Abstract
Purpose of review
Cardiac output (CO) is a fundamental physiological parameter that measures the volume of blood that is pumped by the heart per unit of time, and helps define how oxygen is delivered to the tissues of the human body. In this paper, we discuss current methods of continuous CO monitoring while defining low CO syndrome (LCOS) and how analytical tools may help improve CO management in the subpopulation of patients with congenital heart disease (CHD).
Recent findings
Non-invasive methods of measuring CO have become increasingly available in recent years. Advantages of non-invasive over invasive techniques include decreased risk of procedural complications, decreased exposure to sedative and/or anesthetic agents, and increased patient comfort. Pediatric patient populations are particularly sensitive to the risks and complications of invasive techniques given the relative size of current technologies to pediatric vascular and cardiac dimensions.
Summary
Novel device technologies, combined with emerging analytical techniques, may help improve measurement of CO in children and those with CHD, and allow earlier detection of LCOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac output (CO) is a fundamental physiological parameter that measures the volume of blood that is pumped by the heart per unit of time. It is an essential component of the cardiovascular system, as it ensures sufficient oxygen and nutrient delivery to the body’s organs and tissues (Fig. 1). CO is typically measured in liters per minute (L/min) and is calculated by multiplying the heart rate (HR), measured in beats per minute (bpm), by the stroke volume (SV), measured in milliliters (mL) or liters (L), of blood that is ejected from the systemic ventricle during each heartbeat. The systemic ventricle is most commonly the morphologic left ventricle; however, in some severe forms of congenital heart disease (CHD), the right ventricle may serve as the systemic ventricle due to an absent or hypoplastic left ventricle. In pediatrics, cardiac index (CI) is often used instead of CO, as this can correct for anthropometric changes as a child grows. CI is calculated by dividing CO by the body surface area (BSA) and is measured in liters per minute per square meter (L/min/m2). A normal CI is generally considered to be 3–4 L/min/m2.
Physiology of cardiac output. Decreased cardiac output (CO) can cause decreased oxygen delivery and tissue perfusion. These in turn can lead to anaerobic metabolism and lactic acidosis, end-organ injury including acute kidney injury (AKI), and the need for vasopressors for patient management. Ultimately, prolonged low cardiac output can lead to other morbidities and mortality. Non-invasive continuous cardiac output monitoring (NICCOM) technologies may help monitor this process at the most upstream end, because current methods of bedside monitoring use more downstream metrics, especially in children, including lactic acidosis (low cardiac output syndrome score, LCOSS) and vasopressor dosing (vasopressor-inotrope score, VIS).
Non-invasive methods of measuring CO/CI have become increasingly available in recent years, as they offer several advantages over invasive techniques, such as decreased risk of procedural complications, decreased exposure to sedative and anesthetic agents, and increased patient comfort. In this paper, we discuss current methods of continuous CO/CI monitoring, low CO syndrome (LCOS), and how analytical tools may help improve CO/CI management in congenital heart disease (CHD).
Cardiac output monitoring: current solutions
The ideal method of tracking changes in CO/CI would be non-invasive, continuous, accurate, actionable, and specifically designed for children and those with CHD. Existing CI/CO measurement methods have significant limitations in the CHD population, some of which are due to the unique physiology of those with CHD. For instance, patients with CHD span the age and size spectrum, meaning that device size is important. Many patients with CHD have intracardiac or extracardiac shunting (blood flowing outside the normal series circuit), meaning that some of the assumptions about thermodilution and/or dye methods are violated. For patients with either disease-based or surgical aortopulmonary shunt placement, and especially those with a single ventricle, the ventricle pumps to both the systemic and pulmonary vascular beds at once, so CO/CI may refer to systemic flow, pulmonary flow, or both. Aortopulmonary shunting also means that these patients are highly sensitive to changes in vascular resistance in the pulmonary and systemic vascular beds, which can lead to significant, rapid changes in CO/CI.
Currently used clinical metrics
The standard methods used most often in the post-operative period include calculating CO/CI using the Fick principle, which involves mixed venous oxygen saturation and arterial saturation, and assuming an oxygen consumption rate. Another surrogate method is the vasopressor-inotrope score (VIS), which measures the type and dose of vasoactive and inotropic medications a patient needs to maintain adequate blood pressure and organ perfusion, though this really measures physician response to low CO/CI [1]. Additionally, the low cardiac output syndrome score (LCOSS) utilizes multiple parameters like heart rate and urine output for score calculation, but does not directly measure CO/CI (Fig. 2) [2••]. Cardiovascular magnetic resonance (CMR) imaging is the gold standard for CO measurement in children and those with CHD with high accuracy and excellent reproducibility [3], but is not feasible for continuous CO monitoring due to technical limitations, including needing to be performed in an MRI magnet, often with sedation and/or anesthesia, and cost.
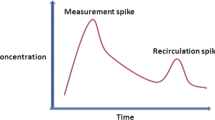
Theoretical low cardiac output syndrome (LCOS) timeline. After surgery for congenital heart disease, patients can develop low cardiac output syndrome (LCOS). Often, this manifests some hours after getting out of the operating room. Measured cardiac output and oxygen delivery decrease, and then lagging clinical indicators change, including low cardiac output syndrome score (LCOSS), lactate, and creatinine. An ideal monitoring system would provide data more continuously and earlier in the process of the development of LCOS.
Other tools are often used for adults. For instance, thermodilution-based pulmonary artery catheterization is relatively accurate, but is not commonly used in children due to the large size of catheters, the risk of vascular and arrhythmia complications, and inaccuracies in patients with shunts [4]. Transesophageal Doppler echocardiography is less invasive, but cannot often be used continuously, is angle-dependent and therefore less accurate, can be bulky, and requires a trained professional for interpretation [5•].
Wearable, non-invasive tools for CO/CI measurement
The development and use of wearable, non-invasive tools to monitor CO/CI has the potential to dramatically improve patient outcomes by enabling earlier diagnosis and treatment of cardiac diseases through less invasive and risky methods (Table 1). Wearable technology may allow for truly non-invasive, continuous cardiac output monitoring (NICCOM), both in hospital and ambulatory settings, providing clinicians with a more comprehensive view of a patient’s health status. The progress in semiconductor technology, embedded computing, machine learning, and connectivity has made it possible to integrate more features while consuming less power in small-sized circuits. These advances have led to wearable devices becoming increasingly reliable and accurate, to the extent that they may now be comparable to clinical devices. Wearable devices can also now incorporate multiple non-invasive techniques to measure biometric parameters, allowing for a comprehensive evaluation of human health through sophisticated data analysis. Lastly, the continuous advancement in manufacturing techniques and the development of novel materials for clinical use have created a distinctive prospect for wearables to offer comfortable, uninterrupted, and inconspicuous health monitoring. Though promising, much work remains to be done to bring these technologies to fruition.
Previous work by Wang et al. [11] has examined using photoplethysmography (PPG) to derive a metric that is closely correlated to CO/CI. PPG sensors are found in many commercial wearable devices, like smartwatches and smart rings, and is fundamentally a measure of blood volume pulse at the periphery (e.g., at a fingertip, toe, earlobe, or wrist). Thus, this approach can be easily deployed on a large scale. However, since this approach utilizes PPG waveform analysis, it is heavily affected by any kind of noise that might distort the waveform including motion noise, ambient light changes, skin tone variability, and sensor position. Moreover, the PPG waveform includes characteristics that reflect not only CO/CI but also other factors such as arterial compliance and perfusion index, a measure of peripheral perfusion [15]. A recent study used a combined PPG and finger cuff and showed reasonable correlation to upper arm arterial pressure in adults in the intensive care unit setting [16]. Seismocardiogram (SCG) signals have become popular due to improvements in micro-electromechanical systems (MEMS) technology that enables fabrication of small, high-resolution, low-noise accelerometers. SCG waveforms, a measure of the vibrations of the chest wall in response to the heartbeat, have been shown to be correlated to CO changes in healthy individuals after exercise [17]. A chest worn device incorporating dual-mode electrocardiography (ECG) and SCG has been used to assess CO in patients with CHD [6••]. Recent work on developing ultra-thin (<200 μm) thick, soft, chest-conformable non-invasive e-tattoos has the potential to improve the wearability and signal quality of mobile CO/CI sensors for children [18, 19]. Some of these technologies are capable of synchronous ECG and SCG recordings. As with PPG signals, SCG measurements contain information that is both pertinent to CO/CI but also relating to other factors such as valve timings and filing characteristics of the heart. Recently, wearable ultrasound patches have been designed, which can perform B-mode echocardiography on human subjects [20]. While the ultrasound beam forming and data processing require a wired device connection, this work represents a significant advancement in achieving comprehensive cardiac monitoring through wearable devices.
Other current solutions estimate CO/CI from models that use demographic information combined with either the impedance cardiogram or the finger arterial pressure waveform, obtained through bioimpedance and the vascular unloading technique, respectively [5, 21]. However, these approaches are obtrusive, require strict placement of multiple electrodes or cuff sizes, have diminished accuracy in critically ill patients, and are rarely tested in children, so their practicality remains in doubt when used for monitoring CO/CI in patients with CHD [21]. Quain et al. [22] evaluated a thoracic impedance tool and found poor correlation with right heart catheter Fick CO/CI calculations in adults with Fontan palliation. CO/CI estimated by PRAM (Pressure Recording Analytical Method) after pediatric cardiac surgery was found to be reliably associated with clinical indicators of tissue perfusion, vasoactive and diuretic drug requirements, and predicted longer mechanical ventilation duration [23]. A study of pediatric CICU patients found that estimations of normal CO/CI via Pulse Index Contour Cardiac Output (PiCCO) were associated with shorter duration of mechanical ventilation and length of stay [24].
As shown, there are physiologic and technical limitations to many of the current methods for NICCOM in CHD. Improving these technologies to make them more accurate, accessible, and deployable in hospital and outpatient clinical settings remains a critical need in the field.
Low cardiac output syndrome
Low cardiac output syndrome (LCOS) physiology
The clinical significance of CO and CI lies in their ability to reflect the adequacy of tissue perfusion. Oxygen delivery to the tissues is calculated by multiplying the CO/CI by the oxygen content of the blood. Thus, low CO/CI can result in inadequate oxygen delivery to the tissues, leading to a state of tissue hypoxia. Tissue hypoxia leads to increased anaerobic metabolism, resulting in the production of lactic acid and metabolic acidosis. In severe and persistent cases, impaired oxygen delivery can lead to end-organ damage, cardiac arrest, and death (Fig. 1).
To describe this state of impaired oxygen delivery due to low CO/CI, the term Low Cardiac Output Syndrome (LCOS) has been used and best describes the altered ratio of oxygen delivery to consumption. LCOS is a common problem in patients with CHD following cardiac surgery, as their cardiac function is often compromised due to the structural defects in their heart and negative effects of cardiopulmonary bypass. Accurate and timely measurement of CO/CI is crucial in the management of these patients in the hospital and outpatient settings, as it helps clinicians to detect early signs of hemodynamic instability and to guide therapeutic interventions aimed at improving tissue perfusion.
Definitions of LCOS
Despite its clinical significance, there is no universally accepted definition of LCOS in the literature or in clinical practice. Various definitions of LCOS have been proposed in the literature, each with its own set of criteria. Wernovsky et al. [25••] defined LCOS as having a CI of <2 L/min/m2, as determined by thermodilution catheter use. Other definitions focus on the clinical picture: Hoffman et al. [26] described LCOS as a clinical syndrome with manifestations such as tachycardia, oliguria, cold extremities, and/or cardiac arrest, with or without a ≥30% difference in arterial-mixed venous oxygen saturation or metabolic acidosis (an increase in the base deficit of >4 or an increase in the lactate of >2 mg/dL) on 2 successive blood gas measurements. Other commonly used definitions use a combination of clinical findings, care team interventions, and lab values: Gaies et al. [27] used the Pediatric Cardiac Critical Care Consortium (PC4) definition of LCOS, which includes vasoactive-inotropic score (VIS) > 15; VIS tripled in 48 h to 10+; arterio-venous oxygen difference (AVO2 difference) > 40% with hemoglobin > 8; and LCOS described in the physician note. There are other varied examples in the literature as well [1, 2, 28,29,30,31,32,33].
Although there is no consensus on a single definition of LCOS, the criteria used in various definitions share some common features, such as signs and symptoms of inadequate tissue perfusion, metabolic acidosis, and low CO/CI. These criteria emphasize the importance of timely detection and management of LCOS, as it can lead to severe morbidity and mortality in patients with CHD, but also the challenge that a lack of objective measures poses in clinical management.
Incidence and sequelae of LCOS
LCOS is a common problem in the post-operative period for patients with CHD. The incidence of LCOS varies widely across studies. For example, Parr et al. [34] and Wernovsky et al. [25] both reported a cardiac index of <2 L/min/m2 in 24% of patients. Butts et al. [30] found that 42% of neonates undergoing CHD surgery developed LCOS, while Gaies et al. [27] reported that 71% of patients had LCOS at some point in the post-operative period, with 38% having LCOS as the initial complication. Despite the widely varying incidence, LCOS is the leading cause of morbidity and death in the post-operative period for patients with CHD. Parr et al. [34] reported that 12% of post-surgical patients died of LCOS, representing 59% of post-operative deaths, and those with lower calculated cardiac index had higher mortality rates. Ma et al. [35] reported that LCOS was the cause of death in 52% of the last 100 patients who died, making it the leading cause of mortality. Gaies et al. [27] reported that systemic circulatory failure (51%) was the leading cause of death, with inadequate pulmonary blood flow (13%) and cardiac arrest (12%) being the next most common causes of death (which could also be related to LCOS). The development of LCOS is also associated with acute kidney injury, acute liver injury, prolonged hospital length of stay, and the need for mechanical circulatory support (such as extracorporeal membrane oxygenation, ECMO).
Although management strategies that mitigate the severity and duration of LCOS have not been clearly elucidated in the literature [29, 36], objective measurement of CO/CI may help bedside providers recognize and respond to LCOS early, which can lead to improved patient outcomes.
Mathematical modeling and machine learning in LCOS in CHD
Mathematical modeling is a valuable approach employed in scientific research to gain insights, make predictions, and address complex problems. In this section, we present a concise overview of the modeling process, condensed into five essential steps (Fig. 3). These steps encompass the entire modeling process, starting with problem identification, where researchers define the specific problem they aim to solve. Next, variables representing the system are selected, and assumptions are made to simplify the model. Mathematical equations are then formulated to describe the interactions between these variables. Parameter estimation follows, allowing researchers to determine the values of model parameters based on available data or literature. Model validation is crucial to assess the accuracy and reliability of the model’s predictions by comparing them to independent data or experimental observations. Refinement of the model involves iterative adjustments based on feedback from validation and analysis. The model is then analyzed to gain insights into its behavior and properties. Once validated and refined, the model can be utilized for various purposes, such as prediction, optimization, or decision-making. Model verification ensures the correct implementation of the model, while effective communication of the model’s results, findings, and limitations to stakeholders is crucial for understanding and application. Through this comprehensive modeling process, researchers can leverage the power of mathematical modeling to tackle complex scientific challenges effectively.
Summarized steps of mathematical modeling process. Caption: The steps involved in the modeling process, highlighting problem identification, assumptions and variable selection, equation formulation, parameter estimation, model validation, refinement, analysis, verification, implementation, and effective communication. These steps enable researchers to leverage mathematical modeling to tackle complex scientific challenges
As described above, another potential tool for estimating CO/CI is mechanistic mathematical modeling, which leverages advanced computational techniques to simulate the complex cardiovascular system. By incorporating physiological data and mathematical equations describing the balance between tissue oxygen supply and tissue oxygen demand, these models may predict cardiac output and other important clinical parameters such as urine output [37]. This approach can be particularly beneficial for individualized patient care, as it allows for the consideration of unique patient characteristics and the effects of various interventions. Mathematical models can also facilitate a deeper understanding of the underlying mechanisms driving cardiac function, supporting the development of more targeted therapies and treatment strategies. Mechanistic mathematical models can provide fundamental insight into pathophysiology, potentially leading to the discovery of new therapeutic or prophylactic clinical management strategies. Also, the increasing rate of development of computational technology allows for more intricate and faster simulations in real time. However, the scale of the model is critical for its clinical applicability. Larger, more complex models can be challenging for clinicians to understand and use at the bedside.
To address this challenge, machine learning techniques, such as neural networks, have been used to process large amounts of data and produce good predictive results. However, neural networks are often considered a black box, providing little insight into pathophysiology and thus hindering their acceptance among clinicians. A new approach is to combine mechanistic mathematical modeling and neural networks, known as physically and biologically informed neural networks (PINNs and BINNs, respectively) [38]. This hybrid approach has the potential to combine the understandability and transparency of mechanistic mathematical models with the power of neural networks to discover unknown variables and parameter estimation, making them a promising tool for clinical applications. Both mechanistic mathematical modeling and BINNs have the potential to be useful tools in understanding LCOS pathophysiology and management of patients with LCOS due to ever increasing computational capacity and the further application of these methods in data science.
Conclusion
Among critically ill children and adults with CHD, maintaining CO/CI is fundamental to ensuring sufficient oxygen delivery to tissues, as evidenced by the potentially devastating consequences of LCOS. Methods exist to measure CO/CI, but there is a critical need in the field to make the technologies non-invasive, more accurate, and useful and to test them in children and those with CHD. Mathematical modeling provides another approach to using physiologic data to detect LCOS. We believe that multi-disciplinary teams of physicians, biomedical engineers, and mathematicians can solve the need for improved CO/CI monitoring through collaborative efforts.
Data availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, Donohue JE, Yu S, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med. 2014;15(6):529–37. https://doi.org/10.1097/PCC.0000000000000153
Ulate KP, Yanay O, Jeffries H, Baden H, Di Gennaro JL, Zimmerman J. An elevated low cardiac output syndrome score is associated with morbidity in infants after congenital heart surgery. Pediatr Crit Care Med. 2017;18(1):26–33. https://doi.org/10.1097/PCC.0000000000000979. This study shows that a low cardiac output syndrome scoring system is associated with morbidity after surgery for congenital heart disease.
Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90(1):29–34. https://doi.org/10.1016/s0002-9149(02)02381-0
Connors AF Jr, Speroff T, Dawson NV, Thomas C, Harrell FE Jr, Wagner D, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276(11):889–97. https://doi.org/10.1001/jama.276.11.889
Kerstens MK, Wijnberge M, Geerts BF, Vlaar AP, Veelo DP. Non-invasive cardiac output monitoring techniques in the ICU. Neth. J Crit Care. 2018;26(3):104–10. Excellent review of non-invasive continuous cardiac output monitoring (NICCOM) techniques.
Ganti VG, Gazi AH, An S, Srivatsa AV, Nevius BN, Nichols CJ, et al. Wearable seismocardiography-based assessment of stroke volume in congenital heart disease. J Am Heart Assoc. 2022:e026067. https://doi.org/10.1161/jaha.122.026067. A study showing a wearable biosensor being used for children with congenital heart disease.
Mahajan A, Shabanie A, Turner J, Sopher MJ, Marijic J. Pulse contour analysis for cardiac output monitoring in cardiac surgery for congenital heart disease. Anesth Analg. 2003;97(5):1283–8. https://doi.org/10.1213/01.ANE.0000081797.61469.12
Taylor K, La Rotta G, McCrindle BW, Manlhiot C, Redington A, Holtby H. A comparison of cardiac output by thoracic impedance and direct fick in children with congenital heart disease undergoing diagnostic cardiac catheterization. J Cardiothorac Vasc Anesth. 2011;25(5):776–9. https://doi.org/10.1053/j.jvca.2011.05.002
Yazdi D, Sridaran S, Smith S, Centen C, Patel S, Wilson E, et al. Noninvasive scale measurement of stroke volume and cardiac output compared with the direct Fick method: a feasibility study. J Am Heart Assoc. 2021;10(24):e021893. https://doi.org/10.1161/JAHA.121.021893
Dvir A, Goldstein N, Rapoport A, Balmor RG, Nachman D, Merin R, et al. Comparing cardiac output measurements using a wearable, wireless, noninvasive photoplethysmography-based device to pulse contour cardiac output in the general ICU: a brief report. Crit Care Explor. 2022;4(2):e0624. https://doi.org/10.1097/CCE.0000000000000624. An interesting recent example of using non-invasive methods for cardiac output monitoring.
Wang L, Pickwell-Macpherson E, Liang YP, Zhang YT. Noninvasive cardiac output estimation using a novel photoplethysmogram index. Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:1746–9. https://doi.org/10.1109/IEMBS.2009.5333091
Bernstein DP, Henry IC, Lemmens HJ, Chaltas JL, DeMaria AN, Moon JB, et al. Validation of stroke volume and cardiac output by electrical interrogation of the brachial artery in normals: assessment of strengths, limitations, and sources of error. J Clin Monit Comput. 2015;29(6):789–800. https://doi.org/10.1007/s10877-015-9668-9
Godoy A, Contreras A, Tabares A. Agreement analysis of stroke volume and cardiac output measurement between a oscillometric device and transthoracic echocardiogram in normotensive individuals: a preliminary report. Braz J Anesthesiol. 2023;73(4):380–4. https://doi.org/10.1016/j.bjane.2021.09.006
Saugel B, Meidert AS, Langwieser N, Wagner JY, Fassio F, Hapfelmeier A, et al. An autocalibrating algorithm for non-invasive cardiac output determination based on the analysis of an arterial pressure waveform recorded with radial artery applanation tonometry: a proof of concept pilot analysis. J Clin Monit Comput. 2014;28(4):357–62. https://doi.org/10.1007/s10877-013-9540-8
Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med. 2005;31(10):1316–26. https://doi.org/10.1007/s00134-005-2790-2
Lakhal K, Dauvergne JE, Kamel T, Messet-Charriere H, Jacquier S, Robert-Edan V, et al. Noninvasive monitoring of arterial pressure: finger or lower leg as alternatives to the upper arm: a prospective study in three ICUs. Crit Care Med. 2023; https://doi.org/10.1097/CCM.0000000000005945
Castiglioni P, Faini A, Parati G, Di Rienzo M. Wearable seismocardiography. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:3954–7. https://doi.org/10.1109/IEMBS.2007.4353199
Bhattacharya S, Nikbakht M, Alden A, Tan P, Wang J, Alhalimi TA, et al. A Chest-conformable, wireless electro-mechanical E-tattoo for measuring multiple cardiac time intervals. Advanced Electronic Materials. 2023:2201284. https://doi.org/10.1002/aelm.202201284
Ha T, Tran J, Liu S, Jang H, Jeong H, Mitbander R, et al. A chest-laminated ultrathin and stretchable E-tattoo for the measurement of electrocardiogram, seismocardiogram, and cardiac time intervals. Adv Sci. 2019;6(14):1900290. https://doi.org/10.1002/advs.201900290
Hu H, Huang H, Li M, Gao X, Yin L, Qi R, et al. A wearable cardiac ultrasound imager. Nature. 2023;613(7945):667–75. https://doi.org/10.1038/s41586-022-05498-z
Saugel B, Cecconi M, Wagner JY, Reuter DA. Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine. Br J Anaesth. 2015;114(4):562–75. https://doi.org/10.1093/bja/aeu447
Quain A, Hoyer M, Ephrem G, Kay WA. Non-invasive cardiac output monitoring (NICOM) in adult congenital heart disease patients with Fontan palliation. International Journal of Cardiology Congenital Heart Disease. 2021;6:100287. https://doi.org/10.1016/j.ijcchd.2021.100287
Favia I, Rizza A, Garisto C, Haiberger R, Di Chiara L, Romagnoli S, et al. Cardiac index assessment by the pressure recording analytical method in infants after paediatric cardiac surgery: a pilot retrospective study. Interact Cardiovasc Thorac Surg. 2016;23(6):919–23. https://doi.org/10.1093/icvts/ivw251
Gil-Anton J, Lopez-Bayon J, Lopez-Fernandez Y, Morteruel E, Perez-Estevez E, Lopez-Herce J. Cardiac index monitoring by femoral arterial thermodilution after cardiac surgery in children. J Crit Care. 2014;29(6):1132.e1–4. https://doi.org/10.1016/j.jcrc.2014.06.004
Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92(8):2226–35. Classic study outlining the hemodynamics after congenital heart disease surgery
Hoffman TM, Wernovsky G, Atz AM, Bailey JM, Akbary A, Kocsis JF, et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. Prophylactic Intravenous Use of Milrinone After Cardiac Operation in Pediatrics. Am Heart J. 2002;143(1):15–21. https://doi.org/10.1067/mhj.2002.120305
Gaies M, Pasquali SK, Donohue JE, Dimick JB, Limbach S, Burnham N, et al. Seminal postoperative complications and mode of death after pediatric cardiac surgical procedures. Ann Thorac Surg. 2016;102(2):628–35. https://doi.org/10.1016/j.athoracsur.2016.02.043
Bhalala US, Nishisaki A, McQueen D, Bird GL, Morrison WE, Nadkarni VM, et al. Change in regional (somatic) near-infrared spectroscopy is not a useful indicator of clinically detectable low cardiac output in children after surgery for congenital heart defects. Pediatr Crit Care Med. 2012;13(5):529–34. https://doi.org/10.1097/PCC.0b013e3182389531
Burkhardt BE, Rucker G, Stiller B. Prophylactic milrinone for the prevention of low cardiac output syndrome and mortality in children undergoing surgery for congenital heart disease. Cochrane Database Syst Rev. 2015;(3):CD009515. https://doi.org/10.1002/14651858.CD009515.pub2.
Butts RJ, Scheurer MA, Atz AM, Zyblewski SC, Hulsey TC, Bradley SM, et al. Comparison of maximum vasoactive inotropic score and low cardiac output syndrome as markers of early postoperative outcomes after neonatal cardiac surgery. Pediatr Cardiol. 2012;33(4):633–8. https://doi.org/10.1007/s00246-012-0193-z
Cavigelli-Brunner A, Hug MI, Dave H, Baenziger O, Buerki C, Bettex D, et al. Prevention of low cardiac output syndrome after pediatric cardiac surgery: a double-blind randomized clinical pilot study comparing dobutamine and milrinone. Pediatr Crit Care Med. 2018;19(7):619–25. https://doi.org/10.1097/PCC.0000000000001533
Hickok RL, Spaeder MC, Berger JT, Schuette JJ, Klugman D. Postoperative abdominal NIRS values predict low cardiac output syndrome in neonates. World J Pediatr Congenit Heart Surg. 2016;7(2):180–4. https://doi.org/10.1177/2150135115618939
Rogers L, Ray S, Johnson M, Feinstein Y, Dominguez TE, Peters MJ, et al. The inadequate oxygen delivery index and low cardiac output syndrome score as predictors of adverse events associated with low cardiac output syndrome early after cardiac bypass. Pediatr Crit Care Med. 2019; https://doi.org/10.1097/PCC.0000000000001960
Parr GV, Blackstone EH, Kirklin JW. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation. 1975;51(5):867–74. https://doi.org/10.1161/01.cir.51.5.867
Ma M, Gauvreau K, Allan CK, Mayer JE Jr, Jenkins KJ. Causes of death after congenital heart surgery. Ann Thorac Surg. 2007;83(4):1438–45. https://doi.org/10.1016/j.athoracsur.2006.10.073
Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107(7):996–1002. https://doi.org/10.1161/01.cir.0000051365.81920.28
Baloglu O, Ryan SD, Onder AM, Rosen D, Mullett CJ, Munther DS. A clinical mathematical model estimating postoperative urine output in children underwent cardiopulmonary bypass for congenital heart surgery. J Pediatr Intensive Care. 2022; https://doi.org/10.1055/s-0042-1758474
Yazdani A, Lu L, Raissi M, Karniadakis GE. Systems biology informed deep learning for inferring parameters and hidden dynamics. PLoS Comput Biol. 2020;16(11):e1007575. https://doi.org/10.1371/journal.pcbi.1007575
Funding
Dr. Tandon acknowledges the support from Thrasher Foundation Early Career Research Award; Brett Boyer Foundation; Children’s Health Innovation Grant; Cleveland Clinic Caregiver Catalyst Grant; Department of Heart, Vascular, and Thoracic C4AI Fund; UT Southwestern Cary Council/DocStars/Southwestern Medical Foundation; and UT Southwestern Center for Translational Medicine Swim with the Sharks Grant. SB and NL acknowledge the support from US NSF under Grant ASCENT 2133106 and US ONR under Grant N00014-20-1-2112.
Author information
Authors and Affiliations
Contributions
All authors conceived of this manuscript, provided content, and revised it. All authors agree with the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Animesh Tandon is a consultant for Synergen Technology Labs LLC and Siemens Healthineers. Dr. Omer Inan is a co-founder and board member for Cardiosense, Inc. Sarnab Bhattacharya, Ayse Morca, Daniel S Munther, Shawn D Ryan, Samir Q Latifi, Nanshu Lu, Javier J. Lasa, Bradley S Marino, and Orkun Baloglu report no competing interests.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tandon, A., Bhattacharya, S., Morca, A. et al. Non-invasive Cardiac Output Monitoring in Congenital Heart Disease. Curr Treat Options Peds 9, 247–259 (2023). https://doi.org/10.1007/s40746-023-00274-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-023-00274-1