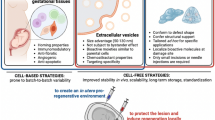
Abstract
Purpose of Review
This review details the discoveries that have propelled forward treatment for MMC, from medical management to postnatal surgical repair to open fetal surgery, paving the way for novel scientific research on non-invasive therapeutic approaches.
Recent Findings
Current investigations to treat MMC in less invasive ways and earlier in gestation include intra-amniotic particle injections, scaffold-based tissue engineering, and cell-based therapy. The common goal of each experimental approach is protecting the exposed spinal cord prior to its damage—to avoid the devastating clinical manifestations associated with the disease.
Summary
Significant discoveries in translational science are underway. Promising research utilizing fetal stem cells, tissue engineering, and non-invasive particle delivery could form the basis for a new approach to MMC or could augment current therapy to better patient outcomes.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fidas A, MacDonald H, Elton RA, Wild SR, Chisholm GD, Scott R. Prevalence and patterns of spina bifida occulta in 2707 normal adults. Clin Radiol. 1987;38(5):537–42.
Sharrard, W.J.W., Zachary, B., Lorber, J., Survival and paralysis in open Myelomeningocele with special reference to the time of repair of the spinal lesion - SHARRARD - 1967 - Developmental Medicine & Child Neurology - Wiley Online Library. Dev Med Child Neurol, 1967. 9(s13): p. 35–50.
Boone D, Parsons D, Lachmann SM, Sherwood T. Spina bifida occulta: lesion or anomaly? Clin Radiol. 1985;36(2):159–61.
Heffez DS, et al. The paralysis associated with myelomeningocele: clinical and experimental data implicating a preventable spinal cord injury. Neurosurgery. 1990;26(6):987–92.
Adzick NS. Fetal myelomeningocele: natural history, pathophysiology, and in-utero intervention. Semin Fetal Neonatal Med. 2010;15(1):9–14.
Pruitt LJ. Living with spina bifida: a historical perspective. Pediatrics. 2012;130(2):181–3.
Zachary RB. Spina bifida: to treat or not to treat? Give every baby a chance. Nurs Mirror. 1978;147(11):17–9.
Lorber J. Results of treatment of myelomeningocele. An analysis of 524 unselected cases, with special reference to possible selection for treatment. Dev Med Child Neurol. 1971;13(3):279–303.
Danzer, E. and A.W. Flake, In utero repair of myelomeningocele: rationale, initial clinical experience and a randomized controlled prospective clinical trial, in Neuroembryology Aging. 2008. p. 165–74.
•• Adzick NS, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1004 Randomized controlled trial which established outcomes from fetal surgery as superior to those from postnatal repair of MMC.
• Danzer E, et al. Fetal myelomeningocele surgery: preschool functional status using the Functional Independence Measure for children (WeeFIM). Childs Nerv Syst. 2011;27(7):1083–8 Demonstrated the functional and self-care limitations still present after fetal repair of MMC.
UCSF Fetal Treatment Center | Spina Bifida (Myelomeningocele). 2019(February 13, 2019).
Peranteau WH, Adzick NS. Prenatal surgery for myelomeningocele. Curr Opin Obstet Gynecol. 2016;28(2):111–8.
• Kohl T. Percutaneous minimally invasive fetoscopic surgery for spina bifida aperta. Part I: surgical technique and perioperative outcome. Ultrasound Obstet Gynecol. 2014;44(5):515–24 Demonstrates technical feasibility and clinical safety of fetoscopic surgery after extensive training.
•• Pedreira DA, et al. Endoscopic surgery for the antenatal treatment of myelomeningocele: the CECAM trial. Am J Obstet Gynecol. 2016;214(1):111.e1–111.e11 Evidence of a watertight fetoscopic surgery using a patch and skin-closure technique that results in reversal of hindbrain herniation.
Bruner JP, et al. Endoscopic coverage of fetal myelomeningocele in utero. Am J Obstet Gynecol. 1999;180(1 Pt 1):153–8.
Joyeux L, Engels AC, Russo FM, Jimenez J, van Mieghem T, de Coppi P, et al. Fetoscopic versus open repair for spina bifida aperta: a systematic review of outcomes. Fetal Diagn Ther. 2016;39(3):161–71.
Pedreira, D.A.L., et al., Fetoscopic repair of spina bifida: safer and better?, in Ultrasound Obstet Gynecol. 2016. p. 141–7.
Moldenhauer JS, Soni S, Rintoul NE, Spinner SS, Khalek N, Martinez-Poyer J, et al. Fetal myelomeningocele repair: the post-MOMS experience at the Children’s Hospital of Philadelphia. Fetal Diagn Ther. 2015;37(3):235–40.
Graf K, Kohl T, Neubauer BA, Dey F, Faas D, Wanis FA, et al. Percutaneous minimally invasive fetoscopic surgery for spina bifida aperta. Part III: neurosurgical intervention in the first postnatal year. Ultrasound Obstet Gynecol. 2016;47(2):158–61.
Luks FI, Deprest J, Marcus M, Vandenberghe K, Vertommen JD, Lerut T, et al. Carbon dioxide pneumoamnios causes acidosis in fetal lamb. Fetal Diagn Ther. 1994;9(2):105–9.
Gratacos E, et al. Effects of amniodistention with carbon dioxide on fetal acid-base status during fetoscopic surgery in a sheep model. Surg Endosc. 2001;15(4):368–72.
•• Watanabe M, et al. A tissue engineering approach for prenatal closure of myelomeningocele with gelatin sponges incorporating basic fibroblast growth factor. Tissue Eng Part A. 2010;16(5):1645–55 Demonstrated the utility of tissue engineering with growth factors for early and effective coverage of the MMC defect.
•• Watanabe M, et al. Complete tissue coverage achieved by scaffold-based tissue engineering in the fetal sheep model of Myelomeningocele. Biomaterials. 2016;76:133–43 Gelatin-based sponges placed over the fetal MMC defect resulted in granulation tissue and complete coverge of the MMC defect--further enhanced with addition of bFGF.
Wang, A., et al., Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele, in Stem Cells Transl Med. 2015. p. 659–69.
Klein, J.D., et al., Amniotic mesenchymal stem cells enhance normal fetal wound healing. https://home.liebertpub.com/scd, 2010.
•• Dionigi B, et al. Partial or complete coverage of experimental spina bifida by simple intra-amniotic injection of concentrated amniotic mesenchymal stem cells. J Pediatr Surg. 2015;50(1):69–73 Intra-amniotic injections of concentrated amniotic stem cells can induce coverage of MMC.
•• Dionigi B, et al. Trans-amniotic stem cell therapy (TRASCET) minimizes Chiari-II malformation in experimental spina bifida. J Pediatr Surg. 2015;50(6):1037–41 A novel stem cell–based minimally invasive approach to MMC repair reduces Chiari II malformation.
•• Farrelly JS, et al. Alginate microparticles loaded with basic fibroblast growth factor induce tissue coverage in a rat model of myelomeningocele. J Pediatr Surg. 2019;54(1):80–5 Intrauterine injections of alginate, a microparticle, with FGF induces tissue coverage in 30% of MMC in a rat model.
I. Marwan, et al., Reverse thermal gel for in utero coverage of spina bifida defects: an innovative bioengineering alternative to open fetal repair. Vol. 17. 2017. 1600473.
Bardill J, Williams SM, Shabeka U, Niswander L, Park D, Marwan AI. An injectable reverse thermal gel for minimally invasive coverage of mouse myelomeningocele. J Surg Res. 2019;235:227–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have grant funding through The Hartwell Foundation and have a licensed patent (YU6717/7223/7404 PCT) for particle based fetal therapy for myelomeningocele.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Prenatal Therapies
Rights and permissions
About this article
Cite this article
Freedman-Weiss, M.R., Stitelman, D.H. Minimally Invasive Fetal Therapy for Myelomeningocele. Curr Stem Cell Rep 6, 1–5 (2020). https://doi.org/10.1007/s40778-020-00167-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-020-00167-1