Abstract
Necrotizing enterocolitis (NEC) is a serious intestinal disease which primarily affects preterm infants. The pathogenesis of NEC is multifactorial. Thus, it is complicated to study, prevent, and manage.
Purpose of Review
The purpose of this review is to provide a comprehensive summary of recent research and provide recommendations for the prevention and management of NEC. Currently, management is supportive and non-specific and long-term outcomes for surgical NEC are poor.
Recent Findings
The most important strategy to prevent NEC is to provide preterm infants with a human milk diet, minimize exposure to antibiotics and avoid medications that disturb the intestinal microbiome.
Summary
Strategies to optimize the infant’s intestinal microbiome are critical, as disturbances in the intestinal microbiome composition are a major factor in the pathogenesis of this disease. Optimizing maternal health is also vital to prevent prematurity and neonatal morbidity. Ongoing research holds promise for the implementation of new diagnostic modalities, preventive strategies, and medical treatment options to improve outcomes for premature infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Necrotizing enterocolitis (NEC) is a devastating intestinal disease characterized by acute intestinal inflammation which can affect multiple organ systems, causing significant morbidity and mortality [1, 2]. NEC disproportionately affects preterm infants [3], with a mortality rate close to 25% [4]. Among our most premature infants and those who require surgery, mortality rates are over 30% [5]. Despite decades of research, long-term outcomes remain unfavorable [6]. Surviving infants are more likely to experience adverse neurodevelopmental outcomes [7] and gastrointestinal sequelae [8].
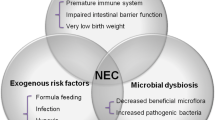
The pathophysiology of NEC is complex and involves many dynamic factors including feeding, intestinal immaturity, microbial dysbiosis, immature immune defenses and local ischemia or hypoxia (Fig. 1) [3, 9]. These factors contribute to a breakdown and dysfunction of the intestinal barrier which can lead to bacterial translocation, excessive inflammation, and intestinal necrosis [9]. Interventions focused on preventing this cascade serve as our primary vehicle in caring for premature infants with NEC. Our goal in this review is to describe the current prevention and management strategies and future directions in NEC research.
NEC Risk Factors and Prevention Strategies. NEC risk factors include preterm birth (causing immature intestinal barrier and immune defenses), formula feeding, microbial dysbiosis and local ischemia or hypoxia. NEC prevention strategies include human milk diet, implementation of feeding protocols, transfusion protocols, probiotics and antibiotic stewardship
Prevention Strategies
Human Milk
Human milk (HM) is the optimal infant nutrition, and the AAP recommends its use in term and preterm infants [10]. HM contains macro and micronutrients essential for infant growth and bioactive factors which promote gastrointestinal development and protect against infection and inflammation [11, 12]. These bioactive factors include commensal bacteria, HM oligosaccharides, immunoglobulins, lactoferrin, and dietary polyunsaturated fatty acids [13]. HM reduces the risk of NEC in a dose dependent fashion [14]. Initiating and sustaining lactation is often challenging for mothers with infants in the Neonatal Intensive Care Unit (NICU).
Donor Human Milk (DHM)
DHM should be used in preterm infants when mother’s own milk (MOM) is unavailable or contraindicated. DHM diet reduces the risk of NEC compared to formula diet in preterm infants but may not confer the other benefits of MOM such as reduction in BPD, ROP, sepsis, and neurodevelopmental benefits [15]. DHM is often composed of human milk donated from mothers of term infants [16, 17], which has lower macro and micronutrients than preterm MOM [18]. Additionally, while pasteurization of DHM is necessary for safety, pasteurization and milk banking reduces the milk’s fat content, immune factors, and enzymes [17, 18].
Human Milk and NEC
HM reduces the risk of NEC. In a recent systematic review and meta-analysis of 6 RCTs with 1,626 infants, the authors found that MOM or DHM compared to a formula diet reduced the risk of NEC (RR = 0.62, 95% CI 0.42–0.93) [19]. Using the same comparison, authors found a similar risk reduction in NEC when evaluating 18 observational studies with 6,405 infants (RR 0.45, 95% CI 0.32–0.62). These results parallel those of a Cochrane review [20]of 11 RCTs with over 1,800 preterm or low birthweight (LBW) infants and found a nearly two-fold higher risk of developing NEC in formula versus DHM-fed infants (RR 1.87, 95% CI 1.23–2.85) [20]. Moreover, the authors estimated one additional case of NEC for every 33 infants who received formula.
Fortification of Human Milk
While HM is the gold standard diet for infants, preterm infants require higher calories, protein, minerals, and electrolytes than term infants [14]. Therefore, HM fortification is necessary for adequate growth and development [21]. HM can be fortified with bovine-milk based fortifier, formula, or DHM-based fortifier. While HM diet clearly reduces NEC, studies do not support an additional protective benefit of DHM-based fortifier over bovine-milk based fortifier. Quigley et al. found HM was protective against NEC even with bovine-milk based fortifier compared to an exclusive formula diet [16]. Two RCTs compared an exclusive HM diet (DHM + HM-based fortifier) to a diet with formula if MOM was not available (MOM + bovine milk-based fortifier or preterm formula if no MOM) and showed decreased rates of NEC in the exclusive HM diet [22, 23]. One RCT compared a HM diet with DHM fortifier to a HM diet with bovine-milk based fortifier [24] found no differences in feeding intolerance or intestinal inflammation assessed by fecal calprotectin. Both groups had the same number of NEC cases, but this study was underpowered to detect a difference in NEC [24]. In a multicenter randomized controlled trial, Jensen et al. evaluated the effect of HM diet with DHM fortification versus HM diet with bovine milk fortification on a composite outcome of NEC (stage II-III), culture positive sepsis or death among extremely preterm infants. The authors of this study report that DHM-based fortification did not reduce the primary outcome compared to bovine-milk based fortification [25•]. Thus, current evidence demonstrates that there is no added benefit of DHM-based over bovine-milk based fortifier on NEC prevention.
Promoting MOM
Provision of MOM is the most effective strategy to reduce NEC [26, 27]. The following are strategies which can improve provision of MOM [28•]:
-
Pumping/hand expression ideally within 1 hour of delivery [29, 30]
-
Pumping every 3 hours
-
Oral colostrum care
-
Promoting skin-to-skin contact in NICU [31]
-
Lactation support through licensed lactation consultant
-
Sharing importance of MOM with parents and support people
Feeding Protocols
Implementation of a standard feeding protocol (SFP) benefits infant growth and decreases NEC incidence [8, 32,33,34,35]. A Cochrane review analyzed 6 observational studies from 1978–2003 before and after SFP implementation. Random effects model revealed SFP implementation reduced the incidence of NEC by 87% [34]. Several observational studies have confirmed these results in VLBW infants [32, 33, 35]. Similar evidence exists for ELBW infants. Shah et al. found the odds of NEC were lowered by 63% in ELBW infants after SFP implementation (OR 0.38, 95% CI 0.142–0.993) [8]. Notably, these studies implemented SFPs that differed in duration of trophic feeds, rate of advancement of feeds, and timing of fortification. Therefore, a unit’s adherence to a protocol is critical to reduce NEC. A SFP should address 1) initiation and duration of trophic feeds, 2) prioritization of HM and 3) feeding advancement rate and timing of fortification [8].
Time to Initiate Feeds
Studies have evaluated the optimal SFP design, specifically timing of feed initiation and advancement rate. A recent meta-analysis compared 14 RCTs that implemented early (< 72 h of life) or delayed (≥ 72 h of life) enteral feeds in preterm or LBW infants [36•]. Early initiation of enteral feeds had no effect on NEC risk (RR 1.05, 95% CI 0.75–1.46). Similarly, a 2022 Cochrane review evaluated timing of enteral feed initiation in VLBW infants with early (< 4 days of life) or delayed (≥ 4 days of life) feeds and found no effect on NEC risk [37•]. However, delayed enteral feeds may increase the risk of invasive infections (RR 1.44, 95% CI 1.15–1.80).
Feeding Advancement
Historically, older studies suggested an association between feed advancement rate and risk of NEC [38, 39]. However, newer evidence from the SIFT trial did not confirm this association [40]. The SIFT trial compared fast (30 ml/kg/day) versus slow (18 ml/kg/day) enteral feed advancement rates in very preterm or VLBW infants and found that advancement rate did not affect NEC risk (aRR 0.88, 95% CI 0.68–1.16). These findings are supported by a recent Cochrane review that concluded that slow (15–24 ml/kg/day) enteral feed advancement rates did not reduce NEC risk when compared to fast (30–40 ml/kg/day) feeding advancement in VLBW infants [41•]. Conversely, a single-center cohort study (using historical controls) of infants < 750 g at birth found a reduced incidence of NEC and death after implementing slow advancement protocol (but higher rates of cholestasis) [42]. This result may due to the implementation of a SFP that didn’t previously exist or indicate that micropremies (< 750 g or < 25 weeks’ gestation) are a unique population [43]. Preterm growth restricted infants and infants with a history of absent or reverse end diastolic flow may also be at increased risk for NEC and TPN-associated liver injury [44,45,46,47]. In these populations, initiating small-volume enteral feeds of MOM early may promote growth, prevent TPN-associated liver injury and reduce the risk of NEC [45].
Probiotics
Probiotic use for NEC prevention has been thoroughly investigated through preclinical and clinical studies [48]. Pre-clinical studies from various animal models show that probiotic supplementation reduces the risk of NEC by nearly 50% [49]. Since 2002, over 50 RCTs of over 10,000 preterm infants have been conducted to assess the effect of probiotic administration on NEC incidence [50]. There is heterogeneity in these RCTs with regards to probiotic used, gestational age/birthweight of infants included; however, several systematic reviews and meta-analyses that have evaluated these RCTs have found that probiotic use in preterm infants reduces the risk of NEC [50]. In the United States (US), probiotics are not FDA regulated. However, in 2023, the FDA issued a warning to NICUs and providers using probiotics to reduce NEC. Following this warning, unfortunately, the use of probiotics in NICUs in the US has all but halted. Based on the extensive data on probiotics, we would recommend probiotic use as an effective tool in reducing the risk of NEC.
Antibiotic Stewardship
Preterm infants are often exposed to empiric antibiotics upon NICU admission [51]. Despite the benefit of treating active bloodstream infections, antibiotics may affect infants’ intestinal microbiome and function, thereby influencing their risk of NEC [52, 53]. In 2009, Wang et al. evaluated the fecal microbiome composition of preterm infants with and without NEC through 16S rRNA gene sequencing. This study found that preterm infants had less diversity and an increase in Gammaproteobacteria just prior to NEC development when compared to preterm infants who did not develop NEC. Additionally, this study found that patients who developed NEC had longer antibiotics exposure [54]. Furthermore, in 2017 Pammi et al. performed a systematic review and meta-analyses of fecal microbiome profiles in preterm infants [55]. This study included 14 studies with 106 NEC cases, 278 controls and over 2000 fecal samples. They found that the fecal microbiome of preterm infants had increased abundance of Proteobacteria and decreased relative abundance of Firmicutes and Bacteroidetes before the onset of NEC [55].
Observational Studies
Several observational studies suggest prolonged early, empiric antibiotic use is associated with an increased NEC risk [56,57,58,59,60,61,62,63]. In a retrospective cohort study of 4,039 ELBW infants, Cotton et al. examined associations between length of first antibiotic course and NEC [56]. The authors concluded that prolonged empiric antibiotics (≥ 5 days) were associated with an increased risk of NEC or death compared to treatment < 5 days (OR 1.30, 95% CI 1.10–1.54). Similarly, a multi-center retrospective case–control study compared 224 NEC cases with 447 matched controls and found early, empiric antibiotic treatment for ≥ 5 days was associated with increased odds of NEC (aOR 2.02, 95% CI 1.55–3.13) [60]. Another large multi-center retrospective cohort study of over 14,000 VLBW infants demonstrated that early antibiotics for > 3 days increased the risk of negative outcomes, composite outcome of mortality, or any major morbidity, including NEC) (aOR 1.24, 95% CI 1.09–1.41 and aOR 1.38, 95% CI 1.25–1.51, respectively) [64].
Randomized Control Trials
RCTs evaluating prophylactic antibiotic use for NEC prevention are limited to older studies from the 1970s-1990s which used primarily oral antibiotics for 7–24 days [65,66,67,68,69,70]. Four of these six studies demonstrated the benefit of oral antibiotics, while the other two found no significant difference. Despite these promising results, due to concern for antibiotic resistance and microbial dysbiosis, the use of oral antibiotics to prevent NEC was not adopted. If oral antibiotics such as Gentamicin can be timed with NEC-associated changes in the infant microbiome (Gammaproteobacter bloom), this may be a future strategy to prevent NEC.
Systematic Reviews and Meta-Analyses
Several systematic reviews and meta-analyses have included both older RCTs and newer observational studies. Rina et al. evaluated 2 RCTs and 11 observational studies and found initial empiric antibiotic therapy ≥ 5 days was associated with an increased NEC incidence (aOR1.51, 95% CI 1.22–1.87) [71]. Similarly, Klerk et al. analyzed 10 observational cohort studies comparing initial empiric antibiotic therapy and prolonged empiric antibiotic therapy (3–14 days) and found prolonged antibiotics were associated with an increased risk of NEC (OR 2.72, 95% CI 1.65–4.47) [72•]. A meta-analysis by Fan and colleagues including 6 RCTs and 3 observational studies also found that prolonged empiric antibiotic treatment increased NEC incidence (1.31, 95% CI 1.08–1.59) [73].
These studies suggest that long courses of empiric antibiotics (≥ 5 days) may increase the risk of NEC. Therefore, we recommend prudent use of prolonged antibiotics, especially in the case of culture negative sepsis.
Medications to Avoid
Promoting a healthy gut microbiome in preterm infants is an important step in preventing NEC. This includes promoting breastfeeding, antibiotic stewardship and avoiding medications that may alter the microbiome such as acid suppression medications. A pooled analysis of observational studies, involving over 11,000 infants showed a significant increase in NEC with administration of acid-suppression medications (OR: 1.78, 95% CI: 1.4–2.27) [74].
Erythropoiesis-Stimulating Agents for Prevention of NEC
Erythropoietin may be beneficial for endothelial cell barriers [75]. A meta-analysis of RCTs of erythropoiesis-simulating agents given to modify transfusion exposure in preterm infants, found that infants who received this therapy < 8 days after birth had decreased NEC incidence (RR: 0.69 95% CI: 0.52–0.91) [76].
Anemia, Transfusions and NEC
Anemia is common in preterm infants due to iatrogenic blood loss from frequent blood draws, impaired red blood cell (RBC) production and bleeding. Because of this, preterm infants often require RBC transfusions. The relationship between NEC, anemia and RBC transfusions has been studied in preclinical and clinical studies.
Pre-Clinical Data
In a model of anemic mouse pups, MohanKumar et al. demonstrated that RBC transfusions triggered gut injury through toll-like receptor 4 (TLR4) activation [77]. The extent of the gut injury was related to duration of RBC storage. Interestingly, when non-anemic mouse pups received RBC transfusions, there was no gut injury. The authors found a positive correlation between the number of RBC transfusions and gut injury. This implies that anemia primes the gut for inflammation and subsequent RBC transfusion(s) result(s) in a secondary inflammatory insult culminating in intestinal inflammation and injury.
Observational Studies
Mally et al. reported a temporal relationship with NEC and RBC transfusion as well as with NEC and the degree of anemia [78]. In a meta-analysis of 12 observational studies there was an association between RBC transfusions and NEC [79]. However, many of these observational studies looked for temporal association between NEC and transfusions. This does not indicate causality or increased risk of NEC after transfusions. Since then, there have been other observational studies with mixed results. Using a matched case–control study, Sharma et al. did not identify an association between RBC transfusions and NEC [80].
Randomized Control Trials
The Prematures in Need of Transfusion (PINT) trial was a RCT conducted to study whether restrictive versus liberal RBC transfusion practices influence death before discharge or survival with severe morbidity [81]. This study found no statistically significant difference in the primary outcome of death or neonatal morbidity. A post hoc analysis identified mild to moderate cognitive delay was reduced in the higher hemoglobin threshold group [82]. This inspired the larger transfusion of prematures (TOP) trial, which aimed to determine whether liberal transfusion practices could improve survival without neurodevelopmental impairment (NDI) in preterm infants [83]. The TOP trial did not identify a difference in the risk of death or NDI at 22–26 months corrected gestational age (CGA) [83]. Another RCT, the ETTNO trial, identified no difference in death or disability at 24 months CGA from liberal versus conservative transfusion practices [84]. In these trials, NEC was assessed as a secondary outcome and no difference in the incidence of NEC was identified between higher versus lower transfusion threshold practices. These data suggest that neither number of transfusions nor degree of anemia prior to transfusions play a role in NEC development clinically.
Meta-Analysis
Hay et al. evaluated the quality of evidence behind transfusion-associated NEC [85]. The authors evaluated 23 observational studies and 3 RCTs which reported NEC 48 h following a RBC transfusion or any time after a transfusion. The pooled outcome of NEC occurring within 48 h of a RBC transfusion was an OR of 1.13 (95% CI 0.99–1.29). For NEC occurring any time after a RBC transfusion, the pooled OR was 1.95 (95% CI 1.6–2.38). In the pooled outcome from RCT data, the OR 0.6 (95% CI 0.3–2.21), with NEC being more frequent in the restrictive transfusion group. However, the quality of evidence was considered very low, suggesting very little confidence in these effect estimates [85]. Meta-analyses of RCTs evaluating the effect of high versus low RBC transfusion threshold strategies have not found a difference in the risk of NEC [86].
Based on these studies, we would recommend implementation of a transfusion protocol based on the infant’s age and level of respiratory support which avoids anemia below that which has been tested in RCTs. By avoiding severe anemia, outside of the range which has been tested by RCTs such as the PINT trial and TOP trial [81, 83], we may be able to decrease risk of intestinal injury.
Docosahexaenoic acid (DHA) for Prevention of NEC
Bernabe-Garcia et al. performed a Phase II RCT with 225 who received an enteral dose of 75 mg/kg DHA for 14 days once enteral feeds were started or control (high-oleic sunflower oil) [87•]. The authors found NEC was lower in the DHA group with no cases of NEC versus 7 cases in the control group (p = 0.007), with RR = 0.93 (95% CI 0.88 to 0.98) [87•].
Optimizing Maternal Health
Last, it is imperative to remember that infant health is inextricably linked to maternal health. By improving maternal health before pregnancy and during pregnancy, we will reduce the incidence of preterm birth and LBW, which are major risk factors for NEC development [88].
Management of NEC
Management of Medical NEC
There is little evidence to guide clinicians in the management of NEC (Table 1). The management of NEC is largely supportive and includes bowel rest, decompression of the gastrointestinal tract, treatment with broad spectrum antibiotics and monitoring for pneumoperitoneum. Generally, clinicians treat medical NEC with 7–14 days of bowel rest and broad-spectrum antibiotics that cover intestinal flora (mostly anaerobes). Length of treatment can be guided by clinical status. Prior to starting antibiotics, a sepsis evaluation should be performed with blood and urine cultures and baseline CBC with differential. Trending inflammatory markers such as CRP or IL-6 can be considered. In the setting of positive cultures, antibiotic therapy may be tailored further. Antibiotics which cover anaerobic bacteria may be considered. Culture results can further direct therapy (tailored if positive or narrowed if negative). Careful monitoring of hemodynamic status and respiratory status is important as patients with NEC may require escalation of care.
Management of Surgical NEC
The most well-accepted indication for surgery in NEC is pneumoperitoneum from intestinal perforation [89]. Other clinical and laboratory indicators include portal venous gas, abdominal wall erythema, hypotension, metabolic acidosis, hyponatremia, neutropenia, and thrombocytopenia, which have been associated with the need for surgical intervention [90], particularly if persistent. Failed medical treatment as an indication for surgery is associated with poor outcomes, suggesting that earlier surgical intervention may be beneficial in these cases [91].
The standard surgical approach is laparotomy for excision of necrotic bowel, but its challenges in critically ill infants prompt discussion about peritoneal drain placement. A recent systematic review concluded that neither RCTs nor observational studies with high quality of reporting showed a difference in mortality when comparing laparotomy versus peritoneal drainage (pooled OR 0.85, 95% CI 0.47–1.54 and pooled OR 0.67, 95% CI 0.37–1.19, respectively) [92]. In the Necrotizing Enterocolitis Surgery Trial (NEST), the authors concluded that with a preoperative diagnosis of NEC, initial laparotomy is more likely than peritoneal drainage to reduce death or NDI, with a Bayesian posterior probability of 97%. This suggests that laparotomy, rather than peritoneal drain, may reduce death or NDI in infants with surgical NEC [93••].
In summary, the surgical approach should be guided by the patient’s condition and be a discussion between the surgeon and neonatologist. Regardless of approach, the goals of surgical intervention include: 1) early intervention to minimize contamination and sepsis, 2) removal of necrotic bowel, and 3) preservation of bowel length to prevent short bowel syndrome [89].
On the Horizon
NEC is a complex, multifactorial disease which has been extensively studied since it was first described. NEC causes significant morbidity and mortality; therefore, continued research is needed to find new modalities for earlier diagnosis of NEC, new therapies to prevent NEC and better therapies for targeted treatment of NEC. Below we summarize exciting research that may change our management of preterm infants.
Pre-clinical Research
Epidermal Growth Factor (EGF) Signaling
Good et al. demonstrated that EGF signaling in amniotic fluid diminished NEC-like injury through inhibition of TLR4 [94].
Human Milk OligosacchaRides (HMOs)
HMOs are being studied for their ability to protect against intestinal inflammation in animal models of NEC [95, 96].
NEC-on-a Chip
Investigators have developed a 3-dimensional system to study the effect of therapies on human intestinal cells [97]. This microfluidic device allows investigators to manipulate the inflammatory environment to test novel NEC therapies.
Bile Acids
Golden et al. demonstrated that ursodeoxycholic acid reduces intestinal injury by promoting intestinal restitution through COX-2 and EGF-signaling pathways [98].
Mesenchymal Stem Cell Therapy
Markel et al. demonstrated that administration of mesenchymal stem cells to animals with NEC-like injury improved survival due to reduced intestinal injury and improved mesenteric perfusion [99].
Interleukin-22 (IL-22)
IL-22 is a cytokine that plays a critical role in maintaining the intestinal barrier, regenerating epithelial cells, and controlling intestinal inflammation. Using a mouse model of NEC, Mihi et al. revealed that IL-22 expression is minimal in neonatal mice [100•]. Additionally, the authors found that human and murine neonates lack IL-22 production during NEC [100•]. Treatment with recombinant IL-22 reduced intestinal inflammation and enhanced epithelial regeneration in their experimental model.
Translational Research
NEC Biorepository
A NEC biorepository in the US has been developed with the goal of improving our understanding of the molecular indicators of NEC through biological signatures and genetic predisposition of infants with NEC [101].
NEC Virome
Kaelin et al. longitudinally evaluated the gut virome of preterm infants who developed NEC versus gestational age matched infants who did not develop NEC [102•]. The authors identified reduced viral beta diversity ten days prior to NEC development. This was driven by specific viral signatures and viral-bacterial interactions suggesting that the early life gut virome may be implicated in NEC development.
Clinical Research
Remote ischemic Conditioning (RIC) for Prevention of NEC
A Phase II Feasibility RCT [103] evaluating the effect of RIC on NEC is ongoing. RIC is a therapy where brief cycles of non-lethal ischemia and reperfusion to a limb results in protection from ischemic damage in distant organs. In pre-clinical models of NEC, the investigators showed that RIC reduces intestinal injury [104].
Bowel/Abdominal Ultrasound (BUS/AUS) for Diagnosis of NEC
Currently, NEC is diagnosed by findings on abdominal radiographs. However, these findings have low sensitivity albeit high specificity for NEC diagnosis [105]. BUS is being investigated as a tool to diagnose or identify medical treatment failures of NEC earlier [106, 107]. One systematic review and meta-analysis of observational studies found that BUS findings such as focal fluid collections, complex ascites, pneumoperitoneum, bowel wall echogenicity, bowel wall thinning or thickening, absent perfusion and dilated bowel were all associated with surgery or death from NEC [108]. Whereas findings of portal venous gas or pneumatosis intestinalis were not associated with surgery or death from NEC [108]. Cuna et al. are performing a pilot RCT to establish the feasibility and pilot the design and delivery of a diagnostic RCT of BUS for NEC diagnosis [109]. There is also an ongoing phase 3 RCT evaluating the use of contrast enhanced ultrasound to evaluate bowel perfusion in infants with NEC [110] or suspected NEC.
GutCheckNEC
A diagnostic tool kit, created with a cohort of over 58,000 infants, provides a weighted composite risk of developing NEC [111]. This tool kit predicts surgical NEC with an area under the curve (AUC) of 0.84 (95% CI 0.82–0.85) and death from NEC with an AUC 0.83 (95% CI 0.81–0.85) [111]. The prediction of medical NEC was not as dependable with an AUC of 0.72 (95% CI 0.70–0.74).
NEC-Zero Project
This tool kit provides communication when deterioration is expected, limits antibiotic duration and promotes adherence to SFP [112].
Antibiotic Use in Preterm Infants
The NICU Antibiotics and Outcomes Trial (NANO) is an upcoming multi-center, double blinded RCT that will evaluate empiric antibiotics after birth or placebo in infants at < = 29 weeks gestation [113]. The primary outcome is composite incidence of NEC, late-onset sepsis, or death during NICU hospitalization.
Withholding Feeds or Continuing Feeds Around Transfusion
The WHEAT trial is an ongoing international multicenter RCT evaluating whether holding enteral feed around the time of a transfusion in babies born less than 30 weeks' gestation will reduce the risk of NEC (NCT05213806).
Conclusion
NEC is a multifactorial disease which requires a multifaceted approach to prevent its development in preterm infants. The most effective way to prevent NEC after preterm birth is to provide a HM diet [26, 27], ideally MOM. By promoting use of MOM, antibiotic stewardship, probiotics, standardized feeding protocols and avoiding acid-reducing medications, we can minimize microbial dysbiosis, a major factor in the development of NEC. Transfusion protocols to reduce severe prolonged anemia that may contribute to NEC risk should also be considered. Improving maternal health is of paramount importance to reduce rates of prematurity and NEC. While not much has changed in NEC treatment, promising research is being done which may lead us to earlier diagnostic and improved preventive and treatment strategies.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–40. https://doi.org/10.1056/NEJMoa1403489.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. https://doi.org/10.1542/peds.2009-2959.
Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368(9543):1271–83. https://doi.org/10.1016/S0140-6736(06)69525-1.
Jones IH, Hall NJ. Contemporary Outcomes for Infants with Necrotizing Enterocolitis-A Systematic Review. J Pediatr. 2020;220(86–92):e3. https://doi.org/10.1016/j.jpeds.2019.11.011.
Han SM, Hong CR, Knell J, Edwards EM, Morrow KA, Soll RF, et al. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: A multicenter cohort analysis. J Pediatr Surg. 2020;55(6):998–1001. https://doi.org/10.1016/j.jpedsurg.2020.02.046.
Bazacliu C, Neu J. Necrotizing Enterocolitis: Long Term Complications. Curr Pediatr Rev. 2019;15(2):115–24. https://doi.org/10.2174/1573396315666190312093119.
Adams-Chapman I. Necrotizing Enterocolitis and Neurodevelopmental Outcome. Clin Perinatol. 2018;45(3):453–66. https://doi.org/10.1016/j.clp.2018.05.014.
Shah TA, Meinzen-Derr J, Gratton T, Steichen J, Donovan EF, Yolton K, et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J Perinatol. 2012;32(7):552–8. https://doi.org/10.1038/jp.2011.176.
Snyder KB, Hunter CJ. Bugs and the barrier: A review of the gut microbiome and intestinal barrier in necrotizing enterocolitis. Semin Pediatr Surg. 2023;32(3):151310. https://doi.org/10.1016/j.sempedsurg.2023.151310.
Meek JY, Noble L, Section on B. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics. 2022;150(1). https://doi.org/10.1542/peds.2022-057988.
Van Gysel M, Cossey V, Fieuws S, Schuermans A. Impact of pasteurization on the antibacterial properties of human milk. Eur J Pediatr. 2012;171(8):1231–7. https://doi.org/10.1007/s00431-012-1750-4.
Shoji H, Shimizu T, Shinohara K, Oguchi S, Shiga S, Yamashiro Y. Suppressive effects of breast milk on oxidative DNA damage in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F136–8. https://doi.org/10.1136/adc.2002.018390.
Vizzari G, Morniroli D, Ceroni F, Verduci E, Consales A, Colombo L, et al. Human Milk, More Than Simple Nourishment. Children (Basel). 2021;8(10). https://doi.org/10.3390/children8100863.
Adamkin DH. Use of human milk and fortification in the NICU. J Perinatol. 2023;43(5):551–9. https://doi.org/10.1038/s41372-022-01532-0.
Miller J, Tonkin E, Damarell RA, McPhee AJ, Suganuma M, Suganuma H, et al. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients. 2018;10(6). https://doi.org/10.3390/nu10060707.
Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;7(7):CD002971. https://doi.org/10.1002/14651858.CD002971.pub5.
Meier P, Patel A, Esquerra-Zwiers A. Donor Human Milk Update: Evidence, Mechanisms, and Priorities for Research and Practice. J Pediatr. 2017;180:15–21. https://doi.org/10.1016/j.jpeds.2016.09.027.
Hair AB, Scottoline B, Good M. Dilemmas in human milk fortification. J Perinatol. 2023;43(1):103–7. https://doi.org/10.1038/s41372-022-01502-6.
Altobelli E, Angeletti PM, Verrotti A, Petrocelli R. The Impact of Human Milk on Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Nutrients. 2020;12(5). https://doi.org/10.3390/nu12051322.
Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;6(6):CD002971. https://doi.org/10.1002/14651858.CD002971.pub4.
de Halleux V, Pieltain C, Senterre T, Rigo J. Use of donor milk in the neonatal intensive care unit. Semin Fetal Neonatal Med. 2017;22(1):23–9. https://doi.org/10.1016/j.siny.2016.08.003.
Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163(6):1592-5 e1. https://doi.org/10.1016/j.jpeds.2013.07.011.
Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kiechl-Kohlendorfer U, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562-71 e1. https://doi.org/10.1016/j.jpeds.2009.10.040.
O’Connor DL, Kiss A, Tomlinson C, Bando N, Bayliss A, Campbell DM, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial. Am J Clin Nutr. 2018;108(1):108–16. https://doi.org/10.1093/ajcn/nqy067.
• Jensen GB, Ahlsson F, Domellof M, Elfvin A, Naver L, Abrahamsson T. Nordic study on human milk fortification in extremely preterm infants: a randomised controlled trial-the N-forte trial. BMJ Open. 2021;11(11):e053400. https://doi.org/10.1136/bmjopen-2021-053400. (This is a multicenter randomized controlled performed to assess the effects of a HM diet (with DHM fortifier) versus a HM diet with bovine milk fortifier on a compositive outcome of NEC (stage II or III), culture positive sepsis or death in extremely preterm infants. There were 229 patients enrolled in this study. There was no difference in composite outcomes or adverse events between the two groups.)
Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–23. https://doi.org/10.1016/0140-6736(90)93304-8.
Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol. 2009;29(1):57–62. https://doi.org/10.1038/jp.2008.117.
• Alshaikh BN, Sproat TDR, Wood C, Spence JM, Knauff M, Hamilton C, et al. A Quality Improvement Initiative to Reduce Necrotizing Enterocolitis in Very Preterm Infants.Pediatrics. 2023;152(6). https://doi.org/10.1542/peds.2023-061273. (This study is a quality improvement initiative aimed to increase provision of mother’s own milk, improve compliance with feeding protocols and implement feeding protocols for feeding during transfusions and with treatment of patent ductus arteriosus.)
Meier PP, Johnson TJ, Patel AL, Rossman B. Evidence-Based Methods That Promote Human Milk Feeding of Preterm Infants: An Expert Review. Clin Perinatol. 2017;44(1):1–22. https://doi.org/10.1016/j.clp.2016.11.005.
Parker LA, Sullivan S, Krueger C, Mueller M. Association of timing of initiation of breastmilk expression on milk volume and timing of lactogenesis stage II among mothers of very low-birth-weight infants. Breastfeed Med. 2015;10(2):84–91. https://doi.org/10.1089/bfm.2014.0089.
Gooding JS, Cooper LG, Blaine AI, Franck LS, Howse JL, Berns SD. Family support and family-centered care in the neonatal intensive care unit: origins, advances, impact. Semin Perinatol. 2011;35(1):20–8. https://doi.org/10.1053/j.semperi.2010.10.004.
Stefanescu BM, Gillam-Krakauer M, Stefanescu AR, Markham M, Kosinski JL. Very low birth weight infant care: adherence to a new nutrition protocol improves growth outcomes and reduces infectious risk. Early Hum Dev. 2016;94:25–30. https://doi.org/10.1016/j.earlhumdev.2016.01.011.
McCallie KR, Lee HC, Mayer O, Cohen RS, Hintz SR, Rhine WD. Improved outcomes with a standardized feeding protocol for very low birth weight infants. J Perinatol. 2011;31(Suppl 1):S61–7. https://doi.org/10.1038/jp.2010.185.
Patole SK, de Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta-analysis of observational studies. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F147–51. https://doi.org/10.1136/adc.2004.059741.
Barr PA, Mally PV, Caprio MC. Standardized Nutrition Protocol for Very Low-Birth-Weight Infants Resulted in Less Use of Parenteral Nutrition and Associated Complications, Better Growth, and Lower Rates of Necrotizing Enterocolitis. JPEN J Parenter Enteral Nutr. 2019;43(4):540–9. https://doi.org/10.1002/jpen.1453.
• Chitale R, Ferguson K, Talej M, Yang WC, He S, Edmond KM, et al. Early Enteral Feeding for Preterm or Low Birth Weight Infants: a Systematic Review and Meta-analysis. Pediatrics. 2022;150(Suppl 1). https://doi.org/10.1542/peds.2022-057092E. (This is a systematic review and meta-analysis of RCTs evaluating the effects of early enteral feeding (<72 hours) to delayed enteral feeding (>/= 72 hours) in preterm or low birthweight infants. 14 RCTs were analyzed, and the authors found that early initiation of feeds decreases the risk of mortality at discharge and 28 days with moderate certainty of evidence. Early initiation of feeds may not influence NEC, low certainty evidence.)
• Young L, Oddie SJ, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2022;1(1):CD001970. https://doi.org/10.1002/14651858.CD001970.pub6. ()
Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2003;111(3):529–34. https://doi.org/10.1542/peds.111.3.529.
Henderson G, Craig S, Brocklehurst P, McGuire W. Enteral feeding regimens and necrotising enterocolitis in preterm infants: a multicentre case-control study. Arch Dis Child Fetal Neonatal Ed. 2009;94(2):F120–3. https://doi.org/10.1136/adc.2007.119560.
Dorling J, Abbott J, Berrington J, Bosiak B, Bowler U, Boyle E, et al. Controlled Trial of Two Incremental Milk-Feeding Rates in Preterm Infants. N Engl J Med. 2019;381(15):1434–43. https://doi.org/10.1056/NEJMoa1816654.
• Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2021;8(8):CD001241. https://doi.org/10.1002/14651858.CD001241.pub8. ()
Viswanathan S, Merheb R, Wen X, Collin M, Groh-Wargo S. Standardized slow enteral feeding protocol reduces necrotizing enterocolitis in micropremies. J Neonatal Perinatal Med. 2017;10(2):171–80. https://doi.org/10.3233/NPM-171680.
Thoene M, Anderson-Berry A. Nutrition Support Practices for Infants Born <750 Grams or <25 Weeks Gestation: A Call for More Research. Int J Environ Res Public Health. 2022;19(17). https://doi.org/10.3390/ijerph191710957
Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000;182(1 Pt 1):198–206. https://doi.org/10.1016/s0002-9378(00)70513-8.
Robinson DT, Ehrenkranz RA. Parenteral nutrition-associated cholestasis in small for gestational age infants. J Pediatr. 2008;152(1):59–62. https://doi.org/10.1016/j.jpeds.2007.06.002.
Dorling J, Kempley S, Leaf A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F359–63. https://doi.org/10.1136/adc.2004.060350.
Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Linsell L, et al. Early or delayed enteral feeding for preterm growth-restricted infants: a randomized trial. Pediatrics. 2012;129(5):e1260–8. https://doi.org/10.1542/peds.2011-2379.
Barbian ME, Patel RM. Probiotics for prevention of necrotizing enterocolitis: Where do we stand? Semin Perinatol. 2023;47(1):151689. https://doi.org/10.1016/j.semperi.2022.151689.
Athalye-Jape G, Rao S, Patole S. Effects of probiotics on experimental necrotizing enterocolitis: a systematic review and meta-analysis. Pediatr Res. 2018;83(1–1):16–22. https://doi.org/10.1038/pr.2017.218.
Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX, McGuire W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev. 2020;10:CD005496. https://doi.org/10.1002/14651858.CD005496.pub5
Flannery DD, Puopolo KM. Neonatal Early-Onset Sepsis. NeoReviews. 2022;23(11):756–70. https://doi.org/10.1542/neo.23-10-e756.
Zwittink RD, Renes IB, van Lingen RA, van Zoeren-Grobben D, Konstanti P, Norbruis OF, et al. Association between duration of intravenous antibiotic administration and early-life microbiota development in late-preterm infants. Eur J Clin Microbiol Infect Dis. 2018;37(3):475–83. https://doi.org/10.1007/s10096-018-3193-y.
Kim CS, Grady N, Derrick M, Yu Y, Oliphant K, Lu J, et al. Effect of Antibiotic Use Within First 48 Hours of Life on the Preterm Infant Microbiome: A Randomized Clinical Trial. JAMA Pediatr. 2021;175(3):303–5. https://doi.org/10.1001/jamapediatrics.2020.4916.
Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3(8):944–54. https://doi.org/10.1038/ismej.2009.37.
Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. https://doi.org/10.1186/s40168-017-0248-8.
Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. https://doi.org/10.1542/peds.2007-3423.
Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–7. https://doi.org/10.1016/j.jpeds.2011.02.035.
Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–5. https://doi.org/10.1016/j.jpeds.2011.05.033.
Abdel Ghany EA, Ali AA. Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Ann Saudi Med. 2012;32(5):521–6. https://doi.org/10.5144/0256-4947.2012.521.
Esmaeilizand R, Shah PS, Seshia M, Yee W, Yoon EW, Dow K, et al. Antibiotic exposure and development of necrotizing enterocolitis in very preterm neonates. Paediatr Child Health. 2018;23(4):e56–61. https://doi.org/10.1093/pch/pxx169.
Zhu K, Gao H, Yuan L, Wang L, Deng F. Prolonged antibiotic therapy increased necrotizing enterocolitis in very low birth weight infants without culture-proven sepsis. Front Pediatr. 2022;10:949830. https://doi.org/10.3389/fped.2022.949830.
Chen WY, Lo YC, Huang PH, Chen YX, Tsao PC, Lee YS, et al. Increased antibiotic exposure in early life is associated with adverse outcomes in very low birth weight infants. J Chin Med Assoc. 2022;85(9):939–43. https://doi.org/10.1097/JCMA.0000000000000749.
Dierikx TH, Deianova N, Groen J, Vijlbrief DC, Hulzebos C, de Boode WP, et al. Association between duration of early empiric antibiotics and necrotizing enterocolitis and late-onset sepsis in preterm infants: a multicenter cohort study. Eur J Pediatr. 2022;181(10):3715–24. https://doi.org/10.1007/s00431-022-04579-5.
Ting JY, Roberts A, Sherlock R, Ojah C, Cieslak Z, Dunn M, et al. Duration of Initial Empirical Antibiotic Therapy and Outcomes in Very Low Birth Weight Infants. Pediatrics. 2019;143(3). https://doi.org/10.1542/peds.2018-2286
Rowley MP, Dahlenburg GW. Gentamicin in prophylaxis of neonatal necrotising enterocolitis. Lancet. 1978;2(8088):532. https://doi.org/10.1016/s0140-6736(78)92265-1.
Grylack LJ, Scanlon JW. Oral gentamicin therapy in the prevention of neonatal necrotizing enterocolitis. A controlled double-blind trial. Am J Dis Child. 1978;132(12):1192–4. https://doi.org/10.1001/archpedi.1978.02120370040010.
Egan EA, Mantilla G, Nelson RM, Eitzman DV. A prospective controlled trial of oral kanamycin in the prevention of neonatal necrotizing enterocolitis. J Pediatr. 1976;89(3):467–70. https://doi.org/10.1016/s0022-3476(76)80553-7.
Boyle R, Nelson JS, Stonestreet BS, Peter G, Oh W. Alterations in stool flora resulting from oral kanamycin prophylaxis of necrotizing enterocolitis. J Pediatr. 1978;93(5):857–61. https://doi.org/10.1016/s0022-3476(78)81101-9.
Siu YK, Ng PC, Fung SC, Lee CH, Wong MY, Fok TF, et al. Double blind, randomised, placebo controlled study of oral vancomycin in prevention of necrotising enterocolitis in preterm, very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1998;79(2):F105–9. https://doi.org/10.1136/fn.79.2.f105.
Fast C, Rosegger H. Necrotizing enterocolitis prophylaxis: oral antibiotics and lyophilized enterobacteria vs oral immunoglobulins. Acta Paediatr Suppl. 1994;396:86–90. https://doi.org/10.1111/j.1651-2227.1994.tb13253.x.
Rina P, Zeng Y, Ying J, Qu Y, Mu D. Association of initial empirical antibiotic therapy with increased risk of necrotizing enterocolitis. Eur J Pediatr. 2020;179(7):1047–56. https://doi.org/10.1007/s00431-020-03679-4.
• Klerk DH, van Avezaath LK, Loeffen EAH, Hulscher JBF, Kooi EMW. Fetal-neonatal exposure to antibiotics and NEC development: A systematic review and meta-analysis. Front Pediatr. 2022;10:1102884. https://doi.org/10.3389/fped.2022.1102884. (This is a systematic review and meta-analysis which analyzed how prenatal maternal antibiotic administration and neonatal prolonged antibiotic exposure may contribute to development of NEC. Analysis from 12 cohort studies and 10 observational cohort studies showed that prolonged empiric antibiotics versus empirical antibiotics (< 48 hours) were associated with increased incidence of NEC. Three cohort studies evaluating prenatal antibiotics versus no prenatal antibiotics found a decreased incidence of NEC with prenatal maternal antibiotic administration.)
Fan X, Zhang L, Tang J, Chen C, Chen J, Qu Y, et al. The initial prophylactic antibiotic usage and subsequent necrotizing enterocolitis in high-risk premature infants: a systematic review and meta-analysis. Pediatr Surg Int. 2018;34(1):35–45. https://doi.org/10.1007/s00383-017-4207-z.
More K, Athalye-Jape G, Rao S, Patole S. Association of inhibitors of gastric acid secretion and higher incidence of necrotizing enterocolitis in preterm very low-birth-weight infants. Am J Perinatol. 2013;30(10):849–56. https://doi.org/10.1055/s-0033-1333671.
Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Semin Fetal Neonatal Med. 2018;23(6):374–9. https://doi.org/10.1016/j.siny.2018.07.005.
Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev. 2017;11(11):CD004863. https://doi.org/10.1002/14651858.CD004863.pub5.
MohanKumar K, Namachivayam K, Song T, Jake Cha B, Slate A, Hendrickson JE, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun. 2019;10(1):3494. https://doi.org/10.1038/s41467-019-11199-5.
Mally P, Golombek SG, Mishra R, Nigam S, Mohandas K, Depalhma H, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am J Perinatol. 2006;23(8):451–8. https://doi.org/10.1055/s-2006-951300.
Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129(3):529–40. https://doi.org/10.1542/peds.2011-2872.
Sharma R, Kraemer DF, Torrazza RM, Mai V, Neu J, Shuster JJ, et al. Packed red blood cell transfusion is not associated with increased risk of necrotizing enterocolitis in premature infants. J Perinatol. 2014;34(11):858–62. https://doi.org/10.1038/jp.2014.59.
Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301–7. https://doi.org/10.1016/j.jpeds.2006.05.011.
Whyte RK, Kirpalani H, Asztalos EV, Andersen C, Blajchman M, Heddle N, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123(1):207–13. https://doi.org/10.1542/peds.2008-0338.
Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al. Higher or Lower Hemoglobin Transfusion Thresholds for Preterm Infants. N Engl J Med. 2020;383(27):2639–51. https://doi.org/10.1056/NEJMoa2020248.
Franz AR, Engel C, Bassler D, Rudiger M, Thome UH, Maier RF, et al. Effects of Liberal vs Restrictive Transfusion Thresholds on Survival and Neurocognitive Outcomes in Extremely Low-Birth-Weight Infants: The ETTNO Randomized Clinical Trial. JAMA. 2020;324(6):560–70. https://doi.org/10.1001/jama.2020.10690.
Hay S, Zupancic JA, Flannery DD, Kirpalani H, Dukhovny D. Should we believe in transfusion-associated enterocolitis? Applying a GRADE to the literature. Semin Perinatol. 2017;41(1):80–91. https://doi.org/10.1053/j.semperi.2016.09.021.
Wang P, Wang X, Deng H, Li L, Chong W, Hai Y, et al. Restrictive versus liberal transfusion thresholds in very low birth weight infants: A systematic review with meta-analysis. PLoS ONE. 2021;16(8):e0256810. https://doi.org/10.1371/journal.pone.0256810.
• Bernabe-Garcia M, Calder PC, Villegas-Silva R, Rodriguez-Cruz M, Chavez-Sanchez L, Cruz-Reynoso L, et al. Efficacy of Docosahexaenoic Acid for the Prevention of Necrotizing Enterocolitis in Preterm Infants: A Randomized Clinical Trial. Nutrients. 2021;13(2). https://doi.org/10.3390/nu13020648. (This is a randomized double-blind parallel-group (1:1) trial assessing efficacy of DHA for prevention of NEC. A total of 225 infants were recruited and received either enteral DHA or sunflower oil (control) daily for 14 days once enteral feeds were initiated. The authors identified lower NEC among patients who received DHA with zero cases of NEC versus 7 cases of NEC in the control group.)
Newnham JP, Dickinson JE, Hart RJ, Pennell CE, Arrese CA, Keelan JA. Strategies to prevent preterm birth. Front Immunol. 2014;5:584. https://doi.org/10.3389/fimmu.2014.00584.
Thakkar HS, Lakhoo K. The surgical management of necrotising enterocolitis (NEC). Early Hum Dev. 2016;97:25–8. https://doi.org/10.1016/j.earlhumdev.2016.03.002.
Munaco AJ, Veenstra MA, Brownie E, Danielson LA, Nagappala KB, Klein MD. Timing of optimal surgical intervention for neonates with necrotizing enterocolitis. Am Surg. 2015;81(5):438–43.
Bethell GS, Knight M, Hall NJ. BAPS-CASS B-CNIGobo Surgical necrotizing enterocolitis Association between surgical indication, timing, and outcomes. J Pediatr Surg. 2021;56(10):1785–90. https://doi.org/10.1016/j.jpedsurg.2021.04.028.
van Heesewijk AE, Rush ML, Schmidt B, Kirpalani H, DeMauro SB. Agreement between study designs: a systematic review comparing observational studies and randomized trials of surgical treatments for necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2020;33(12):1965–73. https://doi.org/10.1080/14767058.2018.1533948.
•• Blakely ML, Tyson JE, Lally KP, Hintz SR, Eggleston B, Stevenson DK, et al. Initial Laparotomy Versus Peritoneal Drainage in Extremely Low Birthweight Infants With Surgical Necrotizing Enterocolitis or Isolated Intestinal Perforation: A Multicenter Randomized Clinical Trial. Ann Surg. 2021;274(4):e370–80. https://doi.org/10.1097/SLA.0000000000005099. (This is a randomized trial performed in 20 US centers comparing initial laparotomy versus peritoneal drainage in preterm infants with NEC or spontaneous intestinal perforation. The primary outcome was death or neurodevelopment impairment at 18-22 months corrected age. A total of 310 infants were randomized. Among all randomized patients, there was no difference in death or NDI between the two groups. However, with a preoperative diagnosis of NEC, death or NDI was higher in the drainage group than the laparotomy group.)
Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci U S A. 2012;109(28):11330–5. https://doi.org/10.1073/pnas.1200856109.
Wu RY, Li B, Koike Y, Maattanen P, Miyake H, Cadete M, et al. Human Milk Oligosaccharides Increase Mucin Expression in Experimental Necrotizing Enterocolitis. Mol Nutr Food Res. 2019;63(3):e1800658. https://doi.org/10.1002/mnfr.201800658.
Rasmussen SO, Martin L, Ostergaard MV, Rudloff S, Roggenbuck M, Nguyen DN, et al. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J Nutr Biochem. 2017;40:141–54. https://doi.org/10.1016/j.jnutbio.2016.10.011.
Bein A, Shin W, Jalili-Firoozinezhad S, Park MH, Sontheimer-Phelps A, Tovaglieri A, et al. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell Mol Gastroenterol Hepatol. 2018;5(4):659–68. https://doi.org/10.1016/j.jcmgh.2017.12.010.
Golden JM, Escobar OH, Nguyen MVL, Mallicote MU, Kavarian P, Frey MR, et al. Ursodeoxycholic acid protects against intestinal barrier breakdown by promoting enterocyte migration via EGFR- and COX-2-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol. 2018;315(2):G259–71. https://doi.org/10.1152/ajpgi.00354.2017.
Markel TA, Crafts TD, Jensen AR, Hunsberger EB, Yoder MC. Human mesenchymal stromal cells decrease mortality after intestinal ischemia and reperfusion injury. J Surg Res. 2015;199(1):56–66. https://doi.org/10.1016/j.jss.2015.06.060.
• Mihi B, Gong Q, Nolan LS, Gale SE, Goree M, Hu E, et al. Interleukin-22 signaling attenuates necrotizing enterocolitis by promoting epithelial cell regeneration. Cell Rep Med. 2021;2(6):100320. https://doi.org/10.1016/j.xcrm.2021.100320. (The authors of this study evaluated the role of IL-22 in NEC and found that human and murine neonates lack IL-22 production during NEC. Treatment with recombinant IL-22 reduced intestinal inflammation and enhanced epithelial regeneration in their experimental model.)
Ralls MW, Gadepalli SK, Sylvester KG, Good M. Development of the necrotizing enterocolitis society registry and biorepository. Semin Pediatr Surg. 2018;27(1):25–8. https://doi.org/10.1053/j.sempedsurg.2017.11.005.
• Kaelin EA, Rodriguez C, Hall-Moore C, Hoffmann JA, Linneman LA, Ndao IM, et al. Longitudinal gut virome analysis identifies specific viral signatures that precede necrotizing enterocolitis onset in preterm infants. Nat Microbiol. 2022;7(5):653–62. https://doi.org/10.1038/s41564-022-01096-x. (This is a prospective translational study using meta genomic sequencing to characterize the DNA gut virile of preterm infants who developed NEC compared to age-matched controls. The authors identified viral and bacterial signatures in the gut that preceded NEC onset. They found reduced viral beta diversity up to 10 days before NEC onset. This pattern was driven by specific viral signatures and viral-bacterial interactions. Thus, neonatal gut virome may play a role in NEC pathogenesis.)
Ganji N, Li B, Ahmad I, Daneman A, Deshpande P, Dhar V, et al. Remote ischemic conditioning in necrotizing enterocolitis: study protocol of a multi-center phase II feasibility randomized controlled trial. Pediatr Surg Int. 2022;38(5):679–94. https://doi.org/10.1007/s00383-022-05095-1.
Koike Y, Li B, Ganji N, Zhu H, Miyake H, Chen Y, et al. Remote ischemic conditioning counteracts the intestinal damage of necrotizing enterocolitis by improving intestinal microcirculation. Nat Commun. 2020;11(1):4950. https://doi.org/10.1038/s41467-020-18750-9.
Tam AL, Camberos A, Applebaum H. Surgical decision making in necrotizing enterocolitis and focal intestinal perforation: predictive value of radiologic findings. J Pediatr Surg. 2002;37(12):1688–91. https://doi.org/10.1053/jpsu.2002.36696.
Alexander KM, Chan SS, Opfer E, Cuna A, Fraser JD, Sharif S, et al. Implementation of bowel ultrasound practice for the diagnosis and management of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):96–103. https://doi.org/10.1136/archdischild-2019-318382.
Janssen Lok M, Miyake H, Hock A, Daneman A, Pierro A, Offringa M. Value of abdominal ultrasound in management of necrotizing enterocolitis: a systematic review and meta-analysis. Pediatr Surg Int. 2018;34(6):589–612. https://doi.org/10.1007/s00383-018-4259-8.
Cuna AC, Reddy N, Robinson AL, Chan SS. Bowel ultrasound for predicting surgical management of necrotizing enterocolitis: a systematic review and meta-analysis. Pediatr Radiol. 2018;48(5):658–66. https://doi.org/10.1007/s00247-017-4056-x.
Cuna A, Chan S, Jones J, Sien M, Robinson A, Rao K, et al. Feasibility and acceptability of a diagnostic randomized clinical trial of bowel ultrasound in infants with suspected necrotizing enterocolitis. Eur J Pediatr. 2022;181(8):3211–5. https://doi.org/10.1007/s00431-022-04526-4.
Al-Hamad S, Hackam DJ, Goldstein SD, Huisman T, Darge K, Hwang M. Contrast-Enhanced Ultrasound and Near-Infrared Spectroscopy of the Neonatal Bowel: Novel, Bedside, Noninvasive, and Radiation-Free Imaging for Early Detection of Necrotizing Enterocolitis. Am J Perinatol. 2018;35(14):1358–65. https://doi.org/10.1055/s-0038-1655768.
Gephart SM, Spitzer AR, Effken JA, Dodd E, Halpern M, McGrath JM. Discrimination of GutCheck(NEC): a clinical risk index for necrotizing enterocolitis. J Perinatol. 2014;34(6):468–75. https://doi.org/10.1038/jp.2014.37.
Gephart SM, Hanson C, Wetzel CM, Fleiner M, Umberger E, Martin L, et al. NEC-zero recommendations from scoping review of evidence to prevent and foster timely recognition of necrotizing enterocolitis. Matern Health Neonatol Perinatol. 2017;3:23. https://doi.org/10.1186/s40748-017-0062-0.
Morowitz MJ, Katheria AC, Polin RA, Pace E, Huang DT, Chang CH, et al. The NICU Antibiotics and Outcomes (NANO) trial: a randomized multicenter clinical trial assessing empiric antibiotics and clinical outcomes in newborn preterm infants. Trials. 2022;23(1):428. https://doi.org/10.1186/s13063-022-06352-3.
Author information
Authors and Affiliations
Contributions
All authors contributed to the main text. Dr. Colarelli wrote most of the first draft. Dr. Barbian created the Figure. Dr. Denning provided the final review and edits. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Colarelli, A.M., MD, Barbian, M.E. et al. Prevention Strategies and Management of Necrotizing Enterocolitis. Curr Treat Options Peds (2024). https://doi.org/10.1007/s40746-024-00297-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s40746-024-00297-2