Abstract
Introduction
This analysis was conducted to assess the incidence of adverse clinical outcomes, healthcare resource use (HCRU), and the costs associated with systemic corticosteroid (SCS) use in adults with systemic lupus erythematosus (SLE) in the UK.
Methods
We identified incident SLE cases using the Clinical Practice Research Datalink GOLD, Hospital Episode Statistics-linked healthcare, and Office for National Statistics mortality databases from January 1, 2005, to June 30, 2019. Adverse clinical outcomes, HCRU, and costs were captured for patients with and without prescribed SCS.
Results
Of 715 patients, 301 (42%) had initiated SCS use (mean [standard deviation (SD)] 3.2 [6.0] mg/day) and 414 (58%) had no recorded SCS use post-SLE diagnosis. Cumulative incidence of any adverse clinical outcome over 10-year follow-up was 50% (SCS group) and 22% (non-SCS group), with osteoporosis diagnosis/fracture most frequently reported. SCS exposure in the past 90 days was associated with an adjusted hazard ratio of 2.41 (95% confidence interval 1.77–3.26) for any adverse clinical outcome, with increased hazard for osteoporosis diagnosis/fracture (5.26, 3.61–7.65) and myocardial infarction (4.52, 1.16–17.71). Compared to low-dose SCS (< 7.5 mg/day), patients on high-dose SCS (≥ 7.5 mg/day) had increased hazard for myocardial infarction (14.93, 2.71–82.31), heart failure (9.32, 2.45–35.43), osteoporosis diagnosis/fracture (5.14, 2.82–9.37), and type 2 diabetes (4.02 1.13–14.27). Each additional year of SCS use was associated with increased hazard for any adverse clinical outcome (1.15, 1.05–1.27). HCRU and costs were greater for SCS users than non-SCS users.
Conclusions
Among patients with SLE, there is a higher burden of adverse clinical outcomes and greater HCRU in SCS versus non-SCS users.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Corticosteroids have been the mainstay of treatment for patients with systemic lupus erythematosus (SLE) for decades; however, long-term corticosteroid treatment in patients with SLE is associated with increased risk for serious adverse events across many organ systems, subsequent organ damage, and morbidity, as well as heightened healthcare costs according to US-based studies. |
Longitudinal data on adverse events and healthcare costs associated with corticosteroid treatment for patients with SLE in the UK are lacking. |
What was learned from this study? |
The incidence of adverse clinical outcomes across multiple organ systems more than doubled with systemic corticosteroid (SCS) use in patients with SLE in the UK. |
Healthcare resource utilization was greater with SCS use in patients with SLE in the UK. |
Annual costs for patient care were higher with SCS use and increased with exposure. |
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by variable clinical presentation and fluctuating disease activity, in which periods of low disease activity are interspersed by flares, contributing to organ damage and adverse patient outcomes [1,2,3,4,5]. The aim of SLE management is to achieve remission, or maintain low disease activity and prevent flares [1, 6], thereby protecting patients from related organ damage and improving outcomes. Current European Alliance of Associations for Rheumatology (EULAR) guidelines recommend the use of hydroxychloroquine for all patients with SLE to reduce the frequency of flares and contribute to remission maintenance [1]. Corticosteroids may also be prescribed and can provide rapid symptom relief [1, 7]. However, for chronic maintenance treatment corticosteroids should be minimized to ≤ 7.5 mg/day or when possible, withdrawn to prevent organ damage [1, 8,9,10]. Immunosuppressive drugs or biologic therapy may also be applied to facilitate corticosteroid tapering and to prevent disease flares [1]. For non-renal SLE of any severity, the British Society for Rheumatology guidelines also recommend using hydroxychloroquine and the lowest effective corticosteroid dosage (≤ 7.5 mg/day), in addition to immunosuppressive drugs for maintenance therapy [6].
Patients with SLE have high demands for healthcare resources, particularly in the presence of severe/active disease, flares, and organ damage [3, 4, 11,12,13,14,15,16]. Consensus statements from a Delphi panel of US rheumatologists suggest that SLE disease activity increases with prescribed corticosteroid dosages, resulting in greater healthcare resource utilization (HCRU) to manage corticosteroid-related adverse clinical outcomes [17], including osteoporosis, obesity, mood disorders requiring medical intervention, type 2 diabetes, fractures, cataracts, serious/life-threatening infections requiring hospitalization, avascular necrosis, and gastrointestinal ulcer/hemorrhage [17, 18].
Corticosteroid use in patients with SLE in the US is associated with greater HCRU and costs, especially at higher doses [19, 20]. Compared with patients receiving low-dose corticosteroids (< 5 mg/day), annual total healthcare costs were calculated to be 2.8 times higher in patients receiving high-dose corticosteroids (> 20 mg/day) and 0.7 times lower in patients not receiving corticosteroids [20]. Corticosteroid users have also been shown to be ~ 1.5 and ~ 2 times more likely to develop chronic and acute adverse clinical outcomes than non-users, resulting in higher treatment costs [21].
Longitudinal data reporting adverse clinical outcomes and HCRU are limited to US claims database analyses, with equivalent information not available for the UK healthcare system. In this study, we used hospital and primary care data from patients with SLE in the UK to investigate the incidence of adverse clinical outcomes, across multiple organ systems, and the related HCRU and costs over time for patients prescribed systemic corticosteroids (SCS) compared with those who were not.
Methods
Study Design
This was an observational, retrospective cohort study of adult patients with an initial diagnosis of SLE recorded between January 1, 2005, and June 30, 2019.
Data Sources
Patients were identified using routinely collected data from a network of general practices and hospitals across the UK and were followed from the date of SLE diagnosis to the end of the study period (June 30, 2019), date of last recorded visit before database exit, or death.
Three linked data sources were used to provide a longitudinal, representative UK population health dataset: the Clinical Practice Research Datalink (CPRD) GOLD, Hospital Episode Statistics (HES)-linked health care administrative databases, and Office for National Statistics (ONS) mortality files. CPRD GOLD provided primary care information and included data from 416 general practices for 10,800,187 patients eligible for linkage with HES. The HES database provided secondary care information (hospital admissions, accident and emergency department [A&E] visits, and International Classification of Diseases Tenth Revision [ICD-10] coding for diagnosis and type of admission). ONS provided death registration data to identify mortality and therefore patient exit from the data sources. All patient data and linkage using patient identifiers were anonymized.
The study was approved by the CPRD Independent Scientific Advisory Committee (ISAC protocol: 17_281) on January 28, 2021.
Patients
Patients aged ≥ 18 years presenting to a general practitioner or hospital with ≥ 1 diagnosis of SLE during the study period, and who could be linked to HES data, were eligible. To include only incident cases, patients were required to have ≥ 12 months of medical records without SLE codes or SCS prescriptions before the first (index) SLE diagnosis date.
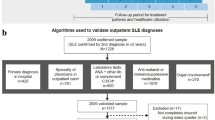
SLE diagnosis was based on diagnostic READ codes (CPRD GOLD) that aligned with those from previous studies where a panel of experts had defined the inclusion list [22], or ICD-10 codes in the HES database (Supplementary Materials Table S1). SLE diagnosis was verified using an adapted algorithm [23] to determine either repeat SLE diagnosis (in CPRD or HES) or rheumatologist outpatient appointment (in HES)/referral (in CPRD), SLE medication use (corticosteroids, immunosuppressive therapy, or hydroxychloroquine), or ≥ 2 records of SLE (≥ 60 days apart) plus ≥ 3 non-steroidal anti-inflammatory drug prescriptions in CPRD.
Patients were excluded if they had READ codes indicating subacute cutaneous, drug-induced, or discoid lupus rather than SLE, and for the HCRU cohort only, did not have ≥ 12 months of follow-up after the SCS index date.
Study Outcomes
SCS Use
SCS exposure, defined as oral or parenteral corticosteroids, was identified as any prescription for systemic prednisolone, methylprednisolone, prednisone, betamethasone, dexamethasone, triamcinolone, hydrocortisone, or cortisone acetate, either as monotherapy or combination therapy (Supplementary Materials Table S2). The population was grouped into patients prescribed SCS (≥ 1 SCS prescription, at any time from the date of first SLE diagnosis to the end of follow-up) and those who were not prescribed SCS.
The SCS index date was defined as the date of the first recorded prescription for a parenteral or oral corticosteroid in the SCS group. To ensure the same average time from SLE diagnosis to SCS index date (start of follow-up for adverse clinical outcomes) for both groups, patients in the non‐SCS group were assigned a proxy SCS index date, corresponding to the average time between first SLE diagnosis and date of first SCS prescription among patients who did receive SCS.
Information on dose and intended duration of treatment for each prescription was used to estimate mean daily exposure (total prednisolone-equivalent dosage divided by number of days of follow-up) and cumulative exposure (number of years at any dose) for the SCS group.
Adverse Clinical Outcomes
The presence of adverse clinical outcomes across multiple organ systems was identified by READ and ICD-10 codes for all patients, from SLE diagnosis to the end of the follow-up period, and included type 2 diabetes, myocardial infarction, heart failure, cerebrovascular accident, osteoporosis and fracture, glaucoma, renal impairment/failure, peptic ulcer, pneumonia, and cataract (Supplementary Materials Table S3).
To ensure only incident adverse clinical outcomes were included, separate risk cohorts for each were constructed. Patients who had the adverse clinical outcome before the SLE diagnosis date (12 months prior) were excluded.
HCRU and Costs
Calculation of HCRU and costs adhered to economic evaluation and cost of illness methodological recommendations [24, 25]. All analyses were conducted from the perspective of the UK National Health Service (NHS), and all-cause costs were estimated by multiplying the number of HCRU events by unit costs, expressed in 2019 British pound sterling (GBP).
Inpatient care was costed using the 2018–2019 Health Resource Group Reference Costs Grouper software [26] before applying reference costs for each category of stay, taken from the UK National Cost Schedule [27]. A&E and outpatient attendances and procedures were assigned the appropriate unit costs from the NHS “National Cost Collection” publication [28] by treatment specialty. All-cause primary care contacts, which included general practitioners (GP) consultations, out-of-hours consultations, telephone contacts, telephone/online consultations, home consultations, and day case consultations, were costed by multiplying the duration of each consultation by the average cost/minute based on a comprehensive estimate of general practice expenses [29]. All-cause medications were costed by mapping CPRD data to British National Formulary codes data and multiplying the prescribed quantity by the unit costs from the British National Formulary [30]. Validity of quantity, strength, and prescription dosages were checked manually.
Statistical Analyses
Descriptive statistics for characteristics at baseline (date of SLE diagnosis) were assessed for the SCS and non-SCS groups. Each of the adverse clinical outcome categories was examined separately (a patient could be included in ≥ 1 analysis) and all were combined into an overall category.
Each patient was followed until the first occurrence of the outcome of interest and was censored at death, study period end, or the date of the patient’s last recorded visit before leaving the database. Cumulative incidence of each specified adverse clinical outcome was calculated over 12-month discrete periods after the SCS index date. Probability of adverse clinical outcomes was also estimated using the Kaplan–Meier method and corresponding plots showed time to first adverse clinical outcome after the SCS index date. The temporal association between SCS use and subsequent hazard of adverse clinical outcomes was estimated using multivariable Cox proportional hazard models.
The primary analyses tested whether SCS treatment in the past 90 days predicted adverse clinical outcomes, for which SCS treatment was defined in two ways: maintenance SCS exposure (≥ 1 prescription in the past 90 days of < 7.5 mg/day prednisolone-equivalent dosage, but no prescriptions of ≥ 7.5 mg/day prednisolone-equivalent dosage) and high-dose SCS exposure (≥ 1 prescription in the past 90 days of ≥ 7.5 mg/day prednisolone-equivalent dosage). Sensitivity analyses were conducted by varying the duration of the prior exposure period to 180 days.
In the secondary analyses, associations between cumulative SCS exposure (number of years on SCS and high-dose SCS) and adverse clinical outcomes were also modeled. Each outcome model accounted for confounders including age, sex, Charlson Comorbidity Index (CCI), number of days of available data up to first SCS prescription, and body mass index (BMI). The original 19-category CCI was modified to 17 categories [31] and the list of specific ICD diagnosis codes used to identify different categories of comorbidity were modified [32] and updated from ICD-9 to ICD-10 coding [33, 34].
Analysis of HCRU
A follow-up period of ≥ 12 months post-SLE diagnosis date was required for the HCRU/cost cohort. Annualized HCRU and costs were compared between SCS and non-SCS groups using generalized estimating equations for all-cause healthcare costs. All models accounted for repeated measures and were adjusted for age, sex, CCI, BMI, and amount of data available up to first SCS prescription. Analyses were conducted using SAS software 9.4 (SAS Institute, Cary, NC, USA). Unadjusted means and standard deviations (SD), and median and interquartile ranges were estimated to summarize annual counts by type of utilization for patients with and without SCS use prior to the SLE diagnosis date and for each 12-month period up to 10 years following the SLE diagnosis date.
Results
Patient Disposition and Characteristics
From the CPRD database, 4414 patients had an SLE code and a patient record linked to HES identified in the study period. Of 936 patients who satisfied all inclusion criteria, 715 had no prior SCS prescriptions in the 12-month period before first SLE diagnosis and constituted the analysis set for assessment of adverse clinical outcomes associated with SCS use (Fig. 1). From the date of first SLE diagnosis to the end of follow-up, 301/715 patients received ≥ 1 prescription for SCS and 414 patients had no recorded SCS prescription. In the SCS group, 91 patients (30%) had ≥ 4 refill prescriptions of SCS/year and mean (SD) daily exposure was 3.2 (6.0) mg. A total of 55 patients (18%) had a mean daily exposure ≥ 7.5 mg and 28 (9%) patients had a mean daily exposure 5.0–< 7.5 mg.
Patient characteristics at SLE diagnosis were similar between the SCS and non-SCS groups, other than the proportion of mean and median duration of follow-up (Table 1).
Incidence of Adverse Clinical Outcomes
The cumulative incidence of any type of adverse clinical outcome from SCS/proxy SCS index date over a 10-year follow-up was 50% in the SCS group and 22% in the non-SCS group (Table 2, Supplementary Materials Figure S1). Among the individual adverse clinical outcomes, osteoporosis diagnosis/fracture was the most frequently reported, with greater cumulative incidence in the SCS than non-SCS group (38% versus 14% respectively) (Table 2).
When exposure to SCS was analyzed as any SCS treatment in the past 90 days, the adjusted hazard ratio (HR) for any type of adverse clinical outcome at any dosage was 2.41 (95% confidence interval [CI] 1.77, 3.26), with no notable difference by dosage level (Fig. 2). Sensitivity analyses did not change the result. Increased hazards associated with SCS use in the past 90 days were observed for osteoporosis diagnosis/fracture and myocardial infarction. When stratifying the SCS group by dosage, patients taking a high dosage of ≥ 1 prescription of ≥ 7.5 mg/day in the past 90 days had a greater hazard for type 2 diabetes, myocardial infarction, heart failure, osteoporosis diagnosis/fracture, and overall hazard of any adverse clinical outcome (2.77 [95% CI 1.67, 4.60]) compared with patients not using SCS (Fig. 2). Those on a low dosage had a greater hazard for osteoporosis diagnosis/fracture, and overall hazard of any adverse clinical outcome (2.28 [95% CI 1.60, 3.23]) than patients not using SCS.
Adjusted hazard ratios* for each adverse clinical outcome associated with SCS treatment† in the past 90 days. *Adjusted for age, sex, Charlson Comorbidity Index, number of days of available data up to first SCS prescription, and body mass index. †For the < 7.5 mg/day group, at least one prescription in the past 90 days of < 7.5 mg/day prednisolone-equivalent dosage, but no prescriptions ≥ 7.5 mg/day prednisolone-equivalent dosage in the same period; for the ≥ 7.5 mg/day group, at least one prescription in the past 90 days of ≥ 7.5 mg/day prednisolone-equivalent dosage. The number of events for peptic ulcer were very low and are not included. CI confidence interval, SCS systemic corticosteroids
Each additional year of SCS exposure was associated with an increased hazard at any dosage compared with no SCS use (adjusted HR 1.12 [95% CI 1.07, 1.17]) and at high dosage compared with no SCS use (adjusted HR 1.15 [95% CI 1.05, 1.27]) (Fig. 3).
Adjusted hazard ratios* for each adverse clinical outcome associated with number of years† using SCS at any dosage and at high dosage‡. *Adjusted for age, sex, Charlson Comorbidity Index, number of days of available data up to first SCS prescription, and body mass index. †Number of years measured as a time-varying predictor. ‡High-dosage SCS: exposure to ≥ 7.5 mg/day prednisolone-equivalent dosage. The number of events for peptic ulcer were very low and are not included. CI confidence interval, SCS systemic corticosteroids
HCRU and Costs
Annualized all-cause HCRU and costs/patient from SCS/proxy SCS index date by SCS exposure, which included only patients with a SCS index date follow-up of ≥ 12 months, are reported in Table 3. Mean annualized all-cause HCRU and unadjusted costs were higher in the SCS group than the non-SCS group for all treatment specialties. All-cause mean (SD) total costs were more than double for patients with low dose SCS (5.0–< 7.5 mg; GBP 7884 [7803]) and more than three times greater for patients on high dose SCS (15.0 mg; GBP 13929 [6453]) than patients in the non-SCS group (GBP 3842 [4455]). After adjusting for confounders, mean annualized costs were significantly greater for SCS users than non-users, increasing with mean daily SCS exposure. Adjusted mean differences in annualized costs/patient versus no SCS exposure were: GBP 1403 (95% CI 416, 2391) for mean daily exposure > 0–< 5.0 mg; GBP 3110 (95% CI 856, 5363) for 5.0–< 7.5 mg; GBP 5242 (95% CI 2492, 7991) for 7.5–< 15.0 mg; and GBP 9096 (95% CI 7039, 11,153) for ≥ 15.0 mg.
Discussion
In this study of newly diagnosed adult patients with SLE in the UK, the probability of experiencing an adverse clinical outcome across multiple organ systems over the follow-up period was higher for patients who used SCS than for those who did not, irrespective of the dosage. Indeed, the cumulative incidence of any adverse clinical outcome over 10 years was more than double in the SCS group (50%, from time of SCS index date) compared with the non-SCS group (22%, from proxy SCS index date). Each additional year of SCS exposure at any dosage and at high dosage was associated with an increased hazard for any adverse clinical outcome compared with no SCS use. During this period, the highest cumulative incidence of adverse outcomes was for osteoporosis diagnosis/fracture. Our study also revealed significantly higher costs in the SCS exposure groups that increased with greater daily SCS exposure.
Our results are consistent with those seen in a study of patients with SLE in the United States, where SCS users were ~ 2 and ~ 1.5 times as likely to develop acute and chronic adverse clinical outcomes than non-users [21]. Moreover, in our study, when exposure to SCS was used as a time-dependent covariate, patients exposed to any SCS dosage in the previous 90 days were predicted to be ~ 2.5 times more likely to have an adverse clinical outcome than patients who did not use SCS. Shah et al. also showed an increased risk of AEs as patients increased the mean daily corticosteroid dose from < 7.5 to ≥ 7.5 mg, compared with no corticosteroid use [21], consistent with our findings. Furthermore, we determined that each additional year of receiving SCS treatment at any dosage was associated with a 12% increase in the risk of any adverse clinical outcome and a 15% increase at higher dosages (> 7.5 mg/day).
The highest cumulative incidence of individual outcomes was for osteoporosis diagnosis/fracture. This was not unexpected given the associated increased risk of osteoporosis with corticosteroid use [35], sex (88.5% women) and age (53.4% > 45 years). Previous studies have reported the association between older age and increased incidence of fractures and osteoporosis in both men and women receiving corticosteroids [36, 37]. One UK study found that hip fracture rates in women receiving medium- (2.5–< 7.5 mg/day) or high-dose corticosteroids (≥ 7.5 mg/day) increased from 0.1 cases/100 patients in women aged 45–54 years to 1.4 cases/100 patients in those aged ≥ 85 years [36]. Another study of South African data showed that hip fractures were more frequent in men than in women; however, women receiving corticosteroids were more susceptible to osteoporosis [38]. Other individual adverse outcomes with notable increases in hazard with high-dosage SCS included type 2 diabetes, myocardial infarction, and heart failure, which have previously been reported to have a dose– or duration–risk relationship with SCS use [39, 40]. The diverse range of organs affected reflects the complex and multifactorial actions of SCS on different tissues [41]. Taken together, these data highlight the risks of different adverse outcomes for patients with SLE using SCS and strengthen the notion that although current EULAR guidelines recommend the use of corticosteroids for rapid symptom relief, they also recommend minimizing the daily dose or, where possible, discontinuing corticosteroids due to the associated risks [1]. In efforts to minimize adverse clinical outcomes, there is a need for SCS-sparing efficacy in SLE treatment strategies.
The higher costs seen in the SCS exposure group compared with the non-SCS group are consistent with observations from previous analyses of US claims databases [19,20,21]. We found that patients receiving SCS doses of 7.5–15.0 mg, and those receiving high doses ≥ 15.0 mg, had increased annual costs of GBP 5242 and GBP 9096, respectively, compared with patients not receiving SCS. These are similar to the increased costs identified by Chen et al. [19]. Higher costs in patients receiving SCS could be attributed to more active disease in this patient group. Although it was not possible to report the severity of SLE for patients in this study as these data are not routinely collected in the CPRD database), a previous study using an algorithm to assess severity in a similar cohort of patients found a high proportion were classified as having mild disease [2], and this may account for why only ~ 40% of patients in this study had received SCS, which is comparable to a recent retrospective database study conducted in the US [42]. An alternative or additional explanation could be that the adverse clinical outcomes resulting from SCS administration are increasing HCRU and therefore costs [17].
One of the strengths of this study was the use of individual patient-level data extracted from an established large nationwide general practice records database. This database contains validated high-quality data and extensive patient records linked to hospital and mortality records, making these findings generalizable to patients with SLE treated in the primary care setting in the UK. Following patients over 10 years also improves real-world understanding of longitudinal trends in adverse clinical outcomes associated with corticosteroid use. The CPRD GOLD database has been widely used in SLE-related studies, mostly to characterize the disease and its incidence in the UK [2, 22, 43, 44], but also to investigate risks associated with SLE [45,46,47], recently using a case-identification algorithm that was adapted for this study [23]. Another study strength was examination of patients at initiation of SCS use, enabling analysis of the ongoing effects of SCS use, while excluding patients who had been exposed to SCS prior to SLE diagnosis.
This study has a number of limitations. Disease activity is not accounted for in this study. Previous studies have reported an association of disease activity and corticosteroid use on adverse clinical outcomes [48], with corticosteroid use being among the most important predictors of damage accrual [49]. Corticosteroid use is recommended for rapid symptom relief in patients with higher disease activity [1, 6]. Therefore, there is likely to be an interplay between corticosteroid use and disease activity in adverse clinical outcomes. Our results did adjust for comorbidities; however, given the retrospective nature of the study design, it was not possible to attribute all adverse outcomes to use of corticosteroid therapy alone and therefore caution should be employed when interpreting the results.
In addition, the retrospective nature of this study and the use of an administrative database leads to additional challenges. The CPRD GOLD database has HES linkage data available for only 50% of contributing practices; records may have had missing data (e.g., SCS dose and recording of prescription), other potential biases are possible (e.g., misclassification biases, or inconsistencies in coding within/among practices and over time), and the dataset does not take into account either adherence to treatment or potential losses of prescriptions. To reduce potential misclassification, this study required linkage with HES and, for the HCRU cohort, ≥ 12 months of follow-up. The requirement for a verification of diagnosis of SLE may underestimate the true number of SLE cases and enrich for patients with more active disease. However, this potential bias was small, as only 133 patients (2.4%) were excluded because verifying information was missing from their record.
Furthermore, the coding system in the CPRD GOLD is not granular enough to distinguish between different types of fractures (e.g., minimal trauma fractures with vs without osteoporosis, or fractures by external force). We accept that separating out osteoporosis and fractures should be included in future studies, where this is possible. This was out of scope of the current study.
Although index dates were aligned between the SCS and non-SCS groups and accounted for several confounders in the adjusted analyses (age, sex, CCI, number of days of available data up to first SCS prescription, and BMI), there is the potential for residual confounding from unrecognized and/or unmeasured factors, including the presence of comorbid conditions that frequently require corticosteroids that are not measured by the CCI (e.g., allergies). There could also be bias in the identification for certain outcomes in the SCS arm as patients may be more likely to be screened for these conditions while receiving SCS (e.g., type 2 diabetes and osteoporosis). Other limitations include small patient numbers for some analyses, and drugs such as biologics, which are prescribed in the specialist setting, not being routinely available in the CPRD and therefore not included in this study.
Conclusions
In patients with SLE in the UK, there is a higher burden of adverse clinical outcomes and greater use of healthcare resources in SCS users, especially among patients receiving high SCS dosages, compared with non-SCS users. This could, in part, also be due to higher disease activity in this group or higher comorbidity burden potentially increasing SCS use and thus adverse outcomes. The lower incidence of adverse clinical outcomes in the non- and low-SCS groups than in the SCS users group may indicate the need for SCS-sparing efficacy in SLE treatment. Awareness of SCS-related adverse clinical outcomes and their burden on the patient and the healthcare system is an important consideration for patients with SLE, and future patient management should identify SCS-sparing treatment strategies early in the treatment pathway.
References
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–45.
Langham J, Barut V, Samnaliev M, Langham S, Weir S, Wang X, et al. Disease severity, flares and treatment patterns in adults with systemic lupus erythematosus in the UK: a real-world observational retrospective cohort analysis. Rheumatol Adv Pract. 2021;5(3):rkab061.
Katz P, Nelson WW, Daly RP, Topf L, Connolly-Strong E, Reed ML. Patient-reported lupus flare symptoms are associated with worsened patient outcomes and increased economic burden. J Manag Care Spec Pharm. 2020;26(3):275–83.
Hammond ER, Desta B, Near AM, Wang X, Jiang M. Frequency, severity and costs of flares increase with disease severity in newly diagnosed systemic lupus erythematosus: a real-world cohort study, United States, 2004–2015. Lupus Sci Med. 2021;8(1): e000504.
Justiz Vaillant AA, Goyal A, Bansal P, Varacallo M. Systemic lupus erythematosus. StatPearls. Treasure Island: StatPearls Publishing LLC; 2021.
Gordon C, Amissah-Arthur MB, Gayed M, Brown S, Bruce IN, D’Cruz D, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults: executive summary. Rheumatology (Oxford). 2018;57(1):14–8.
Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–8.
Al Sawah S, Zhang X, Zhu B, Magder LS, Foster SA, Iikuni N, et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins Lupus Cohort. Lupus Sci Med. 2015;2(1): e000066.
Tarr T, Papp G, Nagy N, Cserep E, Zeher M. Chronic high-dose glucocorticoid therapy triggers the development of chronic organ damage and worsens disease outcome in systemic lupus erythematosus. Clin Rheumatol. 2017;36(2):327–33.
Apostolopoulos D, Kandane-Rathnayake R, Raghunath S, Hoi A, Nikpour M, Morand EF. Independent association of glucocorticoids with damage accrual in SLE. Lupus Sci Med. 2016;3(1): e000157.
Barber MRW, Hanly JG, Su L, Urowitz MB, St Pierre Y, Romero-Diaz J, et al. Economic evaluation of damage accrual in an international systemic lupus erythematosus inception cohort using a multistate model approach. Arthritis Care Res. 2020;72(12):1800–8.
Clarke AE, Yazdany J, Kabadi SM, Durden E, Winer I, Griffing K, et al. The economic burden of systemic lupus erythematosus in commercially- and Medicaid-insured populations in the United States. Semin Arthritis Rheum. 2020;50(4):759–68.
Jiang M, Near AM, Desta B, Wang X, Hammond ER. Disease and economic burden increase with systemic lupus erythematosus severity 1 year before and after diagnosis: a real-world cohort study, United States, 2004–2015. Lupus Sci Med. 2021;8(1): e000503.
Murimi-Worstell IB, Lin DH, Kan H, Tierce J, Wang X, Nab H, et al. Healthcare utilization and costs of systemic lupus erythematosus by disease severity in the United States. J Rheumatol. 2021;48(3):385–93.
Samnaliev M, Barut V, Weir S, Langham J, Langham S, Wang X, et al. Health-care utilization and costs in adults with systemic lupus erythematosus in the United Kingdom: a real-world observational retrospective cohort analysis. Rheumatol Adv Pract. 2021;5(3):rkab071.
Schwarting A, Friedel H, Garal-Pantaler E, Pignot M, Wang X, Nab H, et al. The burden of systemic lupus erythematosus in Germany: incidence, prevalence, and healthcare resource utilization. Rheumatol Ther. 2021;8(1):375–93.
Petri M, Bechtel B, Dennis G, Shah M, McLaughlin T, Kan H, et al. Burden of corticosteroid use in patients with systemic lupus erythematosus: results from a Delphi panel. Lupus. 2014;23(10):1006–13.
Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204.
Chen SY, Choi CB, Li Q, Yeh WS, Lee YC, Kao AH, et al. Glucocorticoid use in patients with systemic lupus erythematosus: association between dose and health care utilization and costs. Arthritis Care Res. 2015;67(8):1086–94.
Kabadi S, Yeaw J, Bacani AK, Tafesse E, Bos K, Karkare S, et al. Healthcare resource utilization and costs associated with long-term corticosteroid exposure in patients with systemic lupus erythematosus. Lupus. 2018;27(11):1799–809.
Shah M, Chaudhari S, McLaughlin TP, Kan HJ, Bechtel B, Dennis GJ, et al. Cumulative burden of oral corticosteroid adverse effects and the economic implications of corticosteroid use in patients with systemic lupus erythematosus. Clin Ther. 2013;35(4):486–97.
Rees F, Doherty M, Grainge M, Davenport G, Lanyon P, Zhang W. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann Rheum Dis. 2016;75(1):136–41.
Nightingale AL, Davidson JE, Molta CT, Kan HJ, McHugh NJ. Presentation of SLE in UK primary care using the Clinical Practice Research Datalink. Lupus Sci Med. 2017;4(1): e000172.
Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313(7052):275–83.
Onukwugha E, McRae J, Kravetz A, Varga S, Khairnar R, Mullins CD. Cost-of-illness studies: an updated review of current methods. Pharmacoeconomics. 2016;34(1):43–58.
NHS Digital. HRG4+ 2018/19 reference costs grouper application 2019. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/costing---hrg4-2018-19-reference-costs-grouper. Accessed 8 Mar 2022.
NHS Digital. 2018/19 national cost collection data 2019. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/reference-costs. Accessed 8 Mar 2022.
NHS. 2018/19 national cost collection data publication 2019. https://www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/. Accessed 8 Mar 2022.
Curtis LA. Unit costs of health and social care 2019. Canterbury: PSSRU, University of Kent; 2019.
NHS Business Services Authority. Prescription cost analysis (PCA) data: 2019 data 2019. https://www.nhsbsa.nhs.uk/prescription-data/dispensing-data/prescription-cost-analysis-pca-data. Accessed 8 Mar 2022.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Romano P, Roos L, Jollis J. Further evidence concerning the use of a clinical comorbidity index with ICD-9-CM administrative data. J Clin Epidemiol. 1993;46(10):1085–90.
Halfon P, Eggli Y, van Melle G, Chevalier J, Wasserfallen JB, Burnand B. Measuring potentially avoidable hospital readmissions. J Clin Epidemiol. 2002;55(6):573–87.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open. 2015;1(1): e000014.
van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HG. Public health impact of adverse bone effects of oral corticosteroids. Br J Clin Pharmacol. 2001;51(6):601–7.
Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15(4):323–8.
Rothberg AD, Matshidze PK. Monitoring and management of bone status in patients on chronic glucocorticoid treatment—the Medscheme experience. S Afr Med J. 2000;90(11):1125–9.
Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975–94.
Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216–29.
DA Ruiz-Irastorza G, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford). 2012;51(7):1145–53.
Bell CF, Ajmera MR, Meyers J. An evaluation of costs associated with overall organ damage in patients with systemic lupus erythematosus in the United States. Lupus. 2022;31(2):202–11.
Nightingale AL, Farmer RD, de Vries CS. Incidence of clinically diagnosed systemic lupus erythematosus 1992–1998 using the UK General Practice Research Database. Pharmacoepidemiol Drug Saf. 2006;15(9):656–61.
Somers EC, Thomas SL, Smeeth L, Schoonen WM, Hall AJ. Incidence of systemic lupus erythematosus in the United Kingdom, 1990–1999. Arthritis Rheum. 2007;57(4):612–8.
Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol. 2004;93(2):198–200.
Bultink IE, Harvey NC, Lalmohamed A, Cooper C, Lems WF, van Staa TP, et al. Elevated risk of clinical fractures and associated risk factors in patients with systemic lupus erythematosus versus matched controls: a population-based study in the United Kingdom. Osteoporos Int. 2014;25(4):1275–83.
Rees F, Doherty M, Grainge MJ, Lanyon P, Davenport G, Zhang W. Mortality in systemic lupus erythematosus in the United Kingdom 1999–2012. Rheumatology (Oxford). 2016;55(5):854–60.
Urowitz MB, Gladman DD, Ibañez D, Su J, Mursleen S, Sayani A, et al. Effect of disease activity on organ damage progression in systemic lupus erythematosus: University of Toronto Lupus Clinic Cohort. J Rheumatol. 2021;48(1):67–73.
Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum. 2012;64(12):4021–8.
Acknowledgements
Funding
Funding for this work, including the journal’s Rapid Service Fee, was supported by AstraZeneca. The funder of the study had a role in its design, interpretation of the data, and in the writing of the manuscript. The funder had no role in the conduct, collection, or analysis of the data.
Medical Writing, Editorial, and Other Assistance
Writing and editing assistance was provided by Victoria Alikhan, PhD, and Rebecca Jones, PhD, of JK Associates Inc., part of Fishawack Health. This assistance was funded by AstraZeneca.
Author Contributions
Sarowar Golam, Barbara Naisbett-Groet, Julia Langham, Sue Langham, Danny Gibson, and Heide Stirnadel-Farrant designed the research. Julia Langham, Sue Langham, and Mihail Samnaliev conducted the research. Mihail Samnaliev performed the statistical analysis. All authors contributed to the data interpretation and revised each draft for important intellectual content. All authors read and approved the final manuscript. Heide Stirnadel-Farrant had primary responsibility for the final content and is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosures
Heide Stirnadel-Farrant, Sarowar Golam, Barbara Naisbett-Groet, and Danny Gibson are employees of AstraZeneca. Julia Langham and Sue Langham are consultants and have worked on behalf of AstraZeneca. Mihail Samnaliev is currently an employee of Axtria.
Compliance with Ethics Guidelines
All patient data and linkage using patient identifiers were anonymized. The study was approved by the CPRD Independent Scientific Advisory Committee (ISAC protocol: 17_281) on January 28, 2021.
Data Availability
All data relevant to the study are included in the article or Supplementary Material. This study is based in part on data from CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The Office for National Statistics provided the mortality data. The interpretation and conclusions contained in this study are those of the authors alone. The authors do not own these data and hence are not permitted to share the data in the original form.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Stirnadel-Farrant, H.A., Golam, S.M., Naisbett-Groet, B. et al. Adverse Outcomes, Healthcare Resource Utilization, and Costs Associated with Systemic Corticosteroid use Among Adults with Systemic Lupus Erythematosus in the UK. Rheumatol Ther 10, 1167–1182 (2023). https://doi.org/10.1007/s40744-023-00566-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00566-w