Mei, Y. (2020). Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nature Biomedical Engineering, 4(4), 446–462. https://doi.org/10.1038/s41551-020-0539-4
Zhao, D., Lei, W., & Hu, S. (2021). Cardiac organoid - a promising perspective of preclinical model. Stem Cell Research & Therapy, 12(1), 272. https://doi.org/10.1186/s13287-021-02340-7
Mills, R. J., Titmarsh, D. M., Koenig, X., Parker, B. L., Ryall, J. G., Quaife-Ryan, G. A., Voges, H. K., Hodson, M. P., Ferguson, C., Drowley, L., Plowright, A. T., Needham, E. J., Wang, Q. D., Gregorevic, P., Xin, M., Thomas, W. G., Parton, R. G., Nielsen, L. K., Launikonis, B. S., et al. (2017). Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proceedings of the National Academy of Sciences of the United States of America, 114(40), E8372–E8381. https://doi.org/10.1073/pnas.1707316114
Varzideh, F., Pahlavan, S., Ansari, H., Halvaei, M., Kostin, S., Feiz, M. S., Latifi, H., Aghdami, N., Braun, T., & Baharvand, H. (2019). Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials, 192, 537–550. https://doi.org/10.1016/j.biomaterials.2018.11.033
Rao, C., Prodromakis, T., Kolker, L., Chaudhry, U. A., Trantidou, T., Sridhar, A., Weekes, C., Camelliti, P., Harding, S. E., Darzi, A., Yacoub, M. H., Athanasiou, T., & Terracciano, C. M. (2013). The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials, 34(10), 2399–2411. https://doi.org/10.1016/j.biomaterials.2012.11.055
Abadi, P. P. S. S., Garbern, J. C., Behzadi, S., Hill, M. J., Tresback, J. S., Heydari, T., Ejtehadi, M. R., Ahmed, N., Copley, E., Aghaverdi, H., Lee, R. T., Farokhzad, O. C., & Mahmoudi, M. (2018). Engineering of mature human induced pluripotent stem cell-derived Cardiomyocytes using substrates with multiscale topography. Advanced Functional Materials, 28(19). https://doi.org/10.1002/adfm.201707378
Liu, T., Zhang, S., Huang, C., Ma, S., Bai, R., Li, Y., Chang, Y., Hang, C., Saleem, A., Dong, T., Guo, T., Jiang, Y., Lu, W., Zhang, L., Jianwen, L., Jiang, H., & Lan, F. (2021). Microscale grooves regulate maturation development of hPSC-CMs by the transient receptor potential channels (TRP channels). Journal of Cellular and Molecular Medicine. https://doi.org/10.1111/jcmm.16429
Huethorst, E., Hortigon, M., Zamora-Rodriguez, V., Reynolds, P. M., Burton, F., Smith, G., & Gadegaard, N. (2016). Enhanced human-induced pluripotent stem cell derived Cardiomyocyte maturation using a dual microgradient substrate. ACS Biomaterials Science & Engineering, 2(12), 2231–2239. https://doi.org/10.1021/acsbiomaterials.6b00426
Carson, D., Hnilova, M., Yang, X., Nemeth, C. L., Tsui, J. H., Smith, A. S., Jiao, A., Regnier, M., Murry, C. E., Tamerler, C., & Kim, D. H. (2016). Nanotopography-induced structural anisotropy and sarcomere development in human Cardiomyocytes derived from induced pluripotent stem cells. ACS Applied Materials & Interfaces, 8(34), 21923–21932. https://doi.org/10.1021/acsami.5b11671
Silbernagel, N., Korner, A., Balitzki, J., Jaggy, M., Bertels, S., Richter, B., Hippler, M., Hellwig, A., Hecker, M., Bastmeyer, M., & Ullrich, N. D. (2020). Shaping the heart: Structural and functional maturation of iPSC-cardiomyocytes in 3D-micro-scaffolds. Biomaterials, 227, 119551. https://doi.org/10.1016/j.biomaterials.2019.119551
Gorain, B., Choudhury, H., Pandey, M., Kesharwani, P., Abeer, M. M., Tekade, R. K., & Hussain, Z. (2018). Carbon nanotube scaffolds as emerging nanoplatform for myocardial tissue regeneration: A review of recent developments and therapeutic implications. Biomedicine & Pharmacotherapy, 104, 496–508. https://doi.org/10.1016/j.biopha.2018.05.066
Lekshmi, G., Sana, S. S., Nguyen, V. H., Nguyen, T. H. C., Nguyen, C. C., Le, Q. V., & Peng, W. (2020). Recent Progress in carbon nanotube polymer composites in tissue engineering and regeneration. International Journal of Molecular Sciences, 21(17). https://doi.org/10.3390/ijms21176440
Ren, J., Xu, Q., Chen, X., Li, W., Guo, K., Zhao, Y., Wang, Q., Zhang, Z., Peng, H., & Li, Y. G. (2017). Superaligned carbon nanotubes guide oriented cell growth and promote electrophysiological homogeneity for synthetic cardiac tissues. Advanced Materials, 29(44). https://doi.org/10.1002/adma.201702713
Wang, J., Cui, C., Nan, H., Yu, Y., Xiao, Y., Poon, E., Yang, G., Wang, X., Wang, C., Li, L., Boheler, K. R., Ma, X., Cheng, X., Ni, Z., & Chen, M. (2017). Graphene sheet-induced global maturation of Cardiomyocytes derived from human induced pluripotent stem cells. ACS Applied Materials & Interfaces, 9(31), 25929–25940. https://doi.org/10.1021/acsami.7b08777
Shiekh, P. A., Singh, A., & Kumar, A. (2018). Engineering bioinspired antioxidant materials promoting Cardiomyocyte functionality and maturation for tissue engineering application. ACS Applied Materials & Interfaces, 10(4), 3260–3273. https://doi.org/10.1021/acsami.7b14777
Cui, C., Wang, J., Qian, D., Huang, J., Lin, J., Kingshott, P., Wang, P. Y., & Chen, M. (2019). Binary colloidal crystals drive spheroid formation and accelerate maturation of human-induced pluripotent stem cell-derived Cardiomyocytes. ACS Applied Materials & Interfaces, 11(4), 3679–3689. https://doi.org/10.1021/acsami.8b17090
Wang, Y., Cui, H., Wang, Y., Xu, C., Esworthy, T. J., Hann, S. Y., Boehm, M., Shen, Y. L., Mei, D., & Zhang, L. G. (2021). 4D printed cardiac construct with aligned Myofibers and adjustable curvature for myocardial regeneration. ACS Applied Materials & Interfaces, 13(11), 12746–12758. https://doi.org/10.1021/acsami.0c17610
Zhu, Y., Do, V. D., Richards, A. M., & Foo, R. (2021). What we know about cardiomyocyte dedifferentiation. Journal of Molecular and Cellular Cardiology, 152, 80–91. https://doi.org/10.1016/j.yjmcc.2020.11.016
Zhang, Y., Gago-Lopez, N., Li, N., Zhang, Z., Alver, N., Liu, Y., Martinson, A. M., Mehri, A., & MacLellan, W. R. (2019). Single-cell imaging and transcriptomic analyses of endogenous cardiomyocyte dedifferentiation and cycling. Cell Discovery, 5, 30. https://doi.org/10.1038/s41421-019-0095-9
Yu, M., Lei, W., Cao, J., Wang, L., Ma, A., Zhao, Z. A., Yang, H. T., Shen, Z., Lan, F., Cao, F., Liang, P., Pei, X., Xiang, A. P., Yu, J., Zhang, Y., Zhang, Y., Li, Q., Zhou, J., Wei, J., et al. (2021). Requirements for human cardiomyocytes. Cell Proliferation, e13150. https://doi.org/10.1111/cpr.13150
Funding
This study was supported by the National Key R&D Program of China (2021YFA1101902), the National Natural Science Foundation of China (82170364, 91949111, 81970223, 82003756), Jiangsu Province’s Key Discipline/Laboratory of Medicine (XK201118) and Introduction Project of Clinical Medicine Expert Team for Suzhou (SZYJTD201704).
Author information
Authors and Affiliations
Contributions
YW, MY and KH wrote the manuscript and contributed equally to this work. LW revised the manuscript. MT and SH wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable
Consent to Participate
Not applicable
Consent to Publish
All the authors declared their consent to publish.
Competing Interests
All the authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaning Wang, Miao Yu, and Kaili Hao are co-first authors and contribute equally to this work
Rights and permissions
About this article
Cite this article
Wang, Y., Yu, M., Hao, K. et al. Cardiomyocyte Maturation–the Road is not Obstructed. Stem Cell Rev and Rep 18, 2966–2981 (2022). https://doi.org/10.1007/s12015-022-10407-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10407-y