Abstract
Stress is associated with reliable behavioral responses that may be observed by other people. The experience of stress should therefore be easily identified by and potentially shared between individuals under stress and those around them. Considerable research has focused on the physiology and psychology of empathy to better understand how we come to understand the pain of others. Recently, researchers have begun to examine how stress may lead to empathic responses. In the present article, we begin with a selective review on empathy, including physiological, emotional, and cognitive components, followed by an introduction to recent research on empathy for stress. We conclude the article by describing some of the outstanding questions that should be addressed to gain a better understanding of how the stress of others influences our own physiology and behavior.
Similar content being viewed by others
Empathy for the Stressed
The neural bases of socioemotional phenomena, especially empathy, are a topic of intense interest in neuroscience (see Bernhardt and Singer 2012; Decety 2015 for reviews). Considered along with behavioral research, evidence from neuroscience has shed a new light on the complex social processes that comprise empathy. Here, we define empathy as the process whereby the perception of a target individual’s state leads to a similar state in an observing individual (after Hoffman 2000, cited in Preston and de Waal 2002). The primary components of empathy are typically broken down into emotional contagion and cognitive empathy; they are mostly dissociable processes, although they do interact (Decety 2015). Much of the research on empathy has focused on negative emotional states and pain. An area that has received little attention until recently is empathy for those who are under stress. Stress is a ubiquitous phenomenon that affects people worldwide. Here, we define stress as a situation that exceeds one’s ability to cope, often characterized by uncontrollability and negative effect, and elicits offsetting changes in one’s psychobiological state (see Mason, 1968; Dickerson and Kemeny 2004). A stressor, then, is the source of the stress and the stress response is the compensatory physiological and behavioral reactions to the stressor (Lovallo 2016). Stress often occurs in social settings and therefore, we are often exposed to others under stress, even in situations in which we are not directly stressed. These situations may lead us exhibit a stress response ourselves, even in the absence of a threat to our own ability to cope.
In the first section of this review, we briefly discuss research that has addressed the two primary components of empathy, emotional contagion and cognitive empathy. Note that there are a number of recent extensive reviews of the psychology and neuroscience of empathy (Batson 2007; Bernhardt and Singer 2012; Decety 2015), so this first section serves as merely an introduction. Next, we turn our attention to empathy for stress. Only recently have researchers examined how the psychology and physiology of stress may be shared between individuals. The goal of this review is to provide background on how empathy for the stressed may come about, the physiological and behavioral mechanisms that may underlie the phenomenon, and to address the situational characteristics that influence our empathic responses to those under stress.
Emotional Contagion
Emotional contagion is defined as “catching” the emotions of others; more specifically, it is the propensity to spontaneously exhibit convergent affective and physiological responses as another individual to whom we are exposed (Hatfield et al. 1994). Multiple channels of information contribute to emotional contagion, as well as multiple neurophysiological processes (Hatfield et al. 2007), and it tends to produce similar physiological responses between individuals (see Preston and de Waal 2002). Emotional contagion is a critical affective component of empathy that aids in the subjective understanding of another’s state. For instance, Levenson and Ruef (1992) demonstrated that when an observer exhibits similar autonomic activity as a target individual, the accuracy of the observer’s inference of the target’s emotional state increases. Emotional contagion, however, need not include a cognitive understanding of the internal state of another (Hatfield et al. 2007); thus, it is simply a congruency between individuals in emotional state and physiological activity.
There is a great deal of evidence suggesting that emotional contagion is an early developing ability that evolved in humans and other social mammals (see Hatfield et al. 2007), particularly primate and rodent species (Hatfield et al. 1994; Hatfield et al. 2007). Empirical evidence of emotional contagion has also been found in humans in many different cultures (e.g., Tseng and Hsu 1980), as well as across the human lifespan (e.g., Eisenberg et al. 1991; Hurley and Chater 2005; Michalska et al. 2013; Sze et al. 2012). One of the earliest manifestations of emotional contagion in humans is the well-documented phenomenon of newborn infants crying in response to hearing the cry of another infant, referred to as contagious crying (Simner 1971). Contagious crying begins soon after birth and extends throughout at least the first year of life (Geangu et al. 2010). Although it is improbable that infants have a cognitive understanding of the internal state of another crying infant, developmental research suggests that contagious crying represents a crude form of emotional contagion. For example, infants exhibit greater distress when exposed to a recording of another infant crying, compared to listening to a recording of their own cry (Dondi et al. 1999; Martin and Clark 1987), and even when compared to listening to a baby chimpanzee crying (Martin and Clark 1987). These findings indicate that contagious crying is species-specific and specific to the cries of other human infants, supporting the notion that contagious crying is caused by the resonance of emotional expressions. These neural capabilities may form the basis for the gradual development of more complicated empathic responses as the forebrain matures (Decety and Svetlova 2012).
The perception-action model (PAM) of empathy includes emotional contagion as a primary component of empathy (Preston and de Waal 2002). The PAM posits that a target’s emotional states are automatically and obligatorily mapped onto an observer’s representations of the target’s state. Considerable research supports this model of how emotional contagion occurs, both at the level of the brain (Singer et al. 2004; Lamm et al. 2011) and peripheral nervous systems (Krebs 1975; Levenson and Ruef 1992; Dimberg et al. 2000). A recent study by Rütgen and colleagues (2015) provides strong evidence for the neural and pharmacological overlap between a target and observer in empathy for pain. These authors demonstrated a placebo-induced reduction of an observer’s own pain ratings in response to first-person experience of pain as well as for the same observer’s ratings of a target individual in pain. Further, this placebo-induced analgesia led to decreased activity of the anterior insula and the anterior cingulate cortex, areas consistently activated in both first-person pain and pain empathy, in observers. Finally, in a follow-up, double-blind, placebo-controlled study examining the effects of the opioid blocker naltrexone versus placebo, Rütgen and colleagues (2015) showed that blockade of the opioid system blocked the placebo-induced analgesia for both the observer’s own pain as well as the rated pain in response to a target’s pain. This study provides strong support for the PAM by demonstrating that empathy for pain operates on the same neural and pharmacological mechanisms as first-person pain.
Some researchers have proposed that emotional contagion is caused by higher order cognitive processes (e.g., Goubert et al. 2007). In other words, they claim that the ability to understand the personal experience of another is what causes emotional contagion. Although emotional contagion can be elicited by cognitive processes such as imagining someone else in distress (e.g., Danziger et al. 2006; Jackson and Decety 2004), neuroscience research implicates the involvement of a wide array of brain regions associated with affective processing in emotional contagion, such as the anterior cingulate and anterior insula (see Lamm et al. 2011), many of which are not associated with perspective taking or other forms of cognitive empathy. In addition, emotional contagion occurs very rapidly, as shown in the simultaneous matching physiological activity recorded between targets and observers (Konvalinka et al. 2011) and in the facial EMG responses that occur milliseconds after non-conscious exposure to posed facial expressions (Dimberg et al. 2000), suggesting that emotional contagion operates at a very primitive level and is not under conscious control (see Hatfield et al. 2007 for review).
Cognitive Empathy
Along with feeling the emotions of others and resonating their physiology, empathy also involves cognitive factors, including a conscious understanding of what other people know, think, and feel. Even simple conversations require a certain awareness of the other person’s knowledge. When acting as a team, one’s perception of one’s own situation often includes comprehension of the perceptions and knowledge of her teammates (Andersen et al. 2001). Thus, many social interactions require that we infer the mental state of others, and this skill is broadly referred to as cognitive empathy (Goubert et al. 2007). A basic component of cognitive empathy is theory of mind (ToM), the processes involved in psychologically representing the internal state of another, including their knowledge, goals, intentions, beliefs, thoughts, and feelings (Premack and Woodruff 1978). Cognitive empathy corresponds to overlapping activation of higher order cortical structures between individuals, and this cortical activity interacts with signals from affective brain regions to produce the full empathic response (see Preston and de Waal 2002). The automatic and rapidly initiated experience of emotional contagion is accompanied by cognitive processes which modulate the emotional and physiological responses related to empathy, possibly even increasing the accuracy of our empathic inferences (Goubert et al. 2007), and allowing us to differentiate between our own experiences and the experiences of others. Although some theorists have attributed empathy to purely cognitive processes (e.g., Goubert et al. 2007), empirical evidence suggests instead that emotional contagion operates in tandem with cognitive empathy and is modulated by higher order cognitive processes (e.g., Luo et al. 2006). Empathy is not primarily driven by either emotional contagion or cognitive empathy, but instead is the resulting interaction between emotional contagion and cognitive empathy.
Empathy for Stress
Recent work has focused on the intersection between empathy and stress and the propensity of stress to be “contagious” among individuals (e.g., Buchanan et al. 2012; Buchanan & Preston 2014; Ebisch et al. 2012; Engert et al. 2015; Waters et al. 2014). Here, we give a brief introduction to the physiology and psychology of stress and outline some of the work that has examined the intersection between stress and empathy. Finally, we outline potential future directions in this research.
The Psychophysiology of Stress
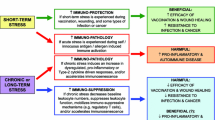
The survival of a species is largely dependent upon a species’ capacity to adaptively respond to environmental threats (Cannon 1935). Stress serves the vital purpose of maintaining a relatively stable internal environment, or homeostasis (see Canon 1935), in the face of a constantly changing external environment that potentially poses a threat to homeostasis. Lovallo (2016) describes two primary physiological systems underlie stress responses in mammals, allowing for concerted control over the wide array of otherwise isolated organic functions throughout the body. These systems are the sympathoadrenomedullary (SAM) system and the hypothalamic-pituitary-adrenocortical (HPA) axis. The SAM system controls smooth muscle cells throughout the body, through the secretion of the hormones epinephrine and norepinephrine, whose effects are mainly due to spillover at peripheral synapses, rather than strictly as a hormone. The primary output of the HPA axis in primates is the hormone cortisol; in rodents, corticosterone is predominant. These hormones are released in response to stress, in addition to their role in normal, non-stress-related metabolic activities. Cortisol exhibits a distinct diurnal cycle in which secretion peaks 30–60 min after waking in the morning, and then drops throughout the day, with small fluctuations related to eating and exercise behaviors (see Lovallo 2016 for a more in-depth discussion of stress).
There is considerable individual variability in cortisol response when confronted with a stressor, and the probability and magnitude of this response differs greatly across contexts and individuals (Kirschbaum et al. 1995a, 1995b, 1999). Acute laboratory stressors characterized by novelty, uncontrollability, as well as a social evaluation component, tend to result in the most pronounced cortisol responses (see Dickerson and Kemeny 2004). The SAM acts more as a general physiological response system, reacting to an array of arousing events such as moderate exercise as well as stressors. The HPA axis, on the other hand, is uniquely activated during stressful situations. In humans, these situations tend to be those that threaten one’s social self, which are characterized by uncontrollability and the potential for negative evaluation from others (Dickerson et al. 2004). Dickerson and colleagues (2004) demonstrated that the presence of social others was necessary for the elicitation of cortisol responses to a laboratory public speaking stressor. While social stress increased both shame and anxiety in stressed speakers, only shame was specifically associated with stress, as a non-social stressor elevated anxiety but not shame. These findings highlight the social nature of stress in humans and suggest that threats to the social self are a major contextual factor influencing stress reactivity.
Contagious Stress?
Stress can affect more than just the individual who directly experiences the stressor. Recent research has focused on the physiological effects of the observation of acute stress elicited in the laboratory in target individuals. Here, we define contagious stress as activation of the physiological stress response (including the SAM and/or HPA) in an observer who is in the presence of a target experiencing stress. Specifically, the target experiences a stressor characterized by social evaluation and uncontrollability (see Dickerson and Kemeny 2004), but the observer is not undergoing the same stressor as the target. Although the observer is not confronted with the stressor, they will often exhibit a similar psychophysiological stress response to the mere observation of the target undergoing the stressor. Buchanan et al. (2012) first demonstrated this phenomenon by showing that simultaneously interacting individuals can exhibit similar HPA axis activation when only one target participant underwent a stressful task while the observer watched. In this study, speaker participants underwent the Trier Social Stress Test (TSST; Kirschbaum et al. 1993), while research assistants observed the speakers undergoing this laboratory stressor. Importantly, these research assistants were both observing the target participant as well as serving as the committee members in the TSST. Both the research assistants and the target participants provided saliva samples before and after the TSST for measures of cortisol and salivary alpha amylase (sAA) responses. Results showed that certain observing individuals exhibited cortisol and sAA responses to the TSST, even though they were not directly targeted by the laboratory stress. Further, the observer’s responses were resonant with the speaker’s responses, in that only the observers paired with the highly stressed speakers (those producing the greatest cortisol and sAA responses) produced convergent physiological stress response. These findings reflect true “resonance” of response: the same physiological signals were measured at the same time in interacting pairs and the observer’s response was comparable to the response of the target. Further, this resonant physiological activity was related to trait empathy levels of the observer, in those observers who reported greater trait levels of empathic concern and perspective taking on the Interpersonal Reactivity Index (IRI; Davis 1983) produced greater cortisol and sAA responses in response to observing stressed speakers.
Engert et al. (2015) further investigated the contagion of stress by testing the physiological resonance between romantic partner dyads as well as dyads composed of strangers. Each dyad was comprised of a target individual, who underwent the TSST, and an observer, who watched the target undergo the TSST either over live video transmission or through a one-way mirror. Twenty-six percent of all observers exhibited a significant cortisol response during the observation of the stressed target. Romantic partner dyads showed greater likelihood of physiological resonance (40 %) than did the stranger dyads (10 %), and the one-way mirror condition was more effective at eliciting physiological resonance (30 %) compared to the video transmission condition (24 %). These findings show that both familiarity and proximity influence the contagious stress responses in individuals exposed to others undergoing a stressor, as predicted by the PAM, which posits that the relationship between a target and an observer will influence the degree of overlapping neural activity, and ultimately physiology and behavior, between individuals (Preston and de Waal 2002). These findings demonstrate that even strangers can show resonant stress responses when viewing stressed targets on video, but that those reporting more trait empathy are more likely to do so, and previous experience between observer and target individuals can influence these responses.
As in Buchanan et al. (2012), Engert and colleagues (2015) documented associations between trait measures of empathic concern and perspective taking from the IRI (Davis 1983) and physiological measures of stress contagion. These results suggest that stress contagion may rely on both emotional contagion (as assessed by the empathic concern subscale of the IRI) and cognitive empathy (as assessed by the perspective taking subscale of the IRI). Interestingly, neither study reported significant associations between stress contagion and the personal distress subscale of the IRI. Given the many emotional and cognitive signals that may convey the experience of stress from a target to an observer (e.g., content of speech, tone of voice, facial expressions, bodily posture), it is perhaps not surprising that observers who are higher in both emotional contagion and cognitive empathy would be more likely to take on the physiological responses of stressed targets. Neither of these studies assessed which specific signals that the observers were picking up on that led to stress contagion. A number of behavioral indices have been associated with stress-induced cortisol output, including verbal fluency (Buchanan et al. 2014), facial fear expression (Lerner et al. 2007), and anxiety-related non-verbal cues (Gray et al. 2008). These indices of stress were only collected from the stressed targets, not from observers and so it remains unknown how and whether such behaviors may serve as an index of stress that may trigger a contagious stress response in observers. Only by measuring the same indices at the same time in both targets and observers can we appreciate the relationships between the response systems of each individual.
Ebisch and colleagues (2012) investigated the contagion of physiological stress between mothers and their children. In their study, thermal facial imprints created by infrared imaging were used as a non-invasive measure of sympathetic activation in mother-child dyads. These researchers found that mothers exposed to their child after the child experienced a stressor exhibited similar facial thermal activity as the child, suggesting autonomic resonance between mother and child even after the offset of the stressful situation. In a related study, these authors found that mothers showed greater and faster contagious responses with their children than women with children who were not their own (Manini et al. 2013). Waters and colleagues (2014) followed up on these findings by examining the influence of a mother’s stress response on an infant’s stress responses after the stressor was terminated. Mothers completed the TSST in a separate room from their infants but were then reunited with their infants a few minutes later. Infants exhibited increased heart rate in response to reuniting with their mothers who had undergone the TSST, but not for those mothers who had undergone a “positive evaluation condition,” or a control condition, during which no stress was manipulated. These results demonstrate that stress contagion can occur even after the cessation of the stressor, perhaps through touch, smell, or other communicative channels. In addition, the study by Waters and colleagues (2014) demonstrates that stress contagion can be initiated by the stress of the infant or the mother and in the absence of higher order cognition, suggesting the transference of stress involves primitive and automatic neurobiological mechanisms.
Emerging research on synchronization at the level of the brain, peripheral physiology, and behavior has begun to examine how physiological parameters such as behavior, heart rate, and cortisol can show resonant responses between individuals (see Konvalinka and Roepstorff 2012 for review). This work goes beyond the typical methods of understanding social cognition through examining how one person thinks about another person in isolation, to examining the emergent responses that arise between two or more interacting people (Butler 2011; Konvalinka and Roepstorff 2012). Butler (2011) has described the interactions that occur in emotional communication as a temporal interpersonal emotion system (TIES) that develops over time and is dependent not on the behavior of one individual or another, but on their interaction. This model is in contrast to historical social psychological research on emotion that tended to focus on only one individual; the TIES model by contrast takes a dynamic approach to understanding emotional communication, as can occur under stress. For example, this research has specifically addressed stress contagion by assessing synchronized arousal (assessed through heart rate measures) in individuals undergoing a fire-walking ritual and kin or non-kin spectators (Konvalinka et al. 2011). Results showed that heart rate synchronization occurred between fire walkers and their kin, but not non-kin spectators during the ritual. The authors speculated that such synchronization may form the basis for increased in-group cohesion and rapport and may serve as an explanation for the ubiquity of such costly rituals across human populations (see Wheatley et al. 2012 for a review of social cognitive and neuroscience approaches to synchrony). We view this synchronization at the level of behavior and the autonomic nervous system as an example of contagious stress that can be measured within a much faster time frame than hormonal responses to stress.
Social Influences on Stress Responses
Some of the empirical evidence that initially suggested a pattern of “contagious stress” between individuals came from examining the physiological consequences of caring for loved ones with dementia. Long-term care for a family member with dementia can have considerable impact on the health and well-being of the caregiver (see Kiecolt-Glaser 1999 for review). Considerable work has demonstrated that such caregiving results in chronic activation of both the SAM system and HPA axis (Cacioppo et al. 2000) as well as a reduction in many markers of immune function and increased incidence of affective disorders (Kiecolt-Glaser 1999). We note that the caregiver stress paradigm is not a “pure” example of contagious stress, as the well-being of the patient most definitely impacts the well-being of the caregiver in a much more substantial manner than merely observing another individual under stress. However, this work demonstrates an extreme example of how the stress of another individual can have a profound impact on our well-being. In a particularly dramatic example of caregiver stress on health, Kiecolt-Glaser and colleagues (1995) documented that dementia caregivers showed significantly slowed wound healing compared to a matched comparison group. The caregivers’ wounds from a standardized punch biopsy took on average 48.7 days to fully heal, while the comparison group showed full healing in 39.3 days. This work only partially represents contagious stress, as the caregiver in this case is the observer, but the target of the observation is a loved one in physical and mental decline, whose stress levels are typically not assessed. Indeed, in the wound healing study, the caregivers reported greater stress than did a separate comparison group who were not caregivers for dementia patients, but stress of the dementia patients were not assessed (Kiecolt-Glaser et al. 1995). In this scenario, the dementia patient target should be the one experiencing stress due to the life-threatening status of their illness, but it is the caregiving observer that often bears the brunt of the detrimental effects of stress due to the negatively affective nature of observing a loved one’s decline. The results of this study are unclear with regard to the source of stress and whether the observer bears more of the burden of the stressor compared to the primary stress target. To address the stress levels of caregivers as well as their targets, Buchanan and colleagues (2004) examined diurnal cortisol levels in neurological patients with medial temporal lobe damage, many of whom had severe anterograde amnesia, as well as their caregivers. This study documented a reduced cortisol awakening response, a marker of altered HPA axis function, in both neurological patient targets as well as their caregiving observers. These findings suggest that the caregiving observer and the cognitively impaired target are both under chronic stress. This work represents a real world model of how empathy can lead to activation of the stress axes, leading to deleterious effects on the health of the observer. The time course and direction of causation of stress contagion has not been addressed in this research however, suggesting a potentially fruitful line of future research. We note that in these examples, stress contagion is only one of many sources of stress in the caregiver-patient relationship.
A complementary line of research has demonstrated that positive social support reduces the physiological response to stress (see Cohen and Wills 1985 for review). This work has been influenced by the “tend and befriend” metaphor coined by Taylor and colleagues (Taylor et al. 2000; Taylor 2006). The tend and befriend framework was developed as a contrast to the fight-or-flight pattern of stress response first described by Cannon (1932) to characterize the pattern of physiology and behavior elicited by stress. In this view, a stress-inducing threat can be acted upon via fighting with, or fleeing from, the source of threat. This pattern, however, is based primarily on research in males. Taylor and colleagues note that the fight-or-flight response to stress may be untenable for female mammalian species due to their primary role in caregiving for their offspring. These females must ensure not only self-survival, but also survival of their offspring. In contrast to fighting or fleeing, these animals show more “tending,” or caring for their offspring and “befriending,” or forming social bonds with conspecifics in response to stress. Support for this idea comes from research in both animals and humans; these behaviors are implemented via the well-characterized neurobiological mechanisms of attachment (see Preston 2013 for review). This tending and befriending results in a reduced stress response in one or both individuals.
In a sense, the tend and befriend framework is counter to the findings on stress contagion reviewed above: whereas stress contagion research highlights the increased stress reactivity in both a target and an observer, stress buffering research demonstrates that the presence of another individual can act to reduce the stress response, specifically in the target individual. We view the findings from the stress buffering research as complementary to those from research on contagious stress, in that the influence of another person’s presence on one’s stress response does not act in only one direction, but may lead to increases or decreases in physiological activity, depending on factors such as relationship status of the observer and target, quality of social support, personality factors, time course of physiological response, as well as a host of other factors (Kirschbaum et al. 1995a, 1995b; Grewen et al. 2005; Hellhammer et al. 1997; Heinrichs et al. 2003; Ditzen et al. 2007; Cardoso et al. 2013). Epidemiological studies demonstrate that social support is linked to lower stress-related morbidity and mortality (Cohen 2004) and that these links are different for men and women (Kiecolt-Glaser and Newton 2001). Women, for example, are more likely to seek social support from friends and family than their spouse, while men are more likely to report their spouse as their main source of social support (Kristenson et al. 2002).
It may be the case that the act of providing social support elicits a stress response that may or may not be synchronous with the individual receiving the support, as in dementia caregivers (see Kiecolt-Glaser 1999). As an example, Kirschbaum and colleagues (1995a, 1995b), examined the effects of pre-stress verbal social support on cortisol and psychological responses to an acute stressor (the TSST). One third of the participants received social support from their significant other, one third received social support from a stranger, and one third received no social support at all. Results from this study showed a pronounced sex difference in the effects of partner support: men who received social support from a partner showed reduced cortisol response to the subsequent stressor compared to men in the other conditions, but women showed the opposite pattern. Women who received support from their partners actually showed increased cortisol reactivity compared to women in the other conditions. These findings, under well-controlled laboratory conditions, demonstrate that sex and relationship status result in different patterns of stress reactivity. The relationship between social support and stress reactivity is complex and differs depending on the provider of the support, the recipient of the support, as well as the nature of the support. A recent study, using a similar design as that of Kirschbaum et al. (1995a, 1995b) examined the effects of verbal versus physical social support on women’s stress reactivity (Ditzen et al. 2007). Results showed that physical support in the form of massage, but not verbal social support from their significant other reduced the cortisol and heart rate response to stress in women. These results, together with those of Kirschbaum and colleagues (1995a, 1995b), demonstrate that the nature of social support is critical in influencing the physiological response to stress. The multifaceted, contextual characteristics that lead to either stress contagion or stress buffering in the real world pose a challenge for researchers to disentangle. These findings suggest that stress contagion is not merely caused by non-conscious neurobiological mechanisms involved in emotional contagion, but that it is modulated by higher order cognitive processes as well. Similar mechanisms such as the closeness of social relationships between targets and observers and stress severity most likely influence both stress contagion and stress buffering. Future work should address these characteristics both in the laboratory and in more ecologically valid contexts in order to better understand the precise factors that influence whether stress responses are resonated or buffered by observing individuals.
Conclusions and Future Directions
Although there are still many lingering questions, research has demonstrated certain consistent findings regarding empathy more generally and how these findings may predict empathy for individuals under stress. Empathy is composed of multiple psychological faculties and neural subsystems that interact to produce empathic responses (see Batson 2007; Blair 2005; Gazzola et al. 2006). The primary components of empathy are emotional contagion and cognitive empathy, each of which relies on overlapping and independent neural networks (see Preston and de Waal 2002; Blair 2005). The perception-action model of empathy (PAM; see Preston and de Waal 2002) provides a parsimonious and well-supported explanation for stress empathy by attributing the emotional, physiological, and cognitive components of empathy to overlapping neural activation between individuals. The PAM proposes that such a mechanism is necessary for both the matching physiological responses and, ultimately, an understanding of a target’s emotional and cognitive state. For example, when an observer is exposed to a stressed target, we hypothesize that the observer experiences activation of similar neural representations as the target, and this congruency of neural activity between individuals results in an empathic response, manifested in both physiology and psychology. As proposed in the PAM, contextual factors such as the relationship status between the target and observer, the expressiveness of the target, and the empathic traits of the observer can alter this initial, automatic response, potentially mitigating the stress contagion, or resulting in incongruent internal experiences between individuals. Thus, as with other examples of empathic responding, multiple channels of information, as well as multiple neural networks in the brain influence empathy for the stressed.
Future research on empathy for the stressed should examine the neural networks responsible for stress empathy and the extent to which these networks overlap with those involved in first-person stress as well as both emotional contagion and cognitive empathy. Emotional and cognitive factors are often considered in isolation, but current research suggests that these factors are constantly interacting during empathic experiences; thus, future research should seek to determine the neurobiological underpinnings of this interaction. Further, this work should focus on how these neural networks interact over time, producing the full empathic experience for individuals under stress and those observing them. Research suggests that empathic responses are constantly modulated by other factors; however, the temporal order of these interacting processes is still unclear. Does stress contagion initially occur automatically and is subsequently enhanced or buffered by other factors such as our relationship with the stressed individual? Understanding the specific cognitive, emotional, and contextual factors that influence how an observer responds to a stressed target over time is a fruitful area of research that could elucidate precisely how stress can spread among individuals. Such research could determine the contexts that result in convergent or divergent emotional and physiological responses in the observer and stressed individual. Investigating the resonance of stress between individuals in real time is of great importance, in order to provide empirical evidence for the precise temporal order of the influence of the stress signals from a target to the responses of a paired observer. Just as the study of empathy for pain has led to a better understanding of both empathy and pain, the study of empathy for the stressed may uncover clues to help us understand the often misunderstood concepts of empathy and stress.
These findings may eventually be applied to the development of psychotherapeutic techniques that may enhance specific components of empathy in individuals with specific deficits in empathy-related capacities, including individuals with autism or psychopathy. In addition, this line of research may lead to new approaches for improving the health and well-being of individuals who are regularly exposed to stressed individuals, including social workers, medical professionals, and caregivers. Lastly, gaining a better understanding of the cognitive components that modulate empathic responses to the stressed may eventually help individuals to respond to stressed individuals with prosocial behaviors, rather than simply being overwhelmed by personal distress.
References
Andersen, H. B., Pedersen, C. R., & Andersen, H. H. K. (2001). Using eye tracking data to indicate team situation awareness. In M. J. Smith, G. Salvendy, D. Harris, & R. J. Koubek (Eds.), Usability evaluation and interface design: cognitive engineering, intelligent agents and virtual reality (pp. 1318–1322). Mahwah, NJ: Erlbaum.
Batson, C. D. (2007). These things we call empathy: eight related by distinct phenomena. In J. Decety & W. Ickes (Eds.), The social neuroscience of empathy (pp. 139–152). Cambridge, MA: The MIT Press.
Bernhardt, B. C., & Singer, T. (2012). The neural basis of empathy. Annual Review of Neuroscience, 35, 1–23.
Blair, R. J. R. (2005). Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Consciousness and Cognition, 14, 698–718. doi:10.1016/j.concog.2005.06.004.
Buchanan, T. W., & Preston, S. D. (2014). Stress leads to prosocial action in immediate need situations. Frontiers in Behavioral Neuroscience, 8, p. 5. doi:10.3389/fnbeh.2014.00005.
Buchanan, T. W., Kern, S., Allen, J. S., Tranel, D., & Kirschbaum, C. (2004). Circadian regulation of cortisol after hippocampal damage in humans. Biological Psychiatry, 56, 651–656.
Buchanan, T. W., Bagley, S. L., Stansfield, R. B., & Preston, S. D. (2012). The empathic, physiological resonance of stress. Social Neuroscience, 7(2), 191–201. doi:10.1080/17470919.2011.588723.
Buchanan, T. W., Laures-Gore, J. S., & Duff, M. C. (2014). Acute stress reduces speech fluency. Biological Psychology, 97, 60–66.
Butler, E. A. (2011). Temporal interpersonal emotion systems: the “TIES” that form relationships. Personality and Social Psychology Bulletin, 15, 367–393.
Cacioppo, J. T., Burleson, M. H., Poehlmann, K. M., Malarkey, W. B., Kiecolt-Glaser, J. K., Berntson, G. G., … & Glaser, R. (2000). Autonomic and neuroendocrine responses to mild psychological stressors: effects of chronic stress on older women. Annals of Behavioral Medicine, 22(2), 140–148.
Cannon, W. B. (1932). The wisdom of the body. New York: Norton.
Cannon, W. (1935). Stresses and strains of homeostasis. American Journal of Medical Sciences, 189, 13–14.
Cardoso, C., Ellenbogen, M. A., Serravalle, L., & Linnen, A. M. (2013). Stress-induced negative mood moderates the relation between oxytocin administration and trust: evidence for the tend-and-befriend response to stress? Psychoneuroendocrinology, 38(11), 2800–2804.
Cohen, S. (2004). Social relationships and health. American Psychologist, 59, 676–684.
Cohen, S., & Wills, T. A. (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98, 310–357.
Danziger, N., Prkachin, K. M., & Willer, J. C. (2006). Is pain the price of empathy? The perception of others’ pain in patients with congenital insensitivity to pain. Brain, 129, 2494–2507. doi:10.1093/brain/awl155.
Davis, M. H. (1983). Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44(1), 113–126.
Decety, J. (2015). The neural pathways, development and functions of empathy. Current Opinion in Behavioral Sciences, 3, 1–6.
Decety, J., & Svetlova, M. (2012). Putting together phylogenetic and ontogenetic perspectives on empathy. Developmental Cognitive Neuroscience, 2, 1–24. doi:10.1016/j.dcn.2011.05.003.
Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391.
Dickerson, S. S., Gruenewald, T. L., & Kemeny, M. E. (2004). When the social self is threatened: shame, physiology, and health. Journal of Personality, 72(6), 1191–1216.
Dimberg, U., Thunberg, M., & Elmehed, K. (2000). Unconscious facial reactions to emotional facial expressions. Psychological Science, 11, 86–89.
Ditzen, B., Neumann, I. D., Bodenmann, G., von Dawans, B., Turner, R. A., Ehlert, U., & Heinrichs, M. (2007). Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology, 32(5), 565–574.
Dondi, M., Simion, F., & Caltran, G. (1999). Can newborns discriminate between their own cry and the cry of another newborn infant? Developmental Psychology, 35, 418–426. doi:10.1037/0012-1649.35.2.418.
Ebisch, S. J., Aureli, T., Bafunno, D., Cardone, D., Romani, G. L., & Merla, A. (2012). Mother and child in synchrony: thermal facial imprints of autonomic contagion. Biological Psychology, 89, 123–129. doi:10.1016/j.biopsycho.2011.09.018.
Eisenberg, N., Fabes, R. A., Schaller, M., Carlo, G., & Miller, P. A. (1991). The relations of parental characteristics and practices to children’s vicarious emotional responding. Child Development, 62, 1393–1408.
Engert, V., Plessow, F., Miller, R., Kirschbaum, C., & Singer, T. (2015). Cortisol increase in empathic stress is modulated by emotional closeness and observation modality. Psychoneuroendocrinology, 45, 192–201. doi:10.1016/j.psyneuen.2014.04.005.
Gazzola, V., Aziz-Zadeh, L., & Keysers, C. (2006). Empathy and the somatotopic auditory mirror system in humans. Current Biology, 16, 1824–1829. doi:10.1016/j.cub.2006.07.072.
Geangu, E., Benga, O., Stahl, D., & Striano, T. (2010). Contagious crying beyond the first days of life. Infant Behavior & Development, 33, 279–288. doi:10.1016/j.infbeh.2010.03.004.
Goubert, L., Craig, K. D., & Buysse, A. (2007). Perceiving others in pain: experimental and clinical evidence on the role of empathy. In J. Decety & W. Ickes (Eds.), The social neuroscience of empathy (pp. 153–166). Cambridge, MA: The MIT Press.
Gray, H. M., Mendes, W. B., & Denny-Brown, C. (2008). An in-group advantage in detecting intergroup anxiety. Psychological Science, 19, 1233–1237.
Grewen, K. M., Girdler, S. S., Amico, J., & Light, K. C. (2005). Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine, 67, 531–538.
Hatfield, E., Cacioppo, J., & Rapson, R. L. (1994). Emotional contagion. New York: Cambridge University Press.
Hatfield, E., Rapson, R. L., & Le, Y. L. (2007). Emotional contagion and empathy. In J. Decety & W. Ickes (Eds.), The social neuroscience of empathy (pp. 19–30). Cambridge, MA: The MIT Press.
Heinrichs, M., Baumgartner, T., Kirschbaum, C., & Ehlert, U. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54(12), 1389–1398.
Hellhammer, D. H., Buchtal, J., Gutberlet, I., & Kirschbaum, C. (1997). Social hierarchy and adrenocortical stress reactivity in men. Psychoneuroendocrinology, 22(8), 643–650.
Hoffman, M. L. (2000). Empathy and moral development: implications for caring and justice. Cambridge, UK: Cambridge University Press.
Hurley, S., & Chater, N. (2005). Perspective on imitation: from neuroscience to social science: vol. 2. Imitation, human development, and culture. Cambridge, MA: MIT Press.
Jackson, P. L., & Decety, J. (2004). Motor cognition: a new paradigm to study self-other interactions. Current Opinion in Neurobiology, 14, 259–263. doi:10.1016/j.conb.2004.01.020.
Kiecolt-Glaser, J. K. (1999). Stress, personal relationships, and immune function: health implications. Brain, Behavior, and Immunity, 13(1), 61–72.
Kiecolt-Glaser, J. K., & Newton, T. L. (2001). Marriage and health: his and hers. Psychological Bulletin, 127, 472–503.
Kiecolt-Glaser, J. K., Marucha, P. T., Mercado, A. M., Malarkey, W. B., & Glaser, R. (1995). Slowing of wound healing by psychological stress. The Lancet, 346, 1194–1196.
Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The trier social stress test - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. doi:10.1159/000119004.
Kirschbaum, C., Klauer, T., Filipp, S. H., & Hellhammer, D. H. (1995a). Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine, 57(1), 23–31.
Kirschbaum, C., Pruessner, J.C., Stone, A.A., Federenko, I., Gaab, J., Lintz, D.,…Hellhammer, D.H. (1995b). Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic Medicine, 57(5), 468–474.
Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C., & Hellhammer, D. H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61(2), 154–162.
Konvalinka, I., & Roepstorff, A. (2012). The two-brain approach: how can mutually interacting brains teach us something about social interaction? Frontiers in Human Neuroscience, 6.
Konvalinka, I., Xygalatas, D., Bulbulia, J., Schjødt, U., Jegindø, E. M., Wallot, S., & Roepstorff, A. (2011). Synchronized arousal between performers and related spectators in a fire-walking ritual. Proceedings of the National Academy of Sciences, 108, 8514–8519.
Krebs, D. (1975). Empathy and altruism. Journal of Personality and Social Psychology, 32(6), 1134–1146.
Kristenson, M., Cain, V. S., & Weidner, G. (2002). What have we learned so far? Implications for prevention. In G. Weidner, M. Kopp, & M. Kristenson (Eds.), Heart disease: environment, stress, and gender. Amsterdam: IOS Press.
Lamm, C., Decety, J., & Singer, T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54, 2492–2502. doi:10.1016/j.neuroimage.2010.10.014.
Lerner, J. S., Dahl, R. E., Hariri, A. R., & Taylor, S. E. (2007). Facial expressions of emotion reveal neuroendocrine and cardiovascular stress responses. Biological Psychiatry, 61, 253–260.
Levenson, R. W., & Ruef, A. M. (1992). Empathy: a physiological substrate. Journal of Personality and Social Psychology, 63(2), 234–246. doi:10.1037/0022-3514.63.2.234.
Lovallo, W. R. (2016). Stress & health: biological and psychological interactions (3rd ed.). Los Angeles, CA: Sage.
Luo, Q., Nakic, M., Wheatley, T., Richell, R., Martin, A., & Blair, R. J. (2006). The neural basis of implicit moral attitude: an IAT study using event-related fMRI. NeuroImage, 30(4), 1449–1457. doi:10.1016/j.neuroimage.2005.11.005.
Manini, B., Cardone, D., Ebisch, S. J. H., Bafunno, D., Aureli, T., & Merla, A. (2013). Mom feels what her child feels: thermal signatures of vicarious autonomic response while watching children in a stressful situation. Frontiers in Human Neuroscience, 7, 299. doi:10.3389/fnhum.2013.00299.
Martin, G. B., & Clark, R. D. (1987). Distress crying in neonates: species and peer specifically. Developmental Psychology, 18, 3–9.
Mason, J. W. (1968). A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosomatic Medicine, 30, 576–607.
Michalska, K. J., Kinzler, K. D., & Decety, J. (2013). Age-related sex differences in explicit measures of empathy do not predict brain responses across childhood and adolescence. Developmental Cognitive Neuroscience, 3, 22–32. doi:10.1016/j.dcn.2012.08.
Premack, D., & Woodruff, G. (1978). Does the chimpanzee have a theory of mind? Behavioral and Brain Sciences, 1(4), 515–526. doi:10.1017/S0140525X00076512.
Preston, S. D. (2013). The origins of altruism in offspring care. Psychological Bulletin, 139, 1305–1341.
Preston, S. D., & de Waal, F. B. M. (2002). Empathy: its ultimate and proximate bases. Behavioral and Brain Sciences, 25, 1–72.
Rütgen, M., Seidel, E. M., Riečanský, I., & Lamm, C. (2015). Reduction of empathy for pain by placebo analgesia suggests functional equivalence of empathy and first-hand emotion experience. The Journal of Neuroscience, 35, 8938–8947.
Simner, M. L. (1971). Newborn’s response to the cry of another infant. Developmental Psychology, 5(1), 136–150. doi:10.1037/h0031066.
Singer, T., Seymour, B., O’Doherty, J., Kaube, H., Dolan, R. D., & Frith, C. D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–1162. doi:10.1126/science.1093535.
Sze, J. A., Gyurak, A., Goodkind, M. S., & Levenson, R. W. (2012). Greater emotional empathy and prosocial behavior in late life. Emotion, 12(5), 1129–1140. doi:10.1037/a0025011.
Taylor, S. E. (2006). Tend and befriend biobehavioral bases of affiliation under stress. Current Directions in Psychological Science, 15(6), 273–277.
Taylor, S. E., Klein, L. C., Lewis, B. P., Gruenewald, T. L., Gurung, R. A., & Updegraff, J. A. (2000). Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411.
Tseng, W.-S., & Hsu, J. (1980). Minor psychological disturbances of everyday life. In H. C. Triandis & J. D. Draguns (Eds.), Handbook of crosscultural psychology (Psychopathology, Vol. 6, pp. 61–97). Boston: Allyn & Bacon.
Waters, S. F., West, T. V., & Mendes, W. B. (2014). Stress contagion: Physiological covariation between mothers and infants. Psychological Science, 1–9. doi: 10.1177/0956797613518352
Wheatley, T., Kang, O., Parkinson, C., & Looser, C. E. (2012). From mind perception to mental connection: synchrony as a mechanism for social understanding. Social and Personality Psychology Compass, 6(8), 589–606.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
White, C.N., Buchanan, T.W. Empathy for the Stressed. Adaptive Human Behavior and Physiology 2, 311–324 (2016). https://doi.org/10.1007/s40750-016-0049-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40750-016-0049-5