Abstract
Introduction
This study sought to analyze the benefit of an early induction therapy with a biological disease-modifying anti-rheumatic drugs (bDMARD) during the first year of treatment with a 5-year follow-up in early rheumatoid arthritis (ERA).
Methods
We included ERA patients from the UCLouvain Brussels cohort who met the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 classification criteria and were naïve to DMARDs. ERA patients were divided into two groups according to whether they received an induction bDMARD therapy or a standard therapy with methotrexate (MTX). Clinical response after the induction treatment at 6 and 12 months followed by a MTX maintenance therapy at 36 and 60 months was evaluated.
Results
Data from 470 ERA patients were collected, 189 received a bDMARD and 281 initiated MTX alone. In the bDMARD group, disease activity and HAQ were higher at baseline. A total of 391 patients were followed up to 5 years. We then divided each group into two subgroups according to the last treatment they received at 5 years: bDMARD > MTX (n = 95), bDMARD > bDMARD (n = 59); MTX > MTX (n = 134), MTX > bDMARD (n = 103). During the induction, we observed a clinical response with a large number of patients achieving DAS28-CRP remission. According to a treat-to-target (T2T) approach, remission rate was stable on MTX monotherapy or rescued by the addition or prolongation of a bDMARD. Interestingly, bDMARD followed by a MTX maintenance therapy experienced a stable and sustained DAS28-CRP remission rate in 53% of the ERA patients at year 5.
Conclusions
Long-term remission is an achievable goal in ERA. Our results suggest that a bDMARD induction therapy followed by MTX maintenance therapy could be an interesting option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Early diagnosis and therapeutic induction are essential in improving the prognosis for rheumatoid arthritis (RA). |
The aim of this retrospective study is to analyze and compare the benefit of a treatment with a biological disease-modifying anti-rheumatic drugs (bDMARD) as an induction therapy versus methotrexate (MTX). |
What was learned from the study? |
Long-term remission is an achievable goal in early stage RA followed by daily clinical treatment. |
When a bDMARD is started as a first-line therapy followed by MTX only, a large number of ERA patients experienced a stable and sustained DAS28-CRP remission rate. |
bDMARD followed by maintenance therapy with MTX may be a good option in early RA. |
Introduction
Rheumatoid arthritis (RA) is an immune-mediated disease characterized by chronic joint inflammation that leads to structural damage [1]. While improving the signs and symptoms of RA is essential, clinical remission is a key goal of treatment in early RA (ERA) [2, 3]. To achieve remission, early diagnosis and therapeutic interventions are essential, since RA can lead to major deformities and irreversible disability. Over the past 30 years, therapeutic developments have led to better outcomes with care strategy such as the “treat-to-target” approach and the availability of more efficacious conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) mainly methotrexate (MTX) and biological therapies (bDMARDs) or JAK inhibitors [2, 4].
Patients with ERA with poor prognostic factors such as rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA), high disease activity, and baseline erosions have poor outcomes [5, 6]. Initiation of early, intensive treatment is recommended for such patients. When a bDMARD is added early to MTX, significantly greater improvements in disease activity status, rates of remission, and radiographic outcomes are observed compared with MTX alone [7,8,9]. However, there is a lack of consensus on when to initiate biologic therapy, and for economic reasons, the access to bDMARDs is restricted to second-line therapy.
The aim of this study is to analyze and compare the benefit of a treatment with a bDMARD as an induction therapy during the first year with a 5-year follow-up in early rheumatoid arthritis (ERA) patients.
Methods
Study Design
We included ERA patients from the UCLouvain Brussels cohort who met the ACR/EULAR 2010 classification criteria and were naïve to DMARDs. ERA UCLouvain Brussels is a single-site cohort and was started in 2005 at our arthritis clinic of academic Saint Luc hospital (UCLouvain) in Brussels. Treatments were initiated based on the decision of a senior rheumatologist. The conventional treatment consists of MTX starting from 7.5 to 15 mg weekly with folic supplementation associated or not with temporary and low dose of prednisolone. ERA patients with poor prognostic factors were allowed to have an early access program with bDMARD limited to 6–12 months. We collected ERA patients’ characteristics (from 2005 to 2021) at baseline and clinical response was analyzed during period 1 (induction) at 6 and 12 months followed by period 2 (maintenance) at 3 and 5 years.
Patients were followed at our academic center. Clinical evaluations performed at every visit included the 28 and 66/68 swollen and tender joint counts, CRP (mg/l), Health Assessment Questionnaire (HAQ) (4), Physician’s Global Assessment of Disease Activity using a visual analogue scale (VAS 0–100 mm), Patient’s Global Assessment of Disease Activity (VAS 0–100 mm).
Along with the clinical evaluations performed on the day of every visit, all physicians completed an evaluation of the 5-year experience. The evaluation provided an assessment of patients on therapy.
All these patients were prospectively assessed for clinical responses and a T2T method with tight control was applied during the 5-year follow-up. As an add-on therapy, bDMARDs were initiated according to the criteria for reimbursement in Belgium (failure to MTX and another csDMARDs with a DAS28-CRP above 3.7).
Data from 470 included patients were analyzed. As a first-line therapy, 189 patients initiated a bDMARD combined or not with MTX (group 1). A total of 281 patients were treated with a standard regimen of MTX (group 2). bDMARDs induction therapy (mainly TNF inhibitors) was usually limited to 6–12 months. In Belgium, bDMARDs are not prescribed and reimbursed as a first-line therapy. The access to such an agent was possible in our academic hospital as an early access program of bDMARDs limited to an induction program in severe RA with poor prognosis factors. We evaluated the different patients characteristics at baseline (before treatment), in the all cohort and in the two groups: age at RA diagnosis, gender, family history of RA, smoking status, presence of rheumatoid factor (RF) or ACPA, presence of radiological erosion and joint space narrowing, tender and swollen joints, CRP, DAS-28CRP, CDAI, SDAI, VAS pain, VAS fatigue and HAQ. DAS28-CRP remission was defined as < 2.6.
For the long-term analysis, we divided the two groups (bDMARD or MTX) into two subgroups according to the last treatment they received at 5 years.
At 5 years, 95 patients from group 1 initiated a bDMARD and then continued with MTX alone and 59 patients continued or were rescued with a bDMARD. In group 2, 134 patients received only MTX and 103 patients received first MTX and then were rescued by a bDMARD.
We then analyzed the number of consecutive bDMARDs administered in each of the subgroups.
The ERA UCLouvain Brussels cohort was approved by our academic institution and our local ethic committee (Ethic Committee of Cliniques Universitaire Saint-Luc, Brussels) for different protocols. All studies were performed in accordance with the Helsinki Declaration of 1964 and all its later amendments. All study participants gave their consent to participate in this study and to use their data in accordance with the Belgian law of July 30, 2018 on the protection of privacy and the European regulations (European General Regulation on the Protection of Personal Data RGPD of May 25, 2018) in force, the law of August 22, 2002 on patients' rights and the law of May 7, 2004 on human experimentation. This global retrospective analysis does not require further approval.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics Version 26 and GraphPad Prism Version 5. The descriptive data are expressed as the average ± SD (95% CI) or the percentage. A p value < 0.05 was considered statistically significant. Differences in continuous variables between two groups were analyzed by t test or Mann–Whitney test depending on normality. Levene’s test was used to analyze the equality of variance.
Chi-squared or Fisher’s exact test was applied to analyze the significance of the association between categorical variables.
Multivariate linear regression analysis was used to predict the disease evolution scores based on the different other variables.
Results
Characteristics of the Baseline
The average age of the population is 48.9 years; 70.4% of the patients are women; 27.3% are smokers and 68.8% are positive for ACPA.
Data from 189 ERA patients (40.2%) who received a bDMARD (group 1) and 281 ERA patients (59.8%) with MTX monotherapy (group 2) were collected and summarized in Table 1. As expected, disease activity, HAQ scores, ACPA, and RF positivity were statistically higher in group 1.
Other parameters such as age, gender, smoking habits, or baseline erosion were similar between groups.
Clinical Response
A total of 391 patients were analyzed during a 5-year follow-up with a 6-monthly visit according to a T2T strategy.
During the induction (period 1), we observed a clinical response with a large number of patients achieving remission. The percentage of DAS28-CRP, CDAI, and SDAI remission (low disease activity) rate was respectively 51.9% (64.6%), 38.6% (70.3%), 38.9% (69.0%) in group 1 and 43.1% (59.5%), 28.4% (64.4%), 27.6% (63.8%) in group 2 at 6 months.
According to the maintenance and last treatment received at year 5 (period 2), four subgroups were defined: group 1a bDMARD at baseline followed by MTX at 5 years (n = 95, 24.3%): bDMARD > MTX; group 1b bDMARDs during 5 years (n = 59, 15,1%): bDMARD > bDMARD; group 2a only MTX during 5 years (n = 134, 34.3%): MTX > MTX; 2b MTX rescued by bDMARDs (n = 103, 26.3%): MTX > bDMARD.
We analyzed the baseline characteristics of the patients in the subgroups. No significant difference was observed between group 1a and 1b, except for age and baseline erosions (Table 2). Patients on long-term MTX therapy (group 2a) have statistically better baseline prognostic factors such as lower swollen joint count (SJC), HAQ score, RF positivity, and absence of erosions compared to the group 2b (Table 3).
The remission and low DAS28-CRP, CDAI, and SDAI rates were calculated in the two groups at baseline, and after 6 and 12 months, as well as at a long term: after 3 and 5-year follow-up.
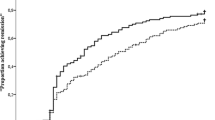
Figure 1 shows the DAS28-CRP, CDAI, and SDAI response rate during 5 years of follow-up in the four subgroups, summarizing the remission rates at 6–12–36 and 60 months. DAS28-CRP response rate was statistically significantly higher in groups 1a and 2a at 12 months compared to the two other groups. As expected, the remission rate was rescued in the group 2b after 12 months.
In addition, ERA patients initially treated by a bDMARD induction followed by a MTX (group 1a) maintenance therapy experienced a stable and sustained rate of DAS28-CRP remission in 53% of the patients at year 5.
Treatment evolution considered as number of bDMARDs prescribed during 5 years in the four groups is analyzed in Table 4.
A higher number of bDMARDs was administered in groups 1b and 2b. The patients from these two groups showed a lower DAS28-CRP remission rate at 6 months (Fig. 1).
Discussion
The aim of our study is to analyze if an induction therapy with a bDMARD is followed by a better outcome during a 5-year follow-up in ERA patients. Remission or at least low disease activity is an important goal for patients with early RA since attaining remission minimize destruction and improve the quality of life [11]. The EULAR guidelines clearly indicate these objectives with the use of MTX as first-line therapy [2]. The remission rate depends on the initial response, which should be achieved within the first 6 months [2]. Data from the CAMERA and Swefot studies have shown that adequate follow-up with MTX significantly reduces the rate of RA activity [13, 14]. However, a recent review indicates that the remission rate with MTX in clinical trials does not exceed 50% [15]. This is reflected in our standard cohort where the response rate varies between 30 and 54% in the MTX group at 6 months. We then divided the patients treated with MTX—the good responders who maintained this treatment for 5 years and those less responders in whom a bDMARD therapy was added. In the MTX good responder, our subgroup 2a, we observed a DAS28-CRP remission rate of 72% at 5 years. In the MTX less responder group 2b, only 40% fulfilled the remission criteria at 12 months and a rescue therapy with a bDMARD was therefore indicated up to 5 years. This step-up approach with different bDMARDs and recently JAK kinase inhibitors has been widely explored in randomized clinical trials to evaluate the clinical efficacy and to register such drugs in RA patients not responding to MTX [16]. The treatment strategy for early RA can be based on different conventional or innovative approaches. In order to optimize the treatment response in ERA, the BEST study has compared four strategies from switch to step-up therapies and more intensive therapies combining glucocorticoids (COBRA regimen) or bDMARDs [12]. In the best study, combination therapies including bDMARD were more effective if given early in the disease as compared with a delayed introduction, giving support to the window of opportunity hypothesis. MTX in a large number of ERA patients could be considered as insufficient to control disease activity. Therefore, induction therapy with a bDMARD as a first-line therapy combined with MTX could be an innovative and valuable strategy in severe ERA patients. We confirmed this in our cohort with a large rate of remission in 51.9% of patients after a bDMARD induction. In daily practice, the access to bDMARDs in ERA is very restricted for economic reasons with access and reimbursement highly variable between countries [17]. In our group 2b, we demonstrated that 71% of our patients received only one bDMARD after 5 years but the other patients have received up to 4–5 bDMARDs. This highlights the need to identify such non-responder patients and to find predictive markers of response to MTX or bDMARDs. Smoking habits, high CRP value, or female gender were associated with poor MTX response in the Swefot study [18]. In our cohort, we confirmed an association between MTX response and a lower DAS28-CRP, HAQ functional index, and presence of autoantibodies (RF and ACPA) at baseline. The retention rate of MTX therapy in our patients was over 50% after 5 years and similar to that observed in the Espoir cohort [19]. In the CareRA-plus follow-up study, 56% of all patients never had their MTX therapy intensified during the 5-year follow-up [20]. In our study, no other DMARD was used and glucocorticoid was only administered in 38% of our patients, mainly in the MTX group without any influence on early and 5-year response [21].
The therapeutic approach based on an early induction with a bDMARD combined or not with MTX is based on previous studies in ERA [7,8,9,10, 24, 25]. Early induction strategy was recently evaluated in the Nord-Star study and all three biological treatment arms and one active conventional arm achieved high remission rates with a superiority for abatacept [26]. Such induction therapy has been applied in other inflammatory rheumatic diseases such as systemic lupus and vasculitis [22, 23]. All bDMARDs and JAK inhibitors have been explored in ERA population naïve to MTX [7,8,9,10, 24, 25]. As expected, this approach was selected for more severe patients with higher DAS28-CRP, HAQ, and presence of RF and ACPA autoantibodies. Such population was evaluated in the AGREE study and reported only 41% of patients achieving remission in the combination arm with abatacept [24]. This percentage was higher in our cohort and reflects the number observed in the COMET and AVERT trial [9, 27]. However, the long-term benefit of a bDMARD induction therapy has not been widely evaluated in a randomized clinical trial. Only the GO-BEFORE study reported data at 5 years but the study design did not allow to discontinue golimumab [28]. Our data analyzed a group (1a) of severe ERA treated with a bDMARD during 6 to 12 months followed by a maintenance treatment on MTX; 72.8 and 65.6% of these patients reached the DAS28-CRP remission criteria at 12 months and 5 years, respectfully. Our data are supported by the second phase of the OPTIMA trial in which a significant higher proportion of patients achieved remission in the combination arm compared to MTX alone [10]. In the best study, discontinuation of infliximab was successful in 52% of patients [29]. Therefore, bDMARD induction therapy followed by MTX could be an attractive therapy in severe ERA and long-term pharmacoeconomics analysis could be valuable in such an approach. Additionally, a reset of the immune system could be induced by early exposure to selected bDMARD or regulatory cells [30].
The strength of our study is the large ERA population followed and analyzed strictly in a single academic center. Another strength is the 5-year follow-up, which allows analyzing the short- and long-term benefit of a bDMARD induction therapy followed by a MTX maintenance.
There are several limitations in our study. The first is the imbalance between the two groups’ population characteristics at baseline according to an early access program of bDMARDs in ERA with poor prognostic factors. The bias is explained by the differences in terms of baseline disease activity and severity (DAS, HAQ scores, ACPA, and RF positivity were statistically higher) observed in the severe ERA population treated with an induction bDMARD strategy. Another limitation is that using bDMARD as the first-line therapy is not possible in many countries and this early induction therapy may not be generalizable to health care settings. Another limitation is the absence of randomization and treatment harmonization guided in this 5-year prospective ERA cohort. We could therefore not compare directly the remission rates, since confounders influence the data observed.
Conclusions
Treatment of ERA with a bDMARD used as a short first-line induction followed by a long-term maintenance therapy with MTX is effective for 5 years. This confirms that the concept of an early short-term use of a bDMARD in ERA patients with poor prognostic factors could be developed as an induction therapy and long-term data could also include pharmacoeconomic aspects of such an approach. At this time, the induction remission approach followed by a MTX maintenance is not feasible in daily care. We need then future work such as a randomized 5-year prospective study to better support this approach in ERA and to demonstrate a better long-term clinical and radiological benefit.
References
Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–22.
Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26.
Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–7.
Kroot EJ, de Jong BA, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000;43(8):1831–5.
van Nies JA, van Steenbergen HW, Krabben A, et al. Evaluating processes underlying the predictive value of baseline erosions for future radiological damage in early rheumatoid arthritis. Ann Rheum Dis. 2015;74(5):883–9.
St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50(11):3432–43.
Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37.
Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–82.
Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet. 2014;383(9914):321–32 (published correction appears in Lancet. 2014 Jan 25;383(9914):308).
Radner H, Smolen JS, Aletaha D. Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Res Ther. 2014;16(1):R56 (Published 2014 Feb 21).
Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52(11):3381–90.
van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374(9688):459–66.
Jurgens MS, Welsing PM, Geenen R, et al. The separate impact of tight control schemes and disease activity on quality of life in patients with early rheumatoid arthritis: results from the CAMERA trials. Clin Exp Rheumatol. 2014;32(3):369–76.
Chatzidionysiou K, Sfikakis PP. Low rates of remission with methotrexate monotherapy in rheumatoid arthritis: review of randomised controlled trials could point towards a paradigm shift. RMD Open. 2019;5(2):e000993 (Published 2019 Jul 27).
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38 (published correction appears in Lancet. 2016 Oct 22;388(10055):1984).
van den Berg R, van der Heijde D, Landewé R, van Lambalgen K, Huizinga T. The METEOR initiative: the way forward for optimal, worldwide data integration to improve care for RA patients. Clin Exp Rheumatol. 2014;32(5 Suppl 85):135–40.
Saevarsdottir S, Wallin H, Seddighzadeh M, et al. Predictors of response to methotrexate in early DMARD naive rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis. 2011;70(3):469–75.
Mathieu S, Pereira B, Saraux A, Richez C, Combe B, Soubrier M. Disease-modifying drug retention rate according to patient age in patients with early rheumatoid arthritis: analysis of the ESPOIR cohort. Rheumatol Int. 2021;41(5):879–85. https://doi.org/10.1007/s00296-020-04770-7.
Stouten V, Westhovens R, Pazmino S, et al. Five-year treat-to-target outcomes after methotrexate induction therapy with or without other csDMARDs and temporary glucocorticoids for rheumatoid arthritis in the CareRA trial. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220857. (published online ahead of print, 2021 Apr 2).
Sapart E, Sokolova T, de Montjoye S, et al. Should we use glucocorticoid in Early Rheumatoid Arthritis?: Results at 5 years from the ERA UCLouvain Brussels cohort. Rheumatology (Oxford). 2021. https://doi.org/10.1093/rheumatology/keab151. (published online ahead of print, 2021 Feb 19).
Tamirou F, Arnaud L, Talarico R, et al. Systemic lupus erythematosus: state of the art on clinical practice guidelines. RMD Open. 2018;4(2):e000793 (Published 2018 Nov 27).
Guillevin L, Mukhtyar C, Pagnoux C, Yates M. Conventional and biological immunosuppressants in vasculitis. Best Pract Res Clin Rheumatol. 2018;32(1):94–111.
Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68(12):1870–7.
Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 2017;69(3):506–17.
Hetland ML, Haavardsholm EA, Rudin A, et al. Active conventional treatment and three biological treatments in early rheumatoid arthritis: phase IV investigator initiated, randomized, observer blinded clinical trial. BMJ. 2020;371: m4328.
Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74(1):19–26.
Emery P, Fleischmann RM, Hsia EC, Xu S, Zhou Y, Baker D. Efficacy of golimumab plus methotrexate in methotrexate-naïve patients with severe active rheumatoid arthritis. Clin Rheumatol. 2014;33(9):1239–46.
van den Broek M, Klarenbeek NB, Dirven L, et al. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score-steered therapy: subanalysis of the BeSt study. Ann Rheum Dis. 2011;70(8):1389–94.
Burmester GR, Feist E, Dörner T. Emerging cells and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:77–88.
Acknowledgements
We thank CAP48 (the funding research project from RTBF), the patients for their contributions to this study, and Mrs. Van Cutsem and Mrs. Henin for their RA donations.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Emilie Sapart: concept and design of the study, data collection, analysis, and writing of the manuscript. Tatiana Sokolova: statistical analysis. Stéphanie de Montjoye: data collection, analysis, and writing of the manuscript. Stéphanie Dierckx: data collection, analysis, and writing of the manuscript. Adrien Nzeusseu: data collection, analysis, and writing of the manuscript. Aleksandra Avramovska: collection, analysis, and writing of the manuscript. Laurent Meric de Bellefon: data collection, analysis, and writing of the manuscript. Patrick Durez: Concept and design, data collection, analysis, and writing of the manuscript.
Prior Presentation
The data has been presented at:
- EULAR June 2–5, 2021 (virtual): oral presentation “Should we use bioDMARDS in first intention in early rheumatoid arthritis: results at 5 years from the ERA Louvain Brussels Cohort”. OP 0119. Ann Rheum Dis, volume 80, supplement 1, year 2021, page 66.
- BCR September 29–30 and October 1, 2021 (Brussels, Belgium): oral presentation: “Early Remission at 6 months as a predictor of long-term remission in new onset rheumatoid arthritis”.
- ACR November 5–9, 2021 (virtual): poster “Early remission at 6 months as a predictor of long-term remission in new-onset rheumatoid arthritis”. Abstract number: 0221.
- SFR December 12–14, 2021 (Paris, France): poster “La rémission précoce à 6 mois comme prédicteur de la rémission à long terme dans la polyarthrite rhumatoïde débutante”. PE.Di-024.
Disclosures
Emilie Sapart, Tatiana Sokolova, Stéphanie de Montjoye, Stéphanie Dierckx, Adrien Nzeusseu, Aleksandra Avramovska, Laurent Meric de Bellefon and Patrick Durez have nothing to disclose.
Compliance with Ethics Guidelines
The ERA UCLouvain Brussels cohort was approved by our academic institution and our local ethic committee (Ethic Committee of Cliniques Universitaire Saint-Luc, Brussels) for different protocols. All studies were performed in accordance with the Helsinki Declaration of 1964 and all its later amendments. All study participants gave their consent to participate in this study and to use their data in accordance with the Belgian law of July 30, 2018 on the protection of privacy and the European regulations (European General Regulation on the Protection of Personal Data RGPD of May 25, 2018) in force, the law of August 22, 2002 on patients' rights and the law of May 7, 2004 on human experimentation. This global retrospective analysis does not require further approval.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sapart, E., Sokolova, T., de Montjoye, S. et al. Should We Use bDMARDs as an Induction Therapy in Early and Severe Rheumatoid Arthritis? Results at 5 years from the ERA UCLouvain Brussels Cohort. Rheumatol Ther 10, 875–886 (2023). https://doi.org/10.1007/s40744-023-00551-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00551-3