Abstract
Introduction
Our aim was to investigate the efficacy and safety of upadacitinib (UPA) in patients with either oligo- or polyarticular active psoriatic arthritis (PsA) using routine clinical practice data from an observational, prospective, multicentre study.
Methods
This interim analysis contains upadacitinib efficacy and safety data from the UPJOINT study, collected from baseline to the week 24 visit with a focus on composite measures, clinical assessments and patient-reported outcomes, amongst others, including minimal disease activity (MDA), very low disease activity (VLDA), Disease Activity Index for Psoriatic Arthritis (DAPSA), Leeds Enthesitis Index (LEI), resolution of dactylitis and nail psoriasis and body surface area affected by skin psoriasis (BSA).
Results
A total of 296 patients with baseline data and 192 with completed week 24 visits were included in the analysis. The proportion of patients achieving MDA increased from 2.7% at baseline to 39.1% at week 24 (95% CI 32.1, 46.3). Similarly, the number of patients in DAPSA remission (DAPSA ≤ 4) increased from 0 at baseline to 32 (16.7%) by week 24. At that time, 59.4% of the patients were either in DAPSA remission or had low disease activity (DAPSA ≤ 14). During the 24 weeks time frame, the proportion of patients with BSA ≤ 3 increased from 80.7% to 91.1%. Furthermore, at weeks 12 and 24, 45.14% and 47.19% of affected patients showed a resolution of enthesitis. Active dactylitis and nail psoriasis at baseline were reported to affect 10.5% and 22.0%, decreasing to 2.6% and 5.7% at week 24, respectively. The safety findings are consistent with the known safety profile of upadacitinib in rheumatoid arthritis and PsA; no new safety risks were identified.
Conclusion
The data from this study confirm the findings of previous randomized controlled trials suggesting UPA is an effective treatment for active PsA without any new safety signals in patients from daily clinical practice.
Clinical Trial Registration
ClinicalTrials.gov identifier, NCT04758117.
Plain Language Summary
Upadacitinib is an antirheumatic medical therapy approved for treating psoriatic arthritis with insufficient response to previous conventional or biological therapies (DMARD-IR). Psoriatic arthritis is a chronic inflammatory disease affecting the joints, spine, tendons/entheses, skin, nails and other parts of the musculoskeletal system. Early diagnosis and treatment initiation are essential for patients with psoriatic arthritis given the potentially irreversible damage to joints, spine, and entheses and the considerable impact on quality of life. The results presented in this manuscript help clinicians evaluate whether the efficacy and the safety profile of upadacitinib found in previous clinical trials can be reproduced in patients seen in daily clinical practice. This analysis presents descriptive data on the real-world efficacy and safety of upadacitinib, measured by clinical and patient-reported outcomes assessed in four visits over 24 weeks. In summary, our findings confirm the results of previous clinical trials showing that upadacitinib effectively reduces symptom severity of PsA and substantially increases the proportion of patients achieving treatment goals relevant to clinical practice, such as remission or very low disease activity. In addition, safety data were consistent with previous studies of upadacitinib in rheumatoid arthritis or psoriatic arthritis; no new risks to the patients' safety were identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
In 2021, upadacitinib, a Janus kinase inhibitor, was approved for the treatment of patients with psoriatic arthritis with inadequate response to non-biological or biological disease-modifying antirheumatic drugs (DMARD) therapy, demonstrating a reasonable benefit–risk profile according to randomized controlled clinical trials (RCTs). |
However, evidence from routine clinical practice investigating the efficacy and safety of upadacitinib in a real-world population is lacking. |
This study aimed to evaluate whether the efficacy and safety of upadacitinib for the treatment of active psoriatic arthritis, given real-world data, resemble the results of RCTs, particularly regarding the achievement of minimal disease activity (MDA). |
What was learned from the study? |
Our interim analysis showed that at weeks 12 and 24, 39.8% and 39.1% of the patients achieved MDA, which is a substantial improvement from baseline and is in line with findings of previous RCTs; no new safety risks were identified from the data currently available |
According to the current study data, upadacitinib is an effective treatment for active PsA in patients with inadequate response to non-biological or biological DMARD therapy. |
Introduction
Psoriatic arthritis (PsA) is a chronic systemic inflammatory disease affecting the joints, spine, tendons/entheses, skin, nails and other parts of the musculoskeletal system. Besides the current lack of curative treatment, varying symptoms and the absence of disease-specific serological markers make diagnosing and managing PsA challenging for physicians [1]. Along with considerably impaired quality of life, untreated PsA may lead to rapid joint destruction and irreversible damage to axial musculoskeletal structures, potentially resulting in disability. In this context, patients with PsA retire earlier than patients with ankylosing spondylitis (AS), for instance [2]. The variety of PsA-related symptoms is reflected by the many clinical measures assessing dermal and musculoskeletal symptoms such as skin and nail psoriasis, arthritis, enthesitis or dactylitis, usually complemented by patient-reported outcomes (PROs) [3, 4]. From a patient perspective, pain at rest and in motion, impaired physical function and fine motor skills, fatigue, reduced quality of sleep, itching skin lesions and feelings of shame, anxiety or depression limiting social participation and promoting social withdrawal were reported to have a significant impact on daily life [5, 6]. Fortunately, with the CASPAR classification criteria and the updated GRAPPA, EULAR and ACR treatment recommendations, established guidelines are available to support proper clinical decision-making and management of PsA [7,8,9,10]. With the approval of Janus kinase inhibitors (JAKi) for rheumatoid arthritis (RA), PsA and axial spondylarthritis (axSpA), the choice of treatments has recently grown beyond conventional synthetic or biological disease-modifying antirheumatic drugs (csDMARDs/bDMARDs). JAKi inhibit autoinflammatory processes associated with PsA, axSPA and RA by blocking the signal transducer and activator of transcription (JAK-STAT) pathway, which can be activated by various pro-inflammatory cytokines [11]. Particularly, cytokines related to the interleukin (IL)-12/23 pathway involved in the pathogenesis of PsA have been shown to be mediated by the JAK-STAT pathway [12, 13].
Upadacitinib (UPA) has been approved for treating adult patients with active PsA and insufficient response to previous csDMARDs or bDMARDs as of June 2021 and December 2021, in Canada and Europe, respectively. It has shown efficacy in patients with inadequate response or intolerance to csDMARD or bDMARD therapy in randomized controlled trials (RCTs) [14,15,16]. Although bDMARDs are considered the current standard of care for patients with PsA and insufficient response to csDMARDs, data from a large cohort study demonstrated that the 3-year persistence for bDMARDS is low across modes of action with an overall persistence rate of 36.2% in PsA [9, 17]. The lack of long-term persistence in patients with a chronic progressive inflammatory disease and the low proportion of patients achieving minimal or low disease activity within 6 months of initiating a bDMARD make the availability of further treatment options crucial for successfully managing PsA [18]. This interim analysis investigates whether data from clinical practice collected within an international, observational, multicentre study confirm the results from the previous RCTs regarding the efficacy and safety of UPA for treating active PsA. The outcome of primary interest was the proportion of patients achieving minimal disease activity (MDA) after 24 weeks of treatment with UPA.
Methods
Patients and Eligibility Criteria
The patients included in this interim analysis were recruited from study sites in Germany and Canada from 4 February 2021 (first patient in) to 28 July 2022 (last patient in). Institutional review board approval was granted by the ethics committee of the Medical Faculty of the Friedrich-Alexander-University Erlangen-Nürnberg on 8 December 2020 (# 458_20B). The UPJOINT study is registered on ClinicalTrials.gov (NCT04758117). All patients were required to complete the informed consent form before any study-related procedures. To be eligible for the study, patients had to meet the following inclusion criteria: (i) ≥ 18 years of age, (ii) diagnosis of active PsA as determined by the treating physician, (iii) swollen joint count ≥ 1 out of 66 joints, (iv) UPA treatment as per local summary of product characteristics (SmPC) in Germany and Canada (Health Canada approved product monograph) and (v) decision on the treatment with UPA was made prior to patient participation in this study. Patients were not able to join the study if any of the following criteria were met: (i) previous treatment with UPA, (ii) missing indication for UPA treatment according to the local SmPC or product monograph, (iii) current or recent (i.e. in the last 30 days) participation in interventional research or (iv) patients unwilling or unable to complete patient-reported questionnaires. As this was a non-interventional study, there was no implementation of follow-up procedures and, thus, patients that discontinued UPA left the study and were no longer monitored. The study was performed according to the Declaration of Helsinki and its later amendments.
Study-Related Procedures and Measures for PsA
UPJOINT is an international prospective open-label multicentre study over 48 weeks of PsA treatment with UPA, including six scheduled study visits, at baseline and weeks 4, 12, 24, 36, and 48. Patient data were recorded in an electronic case report form (eCRF) and included demographics, results from clinical and patient-reported outcome measures, concomitant diseases and medication, laboratory assessments and documentation of adverse events (AEs) or serious adverse events (SAEs). Nevertheless, this is an interim analysis from a study that is still recruiting, and data from weeks 36 and 48 are not yet available. Physicians at participating study sites individually decided on treatment with UPA and in discussions with each patient. AbbVie, the study sponsor, was not involved in deciding in favour for or against treatment with UPA. This pre-specified interim analysis presents efficacy and safety data from clinical practice in patients with PsA refractory to csDMARDs/bDMARDs during the initial 24 weeks of treatment with UPA, where more than 50% of the planned sample size (n = 380) had completed the week 24 visit. According to the previous SELECT-PsA 1 and 2 trial designs and their findings, this time period can be expected to return valid results regarding efficacy [14, 16]. The selection of tools included in this analysis to measure the efficacy of UPA included clinical tools, composite scores and patient-reported outcomes. Clinical tools comprised (i) tender joint count 68/swollen count joint 66 (TJC68/SJC66) [19], (ii) body surface area affected by psoriasis (BSA) [20], (iii) the Leeds Enthesitis Index (LEI) [21] and (iv) the presence of dactylitis and nail psoriasis (yes/no) evaluated by the treating physician. Minimal disease activity (MDA), very low disease activity (VLDA) and remission or low disease activity according to the Disease Activity Index for Psoriatic Arthritis (DAPSA) were chosen as composite scores reflecting treatment targets in clinical routine [22,23,24]. The set of patient-reported outcomes included the Bath Ankylosing Disease Activity Index (BASDAI), the Health Assessment Questionnaire-Disability Index (HAQ-DI), the Dermatology Life Quality Index (DLQI) and two 0–10 numerical rating scales (NRS) assessing patient-reported overall pain (NRS pain) and global disease activity (NRS PtGA) [25,26,27]. The primary endpoint of the study was the proportion of patients achieving MDA after 24 weeks of continuous treatment with UPA.
Statistical Analysis Procedures and Datasets
Besides background data on patients’ characteristics, the presented results refer to two analysis datasets: an efficacy dataset used for intention-to-treat analysis and another for safety analysis containing information regarding AEs to be reported according to Good Clinical Practice guidelines. The safety analysis dataset included AEs reported in the time period between informed consent signature and the database lock categorized according to the Medical Dictionary for Regulatory Activities (MedDRA). The efficacy dataset contained information on all outcome measures specified above for each study visit, except for the DLQI and the dactylitis and nail psoriasis evaluations at week 4, which were not part of the data documentation schedule. If not stated otherwise, continuous descriptive results are presented as arithmetic mean [standard deviation (SD)]. The descriptive results for nominal variables are presented by absolute and relative frequency n (%). Absolute numbers for proportions in the text are shown in the corresponding table. Missing values were not imputed to preserve the original information from the available data. This interim analysis did not include any subgroup analysis specifically addressing sex. Results shown in the tables include additional information reflecting the number of valid data entries for the individual variable in case of missing values. Percentage data refer to the total number of patients having completed the corresponding study visit, including patients with missing data. Patient data are presented as observed, non-responder imputation was not applied. Statistical analyses were conducted using SAS Version 9.4 (Cary, NC, USA) [28].
Results
Baseline Patients’ Characteristics
The efficacy dataset used for intention-to-treat analysis included 296 patients with completed baseline visits, of which 192 had also completed the week 24 visit at the time of the database lock. Most patients (194, 65.5%) included in this dataset were female; 117 (39.5%) and 179 (60.5%) patients had oligo- or polyarticular PsA, respectively. The average age at baseline was 54.1 (SD 11.7) years. The mean DAPSA at baseline was 29.2 (SD 15.0). As expected, the proportion of patients presenting with either MDA or VLDA at the study start was very low, 8 (2.7%) and 0 (0%), respectively, a finding that is also reflected by the corresponding mean values for TJC68 and SJC66 and the considerable number of patients with more than one tender or swollen joint (Tables 1 and 2). A substantial proportion of patients were presenting with nail psoriasis (86, 29.1%), dactylitis (45, 15.2%) or a BSA > 3% (57, 19.3%) at baseline. Regarding physical function impairment, 229 (77.4%) patients had a HAQ-DI exceeding 0.5, suggesting that most patients experienced some limitations in daily physical activities (Table 2, baseline column). Mean baseline DLQI and BASDAI were 6.7 (SD 6.7) and 5.3 (SD 2.2) with the latter being above 4.1 which is the currently proposed cut-off for patients deemed to have an acceptable symptom state [29] (Table 1). At baseline, 198 (66.9%) of patients had at least one comorbidity, among which cardiovascular disease (92, 31.1%), depression (42, 14.2%) and type 2 diabetes (21, 7.1%) were most common (Supplementary Table 1). Regarding current antirheumatic treatment, 87 (31.8%) out of 274 patients with available data were prescribed methotrexate in combination with UPA since baseline, whereas 104 (38.0%) patients were taking glucocorticoids during that period. Regarding previous therapies prior to baseline, 261 (88.2%) out of 296 patients were taking csDMARDs or glucocorticoids as pre-therapy, while 220 (74.3%) patients had at least one pre-therapy with bDMARDs or targeted-synthetic DMARDs (tsDMARDs)—a proportion that nearly remained unchanged until week 24 (75.5%). A combined number of 225 (76.0%) patients took bDMARDs or tsDMARDs as pre-therapy or therapy until baseline (Supplementary Fig. 1).
Efficacy: Composite and Clinical Measures
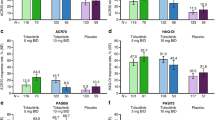
The proportion of patients achieving MDA as the designated outcome of interest increased from baseline (2.7%) to week 24 (39.1%, 95% CI 32.1, 46.3). Moreover, similar numbers were already reached at the previous week 12 visit, with 39.8% of the patients achieving MDA at that time. In line with this result, the proportion of patients achieving DAPSA low disease activity (DAPSA > 4 and ≤ 14) also peaked at week 12 with a value of 49.0% and was slightly decreased to 42.7% at week 24. Furthermore, we discovered a steady increase from baseline to week 24 in the proportion of patients achieving VLDA or DAPSA remission (0% to 16.7% for both outcomes), a pattern that is also reflected by the steady decrease in the mean DAPSA over time (Table 3). The mean change for the DAPSA between baseline and week 24 was − 14.7 (95% CI − 16.4, − 13.0). Notably, at weeks 12 and 24, more than half of the patients were either in DAPSA remission or had low disease activity (week 12: 155, 61.7%; week 24: 114, 59.4%). Further information on results for composite measures are shown in Table 2 and Fig. 1a–d. Concerning the individual components of MDA and VLDA, four of the seven relevant domains, BSA ≤ 3, LEI ≤ 1, NRS pain ≤ 1.5 and NRS PtGA ≤ 2, reached peak values at week 12 with a minor decrease until week 24. The fraction of patients fulfilling the remaining MDA criteria (TJC68 ≤ 1, SJC66 ≤ 1 and HAQ-DI ≤ 0.5) increased steadily until week 24 (Table 2). In line with these findings, the proportion of patients presenting with dactylitis or nail psoriasis decreased noticeably, with numbers halving over time (dactylitis baseline vs week 24, 15.2% vs 6.8%; nail psoriasis baseline vs week 24, 29.1% vs 12.5%). Of those patients affected by dactylitis at baseline, more than 55.2% were found to have symptom resolution at week 24. Comparable findings were made for enthesitis, with more than 45.1% of the previously affected patients assessed no longer showing related symptoms. Concerning skin psoriasis, the proportion of patients with BSA > 3 decreased from 19.3% at baseline to 8.9% at week 24 (Table 2, Fig. 2a–d).
Improvement of composite disease activity indices from baseline to week 24. a Minimal disease activity (MDA), error bars represent 95% CI. b Very low disease activity (VLDA). c Disease Activity in Psoriatic Arthritis Score (DAPSA), error bars represent SD. d DAPSA categories. The number of patients with valid data for MDA/VLDA (a, b): Baseline, N = 296; week 4, N = 274; week 12, N = 251; week 24, N = 192. The number of patients with valid data for DAPSA (c, d): Baseline, N = 289; week 4, N = 228; week 12, N = 238; week 24, N = 179
Improvement of dactylitis, enthesitis, and nail and skin psoriasis. a Proportion of patients with a resolution of dactylitis according to the physician’s evaluation. b Proportion of patients with a resolution of enthesitis according to Leeds Enthesitis Index (LEI). c Proportion of patients affected by nail psoriasis according to the physician’s evaluation. d Proportion of patients with a body surface area (BSA) affected by skin psoriasis > 3%
Efficacy: Patient-Reported Outcomes and Sick Leave Days
Similar to the composite and clinical measure results, patient-reported outcomes for disease activity and physical function also improved remarkably. The initial BASDAI improved from 5.3 (SD 2.2) to 3.6 (SD 2.4) by week 12 and maintained this level throughout week 24. These findings demonstrate that, on average, the BASDAI criterion for minimal clinically important improvement (MCII ≥ 0.7) was met, even if a more conservative MCII from a comparative sample of patients with active ankylosing spondylitis was chosen (MCII ≥ 1.1) [30]. From week 4 onwards, mean BASDAI scores were below the cut-off for a patient-acceptable symptom state (BASDAI ≤ 4.1). Consistent with these findings, the baseline proportion of patients with an NRS PtGA ≤ 2 doubled at week 4 and tripled from week 12 onwards. Similar to the results for NRS PtGA, the fraction of patients with NRS pain ≤ 2 also improved noticeably from 3.7% at baseline to 31.9% and 31.3% at weeks 12 and 24, respectively (Table 2). Despite baseline results suggesting the majority of patients in our sample were not severely limited, mean HAQ-DI scores improved over time. This improvement in physical function is confirmed by the increasing proportion of patients with a HAQ-DI ≤ 0.5 from baseline (20.9%) to week 24 (39.1%) (Fig. 3a, Tables 2 and 3). According to the DLQI results, DQLI improved, with the results for week 12 and week 24 reflecting a shift from a moderate to a small effect of PsA on the patients’ lives compared to the initial DLQI (Fig. 3b, Table 3). However, although the average DLQI improved by one category, the recommended minimal clinically important difference (MCID) for DLQI of 4 was not achieved, as the absolute value of DLQI mean change from baseline was 1.9 (6.1) [31]. At week 24, 6.8% of the patients in the study reported sick leave days, whereas the baseline value was 10.5% (Fig. 3c).
Improvement of patient-reported outcome measures (PROs). a Physical function: Proportion of patients with a Health Assessment Questionnaire-Disability Index (HAQ-DI) ≤ 0.5. b Quality of Life: Dermatology Life Quality Index (DLQI), error bars represent SD. c Proportion of patients reporting to have taken sick leave days. The number of patients with valid data were as follows: HAQ ≤ 0.5 (a): Baseline, N = 291; week 4, N = 269; week 12, N = 237; week 24, N = 180. DLQI (b): Baseline, N = 264; week 12, N = 216; week 24, N = 172. Sick leave (c): Baseline, N = 31; week 4, N = 20; week 12, N = 18; week 24, N = 1
Safety
Data from the safety dataset, covering all reported events of interest so far, showed 126 (42.0%) patients reporting 255 AEs in total, with 45 patients (15.0%) discontinuing UPA because of AEs (see Supplementary Fig. 2). In addition, 19 SAEs were reported for 13 patients (4.3%). From the categorized AEs of particular interest, infections (53 events in n = 46 patients; 15.3%) were most common, followed by gastrointestinal disorders (32 events in n = 25 patients; 8.3%), skin disorders (22 events in n = 19 patients; 6.3%), weight increase (6 events in n = 5 patients; 1.67%) and abnormal liver function (5 events in n = 5 patients; 1.7%). From the infections reported, coronavirus disease (COVID-19) was most frequently reported (n = 20, 6.7%), with the two serious infections both resulting from previous COVID infections. Notably, according to the current data, there were no reports of venous thromboembolism (VTE), major adverse cardiovascular events (MACE) or malignancies (Table 4).
Discussion
The results of this interim analysis confirm the efficacy of UPA for patients with active PsA and inadequate response to previous antirheumatic treatment, as demonstrated in previous RCTs without observing any new safety signals. Particularly for MDA, our findings highlight that UPA improves PsA-related symptom severity during the first 12 weeks of therapy, lasting throughout week 24. Similar findings were obtained for DAPSA remission and low disease activity, with approximately 60% of patients achieving either outcome at weeks 12 and 24. These findings confirm composite scores as highly responsive measures for the treatment efficacy of JAKi therapies [32]. Our findings for MDA at week 24 match well with the numbers from the SELECT-PsA 1 trial and are numerically greater to those from the SELECT-PsA 2 trial [15, 16]. The main reason for this observation is surely the comparison of a real-world open-label observational study versus a placebo-controlled RCT, respectively. Another conceivable explanation is that non-responder imputation in SELECT-PsA 2 is likely to have led to lower response rates. Furthermore, only patients with at least one previous bDMARD were eligible for the SELECT-PsA 2 trial making it more difficult to achieve MDA compared to our sample in which 24% of the patients were biological-naïve. Importantly, baseline rheumatic disease activity in the SELECT-PsA 2 trial was higher than in our study, with TJC and SJC being twice as high in the RCT, making it more difficult to achieve MDA. For future analysis (with increasing numbers of patients included), it will also be interesting to examine response as a function of bDMARD-naïve and bDMARD-experienced patient subgroups and the number of previously failed bMDARDs. With more than 75% of the patients in this study having failed a bDMARD, the available data suggest that UPA is also effective in clinical practice for difficult-to-treat patients. From the longitudinal progression of values for the domains included in MDA and VLDA, the TJC68 ≤ 1, SJC66 ≤ 1 and HAQ-DI ≤ 0.5 show a steady improvement pattern. However, the domain criteria representing patient-reported information, such as NRS pain ≤ 1, NRS PtGA ≤ 2 and HAQ-DI ≤ 0.5, seem more difficult to achieve, hampering the overall achievement of MDA or even VLDA. These results are in line with previous findings in the literature confirming patient-centred MDA domains to frequently not be achieved by MDA non-responders, whereas SJC66 ≤ 1, LEI ≤ 1 or BSA ≤ 3, representing the physician perspective, were rarely a matter of concern [33]. A potential explanation for this observation is the different perspectives patients and physicians may have on PsA [34]. The BASDAI and other PROs showcased herein have available cut-off values equivalent to a patient-acceptable symptom state or MCII/MCID. Our results regarding mean BASDAI values from week 4 onwards demonstrated that for this tool, frequently applied in clinical practice, both criteria were met concerning average outcomes. However, we cannot draw any conclusions concerning the axial involvement of our patients, as no imaging or clinical axial data was collected in this study. UPJOINT is the first prospective study investigating UPA in patients with active PsA and inadequate response to previous antirheumatic treatment in daily practice. Its study duration of 48 weeks will provide valuable information for upcoming efficacy, safety or persistence analyses with underlying data from clinical practice. The generalizability of findings from the interim analysis presented may be limited by the data currently available for week 24, which is 50.5% of the planned total sample size. Hence, results from the final analysis could differ from the numbers presented in this manuscript. The analysis herein consists of descriptive results, focusing on state-of-the-art treatment targets in clinical practice. Upon availability of the full efficacy and safety datasets, the final data analyses will include additional inferential statistical information and measures of sampling adequacy and may also help identify factors independently related to the achievement of MDA, VLDA or DAPSA remission. With this being a non-interventional study, we obviously face further limitations. We mentioned previously that study sites were only selected from rheumatology departments and thus disease duration of PsO was not collected. Furthermore, we cannot provide any follow-up information about our patients as patients that left this particular study were no longer monitored. UPJOINT, as an observational study, includes various concomitant antirheumatic therapies such as glucocorticoids or methotrexate. The role and impact of common therapies in combination with UPA on efficacy and safety remain to be investigated. However, currently available data suggest that the efficacy and safety of UPA were generally consistent when administered as monotherapy or in combination with non-biological DMARDs through 24 weeks, supporting the use of UPA with or without non-biological DMARDs in PsA [35].
Conclusion
Preliminary real-world results from the UPJOINT study have demonstrated the efficacy and safety of UPA for the treatment of active PsA. Results for MDA achievement confirmed previous RCT findings suggesting UPA to be effective in patients with inadequate response or intolerance to csDMARDs or bDMARDs. No new safety signals were identified.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g. protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21–37.
Rodrigues J, Rodrigues AM, Dias SS, Sousa RD, Branco JC, Canhão H. Psoriatic arthritis and ankylosing spondylitis impact on health-related quality of life and working life: a comparative population-based study. Acta Reumatol Port. 2019;44(4):254–65.
Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res Hoboken. 2011;63(Suppl 11):S64–85.
Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med. 2017;17(1):65–70.
Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73(6):1012–9.
Liphardt AM, Manger E, Liehr S, et al. Similar impact of psoriatic arthritis and rheumatoid arthritis on objective and subjective parameters of hand function. ACR Open Rheumatol. 2020;2(12):734–40.
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73.
Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465–79.
Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–12.
Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol Hoboken NJ. 2019;71(1):5–32.
McInnes IB, Szekanecz Z, McGonagle D, et al. A review of JAK-STAT signalling in the pathogenesis of spondyloarthritis and the role of JAK inhibition. Rheumatology (Oxford). 2022;61(5):1783–94.
Fragoulis GE, Siebert S, McInnes IB. Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Annu Rev Med. 2016;67:337–53.
Veale DJ, McGonagle D, McInnes IB, et al. The rationale for Janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatology (Oxford). 2019;58(2):197–205.
McInnes IB, Kato K, Magrey M, et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021;7(3):e001838.
McInnes IB, Kato K, Magrey M, et al. Efficacy and safety of upadacitinib in patients with psoriatic arthritis: 2-year results from the phase 3 SELECT-PsA 1 study. Rheumatol Ther. 2022. https://doi.org/10.1007/s40744-022-00499-w.
Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis. 2021;80(3):312–20.
Pina Vegas L, Penso L, Claudepierre P, Sbidian E. Long-term persistence of first-line biologics for patients with psoriasis and psoriatic arthritis in the French health insurance database. JAMA Dermatol. 2022;158(5):513–22.
Choquette D, Chandran V, Laliberté MC, et al. AB0895 Residual burden and disease activity of Canadian PsA patients treated with advanced therapies: preliminary results from a multiple registry analysis (UNISON-PsA). Ann Rheum Dis. 2022;81(Suppl 1):1575–6.
Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36(6):729–40.
Thomas CL, Finlay AY. The ‘handprint’ approximates to 1% of the total body surface area whereas the ‘palm minus the fingers’ does not. Br J Dermatol. 2007;157(5):1080–1.
Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008;59(5):686–91.
Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis. 2010;69(8):1441–7.
Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(1):48–53.
Coates LC, Helliwell PS. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheumatol. 2016;43(2):371–5.
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21(12):2286–91.
Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9(5):789–93.
Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6.
SAS Institute. SAS® Version 94. SAS Institute, Cary
Godfrin-Valnet M, Prati C, Puyraveau M, Toussirot E, Letho-Gyselink H, Wendling D. Evaluation of spondylarthritis activity by patients and physicians: ASDAS, BASDAI, PASS, and flares in 200 patients. Jt Bone Spine. 2013;80(4):393–8.
Kviatkovsky MJ, Ramiro S, Landewé R, et al. The minimum clinically important improvement and patient-acceptable symptom state in the BASDAI and BASFI for patients with ankylosing spondylitis. J Rheumatol. 2016;43(9):1680–6.
Basra MKA, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the dermatology life quality index (DLQI): further data. Dermatology. 2015;230(1):27–33.
Helliwell P, Coates LC, FitzGerald O, et al. Disease-specific composite measures for psoriatic arthritis are highly responsive to a Janus kinase inhibitor treatment that targets multiple domains of disease. Arthritis Res Ther. 2018;20(1):242.
Lubrano E, Scriffignano S, Perrotta FM. The ‘climb’ towards minimal disease activity in psoriatic arthritis. Rheumatol Ther. 2021;8(3):1443–50.
Dandorfer SW, Rech J, Manger B, Schett G, Englbrecht M. Differences in the patient’s and the physician’s perspective of disease in psoriatic arthritis. Semin Arthritis Rheum. 2012;42(1):32–41.
Nash P, Richette P, Gossec L, et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2022;61(8):3257–68.
Acknowledgements
The authors thank all patients, study sites, and investigators who participated in this clinical trial.
Medical Writing/Editorial Assistance
Excellent statistical analysis support was provided by Daniela Adolf and Maria Kabelitz from StatConsult GmbH, which was funded by AbbVie. Medical writing support was provided by Matthias Englbrecht, which was funded by AbbVie.
Funding
AbbVie funded this trial and participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. The journal’s fee funded by AbbVie.
Author information
Authors and Affiliations
Contributions
Stephanie G Werner, Xenofon Baraliakos, Sabine Reckert, Martin Bohl-Bühler, Louis Bessette provided critical revisions and data acquisition; Marie-Claude Laliberté, Tanya Girard, Katharina Jeromin and Björn Fritz contributed to its conception and critically revised this work; Nikola Baschuk analysed and interpreted the data and provided critical revision; Axel J Hueber contributed to its conception, interpreted the data and critically revised this work.
Corresponding author
Ethics declarations
Conflict of interest
Axel J Hueber consultant or speaker: Abbvie, BMS, Eli Lilly, Galapagos, Gilead, Novartis, UCB; Funding for investigator-initiated studies: Novartis. Stephanie G Werner consultant/honoraria: Abbvie, Janssen-Cilag, Lilly, Pfizer, UCB. Xenofon Baraliakos consultant, speakers bureau, scientific advisory board and honoraria: Abbvie, Amgen, BMS, Chugai, Galapagos, Gilead, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, UCB. Sabine Reckert participated in non-interventional trials of AbbVie and declares no further conflicts of interest. Martin Bohl-Bühler participated in non-interventional trials of AbbVie GmbH & Co. KG, AMS Advanced Medical Services GmbH, BMS via inVentiv Health Clinical#, Charité Universitätsmedizin Berlin, Covance Inc., Galapagos, MSD SHARP & DOHME GMBH, Novartis Pharma GmbH, Pfizer Pharma GmbH, PPD Global Ltd, Rheumatologische Fortbildungsakademie, Roche Pharma AG, Sanofi-Aventis Deutschland GmbH, UCB Biosciences GmbH, Winicker Norimed GmbH and received honoraria for lectures and participation in advisory boards from AbbVie Deutschland GmbH & Co. KG, Amgen GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Galapagos Biopharma Germany GmbH, Gilead Sciences Europe Ltd., GILEAD Sciences GmbH, Hexal AG, Janssen-Cilag GmbH, Lilly Deutschland GmbH, MICE Service GmbH für Mylan Germany Team, MSD SHARP & DOHME GMBH, Novartis Pharma GmbH, Roche Pharma AG, Theramex Germany GmbH, UCB Pharma GmbH. Louis Bessette Speaker: Amgen, BMS, Janssen, UCB, AbbVie, Pfizer, Merck, Lilly, Novartis, Sanofi, Sandoz, Fresenius Kabi, Teva, Organon, JAMP Pharma; Consultant: Amgen, BMS, Janssen, UCB, AbbVie, Pfizer, Merck, Lilly, Novartis, Sanofi, Sandoz, Fresenius Kabi, Teva, Organon, JAMP Pharma; Research: Amgen, BMS, Janssen, UCB, AbbVie, Pfizer, Merck, Celgene, Sanofi, Lilly, Novartis, Gilead, JAMP Pharma. Marie-Claude Laliberté, Nikola Baschuk, Katharina Jeromin, Björn Fritz and Tanya Gerard are employees of AbbVie Corporation and may own AbbVie stocks and/or options.
Ethical approval
Institutional review board approval for the conduct of this study was obtained from the Friedrich-Alexander-University Erlangen-Nürnberg (# 458_20 B) on 8 December 2020. The study was performed according to the Declaration of Helsinki and its later amendments. All subjects provided informed consent to participate in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Werner, S.G., Baraliakos, X., Reckert, S. et al. Treatment with Upadacitinib in Active Psoriatic Arthritis: Efficacy and Safety Data of the First 192 Patients from the UPJOINT Study, a Multicentre, Observational Study in Clinical Practice. Rheumatol Ther 10, 1503–1518 (2023). https://doi.org/10.1007/s40744-023-00589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00589-3