Abstract
Introduction
There is a paucity of data on how patient characteristics may affect the long-term durability of certolizumab pegol (CZP) in patients with rheumatoid arthritis (RA). This study therefore aimed to investigate CZP durability and reasons for discontinuation over 5 years between different subgroups of patients with RA.
Methods
Data were pooled from 27 clinical trials in RA patients. Durability was defined as the percentage of patients randomized to CZP at baseline who were still on CZP treatment at a given timepoint. Post hoc analyses of clinical trial data on CZP durability and reasons for discontinuation among different patient subgroups were conducted using Kaplan–Meier curves and Cox proportional hazards modeling. Patient subgroups included: age (18– < 45/45– < 65/ ≥ 65 years), gender (male/female), prior tumor necrosis factor inhibitor (TNFi) use (yes/no), and disease duration (< 1/1– < 5/5– < 10/ ≥ 10 years).
Results
Among 6927 patients, the durability of CZP was 39.7% at 5 years. Patients aged ≥ 65 years had a 33% greater risk of CZP discontinuation than patients 18– < 45 years (hazard ratio [95% confidence interval]: 1.33 [1.19–1.49]) and patients with prior TNFi use had a 24% greater risk of discontinuing CZP than patients without (1.24 [1.12–1.37]). Conversely, greater durability was observed among patients who had a baseline disease duration of ≥ 1 year. Durability did not differ in the gender subgroup. Of the 6927 patients, the most common reason for discontinuation was inadequate levels of efficacy (13.5%); followed by adverse events (11.9%); consent withdrawn (6.7%); lost to follow-up (1.8%); protocol violation (1.7%); other reasons (9.3%).
Conclusions
CZP durability was comparable with durability data on other bDMARDs in RA patients. Patient characteristics that were associated with greater durability included younger age, TNFi-naïvety, and disease duration ≥ 1 year. Findings may be helpful in informing clinicians on a patient’s likelihood of discontinuing CZP, based on their baseline characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
As rheumatoid arthritis (RA) is a chronic condition, it is important to investigate longer-term retention of certolizumab pegol (CZP) among RA patients. There is currently a paucity of data specifically investigating the proportion of RA patients remaining on CZP in the long term, and how patient characteristics may influence their likelihood of remaining on treatment. |
To investigate how many patients remained on CZP up to 5 years, alongside how individual factors (age, gender) and existing disease condition (prior use of other tumor necrosis factors [TNFi], disease duration) could affect their likelihood of remaining on CZP. |
What was learned from the study? |
To the authors’ knowledge, this is the largest analysis on CZP durability to date; this analysis demonstrated that the number of patients remaining on CZP treatment was comparable to previously published data for other TNFi used in RA. |
Additionally, different patient characteristics may affect CZP treatment durability; specifically, younger patients, patients who had not previously been treated with TNFi, and those with a disease duration of over 1 year at baseline were more likely to remain on CZP. |
It may be useful for clinicians to consider these patient characteristics when managing their RA patients. |
Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune, inflammatory joint disease [1, 2]. If untreated, inflammation can lead to damage of the cartilage and bone, resulting in impaired physical function and poor quality of life [1,2,3,4]. Due to the chronic nature of the disease, RA patients require therapies that are efficacious and durable. Hence, there is an overall need to investigate longer-term drug retention rates among patients with RA, which may be helpful in informing clinicians on how to maximize the long-term efficacy of treatment.
Current treatment guidelines recommend that biologic disease-modifying anti-rheumatic drugs (bDMARDs; including tumor necrosis factor inhibitors [TNFi]) can be added to methotrexate (MTX) for RA patients with an inadequate response to conventional DMARDs [5, 6]. However, inadequate levels of efficacy and occurrence of adverse events (AEs) have been cited as common reasons for discontinuation of bDMARDs [7, 8]. In a real-world retrospective study comparing drug retention rates of seven bDMARDs (including certolizumab pegol [CZP]) among RA patients, 3-year retention rates ranged from 31.1 to 60.7% [7]. Retention rates among TNFi specifically have been reported to vary across different patient subgroups in real-world studies; female sex, older age, concomitant prednisolone, and absence or low dose of combined MTX have been associated with a greater likelihood of TNFi discontinuation [9,10,11,12,13]. The reasons for bDMARD discontinuation have also been noted to differ within a given patient subgroup; for example, among patients stratified by age, inadequate levels of efficacy were more commonly cited among those aged < 60 years compared with those aged ≥ 60 years [14].
CZP is an Fc-free, PEGylated TNFi established for use in early and active RA [15,16,17,18,19,20]. CZP retention rates in RA have previously been reported to range from 42.5 to 59.9% over 3–5 years across both clinical trials and real-world studies [7, 8, 21, 22]. Notably, 49.2% of RA patients from a pooled clinical trial population remained on CZP at 3 years [23]. CZP retention rates have also been reported to vary between patient subgroups, suggesting that differences in patient characteristics may contribute towards specific reasons for discontinuation within a given subgroup [8, 23]. For example, risk of serious infectious events was found to be higher among patients aged ≥ 65 years compared with patients aged < 45–50 years, which could potentially contribute to higher CZP discontinuation rates among older patients [24, 25].
However, there is still a paucity of data specifically investigating longer-term retention rates of CZP, as well as how different patient baseline characteristics might significantly influence CZP durability. Here, we utilized pooled data across multiple clinical trials in RA to investigate CZP durability and associated reasons for discontinuation across different patient subgroups over more than 7 years in this large-scale analysis. We additionally sought to identify characteristics among these subgroups that were specifically associated with a higher likelihood of discontinuing CZP treatment.
Methods
Data Collection
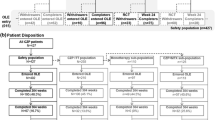
This study pooled observed data on CZP treatment in RA from 27 clinical trials globally, including one open-label, single-dose pharmacokinetic study, 18 randomized clinical trials (RCT), seven open-label extensions (OLE), and one head-to-head study, encompassing data from < 16 weeks to 7.7 years of individual patient exposure (data cut-off August 2017); the full list of studies have been previously reported in Curtis et al. [26]. Patients on CZP as monotherapy or in combination therapy (for example, concomitant MTX) were included. Patients who transitioned from the placebo to CZP arm as part of some trial designs were also included.
Ethics approval was not required for this study as this was a post hoc analysis. The original studies were conducted with patients’ informed consent and in accordance with the Declaration of Helsinki (1964) and its later amendments.
Outcomes
Post hoc analyses of CZP durability and reasons for discontinuation in different patient subgroups were conducted using Kaplan–Meier curves and Cox proportional hazards modeling. Patient subgroups of interest included the age (18– < 45/45– < 65/ ≥ 65 years), gender (male/female), disease duration (< 1/1– < 5/5– < 10/ ≥ 10 years), and history of prior TNFi use (yes/no) subgroups.
Durability Assessments
Durability was defined as the percentage of patients randomized to CZP at baseline who remained on CZP treatment at a given timepoint. Only periods on CZP were considered, and any withdrawal from the study was defined as a discontinuation event. Where a patient had withdrawals in more than one period, only the first withdrawal was considered. Where CZP was administered over multiple periods, the cumulative time on treatment was utilized.
Kaplan–Meier curves were used to provide an estimate of CZP durability for patient subgroups. CZP exposure in patient-years was the number of years of CZP exposure from the first dose of CZP to the end of the treatment exposure period, or to the date of the last dose before first discontinuation. Patients with a reason for discontinuation but no treatment start date were excluded from the analysis.
Although all available data up to the maximum time period of 7.7 years were included in the durability analysis, only data up to 5 years were illustrated in the Kaplan–Meier curves, due to low patient numbers beyond this point.
Reasons for Discontinuation
Reasons for discontinuation of CZP were categorized as follows: ‘Inadequate levels of efficacy’, ‘AEs’, ‘Consent withdrawn’, ‘Lost to follow-up’, ‘Protocol violation’, or ‘Other’ with the opportunity to provide an accompanying description. The full list of AEs which led to discontinuation have previously been reported, in which death was classified as discontinuation due to an AE [26].
Cox Proportional Hazards Models
Cox proportional hazards modeling was used to investigate the association of baseline patient characteristics with the risk of discontinuing CZP. Baseline covariates included in the model corresponded with the patient subgroups of interest; age 18– < 45 years, male gender, disease duration < 1 year, and no prior TNFi use were used as reference for the age, gender, disease duration, and history of prior TNFi subgroups, respectively. Covariates used in each Cox model were entered into and retained in the model if p ≤ 0.25; covariates with p ≤ 0.05 were deemed to be associated with risk of CZP discontinuation. Risk of CZP discontinuation for different patient subgroups are presented as hazard ratios (HRs) with 95% confidence intervals (CIs).
The Cox proportional hazards models illustrated all available data, up to the maximum of 7.7 years.
Results
Patient Baseline Characteristics and CZP Exposure
The pooled analysis included a total of 6927 patients across 27 studies. Most patients were female (79.3%), with a mean disease duration of 6.4 years (standard deviation [SD] 6.9 years), and mean CZP exposure of 2.0 years (range 0.0–7.7 years; Table 1). Approximately half reported concomitant steroid use (46.2%) and most reported concomitant MTX use (78.5%).
Overall Durability of CZP and Reasons for Discontinuation
The overall durability of CZP was 39.7% at 5 years (Table 2). The most common reason for discontinuation was inadequate levels of efficacy (13.5% of patients discontinued for this reason), followed by discontinuation due to AEs (11.9%; Fig. 1).
Overall reasons for CZP discontinuation. aStudy investigators had the opportunity to provide an accompanying description. Some examples of responses received under ‘Other’ reasons included: does not meet all eligibility criteria, hepatitis B virus DNA positive, investigator/sponsor decision, non-compliance, and pregnancy. In some instances, some responses regarding inadequate levels of efficacy and adverse events were included under ‘Other’ reasons, instead of being recorded under their respective main categories. CZP certolizumab pegol, DNA deoxyribonucleic acid
It was also noted that 9.3% of patients discontinued due to ‘Other’ reasons; in some instances, investigators recorded inadequate levels of efficacy and AEs under ‘Other’ reasons, instead of being recorded under their respective main categories. For example, some of cases of discontinuation may be attributable to study designs that forced withdrawal at specific time points. However, individuals who withdrew due to study design were not consistently classified under one particular reason for discontinuation. Additional examples of responses received under ‘Other’ reasons included: does not meet all eligibility criteria, hepatitis B virus deoxyribonucleic acid positive, investigator/sponsor decision, non-compliance, and pregnancy.
Durability of CZP and Reasons for Discontinuation by Subgroups
A higher proportion of patients aged < 65 years (Fig. 2a) or who had a baseline disease duration of > 1 year (Fig. 2b) remained on CZP at 5 years. Prior TNFi use (Fig. 2c) and gender (Fig. 2d) had no apparent impact on CZP durability.
Kaplan–Meier graphs of CZP durability in different patient subgroups over time. (a) Age, (b) Disease duration, (c) Prior TNFi use, (d) Gender. All available data up to 7.7 years were included in durability analyses, but only data up to 5 years are illustrated in the Kaplan–Meier curves due to low patient numbers beyond this point. aPatients who were considered non-responders were withdrawn after a pre-defined period of treatment in certain trials, which may have affected the observed CZP durability. This was particularly evident among the < 1 year baseline disease duration subgroup, which largely consisted of patients enrolled in trials specifically investigating early RA. CZP certolizumab pegol, RA rheumatoid arthritis, TNFi tumor necrosis factor inhibitor
The percentage of patients who discontinued CZP due to AEs increased with age; 16.6% of patients aged ≥ 65 years discontinued CZP, compared with 8.5% of patients aged 18– < 45 years. The proportion of patients discontinuing CZP due to any other reason, however, was comparable among all age groups (Fig. 3a).
Reasons for discontinuation distributed by different patient subgroups. (a) Age, (b) Disease duration, (c) Prior TNFi use, (d) Gender. aStudy investigators had the opportunity to provide an accompanying description. Some examples of responses received under ‘Other’ reasons included: does not meet all eligibility criteria, hepatitis B virus DNA positive, investigator/sponsor decision, non-compliance, and pregnancy. In some instances, some responses regarding inadequate levels of efficacy and adverse events were included under ‘Other’ reasons, instead of being recorded under their respective main categories. DNA deoxyribonucleic acid, TNFi tumor necrosis factor inhibitor
A larger proportion of patients with a baseline disease duration of < 1 year were noted to discontinue CZP due to ‘Other’ reasons (17.3%), compared with all other baseline disease duration subgroups (5.7–8.8%) (Fig. 3b). Furthermore, some of the ‘Other’ reasons had no accompanying description of the reason for discontinuation. Similarly, it was noted that a larger proportion of patients with no prior TNFi exposure discontinued due to ‘Other’ reasons compared with the subgroup with prior TNFi exposure; a common ‘Other’ reason for discontinuation within this subgroup was ‘investigator/sponsor decision’ (Fig. 3c). Conversely, a larger proportion of patients from the subgroup with prior TNFi use (17.1%) discontinued CZP due to inadequate levels of efficacy compared with the subgroup with no prior exposure to a TNFi (12.7%).
All reasons for CZP discontinuation were reported in similar proportions irrespective of gender (Fig. 3d).
Patient Characteristics Associated with Risk of CZP Discontinuation
Cox proportional hazards models, which incorporated all available data up to the maximum of 7.7 years, were used to assess the risk of CZP discontinuation associated with different patient characteristics (Fig. 4). Compared to patients aged 18– < 45 years, patients aged ≥ 65 years had a significantly greater risk of CZP discontinuation (HR [95% CI] 1.33 [1.19–1.49], p < 0.0001). Patients with a baseline disease duration of < 1 year had a significantly greater risk of CZP discontinuation than patients in other disease duration subgroups (HRs [95% CI] compared to disease duration < 1 year were, for the 1– < 5 years subgroup: 0.79 [0.71–0.87], p < 0.0001; 5– < 10 years subgroup: 0.80 [0.72–0.89], p < 0.0001; ≥ 10 years subgroup: 0.84 [0.75–0.93], p = 0.0012). Patients with prior TNFi use also had a significantly greater risk of discontinuing CZP, compared with patients with no prior exposure (1.24 [1.12–1.37], p < 0.0001).
Cox proportional hazards model of time to first CZP discontinuation. All available data up to the maximum of 7.7 years are captured in the Cox model. The above variables result from a stepwise selection procedure with a probability of 0.25 for entry into and retention in the model. Initial variables eligible for entry into the model were: gender, age subgroup, disease duration subgroup, and previous TNFi use (yes/no). Results colored red are significant. CI confidence interval, CZP certolizumab pegol, Ref reference, TNFi tumor necrosis factor inhibitor
Discussion
This study used pooled data across 27 CZP clinical trials in 6927 patients with RA to estimate the long-term durability of CZP treatment and investigate reasons for discontinuation overall, and in subgroups of interest. Patient characteristics that affected the likelihood of discontinuing CZP treatment were also examined.
Overall, the 5-year durability of CZP was 39.7%. These findings are comparable to bDMARD retention rates in RA patients that are reported in other clinical trials of similar durations (3 to > 10 years), which range from 30 to 56% [27,28,29,30,31]. Subgroups found to be associated with poorer CZP durability in our study included patients aged ≥ 65 years, patients with prior TNFi use, and patients who had a baseline disease duration of < 1 year. The most common reason for discontinuation across all patient subgroups was inadequate levels of efficacy, followed by AEs.
Lower rates of CZP retention among patients aged ≥ 65 years were observed compared with patients aged < 65 years—a trend that has also been observed in RA patients on other bDMARDs [12]. Specifically, patients ≥ 65 years were more likely to discontinue CZP treatment due to AEs [14, 24, 25, 32]. Furthermore, increased risk of AEs with older age is likely to be compounded by obesity, increased comorbidity burden, systemic steroid use, an age-related decline in immunity, and general frailty leading to poor health outcomes [24, 33,34,35]. These results may suggest that early intervention, TNFi treatment optimization, and risk mitigation for AEs among older patients with RA may be instrumental to promoting better CZP retention rates among this group [36].
Prior TNFi use was also associated with a higher risk of discontinuation compared with TNFi-naïvety in this study. This is in line with previous literature, which describes a loss of response during TNFi treatment (secondary drug failure) due to the formation of anti-drug antibodies, thereby increasing the likelihood of subsequent treatment discontinuation [36, 37].
Notably, there was a sharp decline in the proportion of individuals with disease duration < 1 year who remained in the trials around the 1-year time point. This observation could partially be attributed to the design of individual trials and different RA populations; for example, C-OPERA and C-EARLY (Period 1) exclusively enrolled patients with a baseline disease duration of ≤ 1 year [15, 38]. Both studies also had a study duration of 1 year, resulting in these patients being categorized as having ‘discontinued’ CZP after the end of the study. As a result of these individual trial design differences, results obtained in this study may not necessarily be an accurate reflection of the true CZP durability in the disease duration < 1 year subgroup. Future investigations could incorporate a sensitivity analysis to determine the impact of these studies on the results. Furthermore, baseline disease duration may also potentially be confounded if patients experienced symptoms prior to official diagnosis, which would lead to misclassification among the disease duration subgroups.
In addition, some trials such as C-OPERA may have also only captured data from a specific ethnic population. Given this, the full effect of racial differences on observed data across the clinical trials cannot be entirely elucidated. Furthermore, regional trial site variability across the pooled studies may have had some influence on CZP durability. Reasons for geographic variation in CZP durability are likely to be multifactorial, such as country-specific socioeconomic factors and practices within the healthcare system. Notably, variable adherence to the treat-to-target (TTT) strategy for RA, which was introduced in 2010, may also account for regional differences in CZP durability [39, 40]. This paradigm shift in the management of RA in the real-world could have influenced clinical trial data, as physicians may opt to discontinue patients achieving suboptimal outcomes from the trial and consider alternative treatments in line with the TTT strategy. Future work could explore regional differences in TTT strategies in further detail, to provide clinicians with region-specific information on the effects of TTT on drug durability.
To the authors’ knowledge, this is the largest analysis of CZP to date, incorporating long-term clinical study data (over 5 years). A key strength of this study therefore lies in its generalizability of results towards the RA patient population, as data from a large number of patients were used. The study additionally captured patients from different populations with a range of baseline characteristics, including disease duration, severity, and prior TNFi exposure. These data can inform clinicians on how long-term CZP treatment plans can be optimized, by highlighting possible relationships between different patient subgroups and treatment durability.
The variability in study design among the pooled trials is a limitation of this study, as it posed a challenge when interpretating trends in these data. Firstly, trials had varying cut-off points, some of which required patients to discontinue CZP if they did not reach a certain efficacy endpoint at a specific time. For example, non-responders in the RAPID trials were withdrawn at Week 16 [18, 19, 21, 22], compared with those from DOSEFLEX and EXXERLERATE who were withdrawn at Week 18 and Week 24, respectively [41, 42].
Secondly, there was a lack of consistency and specificity in how study investigators recorded ‘Other’ reasons for withdrawal, which may have undercut the true impact of other reasons for discontinuation. For example, some investigators included discontinuation due to ‘Inadequate levels of efficacy’ or ‘AEs’ as an ‘Other’ reason. Furthermore, some ‘Other’ reasons for discontinuation were vague (e.g., no further description was provided), precluding interpretation of these data.
Finally, patient baseline data such as length of medical history and changes to patients’ concurrent medication during the trials were also not consistently collected. As a result, the potential effects of altering a patient’s treatment regimen (e.g., reducing or discontinuing concurrent MTX) on CZP efficacy may not have been fully captured.
It is also important to note that studies included in the analysis were RCTs, which have a highly selected patient population and stringent withdrawal criteria. Some trials also included an OLE, in which patients similarly submit to trial procedures and are provided study medication despite fewer visits and procedures overall when compared with an RCT. Therefore, despite the rigorous data that can be captured by robust RCTs and their OLEs, results may not necessarily be reflective of disease management in real-world clinical practice; for example, patients in RCTs tend to have more severe disease due to eligibility criteria of the studies. Additionally, CZP patients who become pregnant or who achieve a major response from disease baseline (but are not necessarily considered to have attained low disease activity) would be continued on CZP in the real world. Nevertheless, previous real-world studies involving CZP have reported retention rates of 42.5–43.3% across 3–5 years, which was comparable with our results as well as those of real-world evidence of other TNFi treatments, barring some differences between patient subgroups [7, 8, 43].
Conclusions
This study is the first to report CZP retention rate data over an extended period of time, and in the largest population of RA patients to date. This is particularly relevant as RA is a chronic condition, necessitating long-term treatment. It is therefore important to establish longer-term retention of therapeutics among these patients. Overall, CZP durability from pooled clinical trial data was comparable to that of other previously reported bDMARDs. Specifically, findings suggest that older patients with RA could benefit from early implementation of risk mitigation strategies against AEs to promote CZP retention. TNFi-naïvety being associated with greater durability may also highlight the importance of early intervention and treatment optimization of TNFis, which may be particularly relevant to the older patient population. These data can additionally inform clinicians on a patient’s likelihood to discontinue CZP given their baseline characteristics.
References
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
Bai B, Chen M, Fu L, et al. Quality of life and influencing factors of patients with rheumatoid arthritis in Northeast China. Health Qual Life Outcomes. 2020;18(1):119.
Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):123–30.
Fraenkel L, Bathon JM, England BR, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73(7):1108–23.
Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis—the ANSWER cohort study. PLoS One. 2018;13(3): e0194130.
Favalli EG, Becciolini A, Sarzi Puttini P, et al. FRI0114 Efficacy and retention rate of certolizumab pegol in rheumatoid arthritis: data from a large real-life multicentre retrospective cohort. Ann Rheum Dis. 2018;77(Suppl 2):601.
Alvarez-Madrazo S, Kavanagh K, Siebert S, et al. Discontinuation, persistence and adherence to subcutaneous biologics delivered via a homecare route to Scottish adults with rheumatic diseases: a retrospective study. BMJ Open. 2019;9(9): e027059.
Gabay C, Riek M, Scherer A, Finckh A. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology (Oxford). 2015;54(9):1664–72.
Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62(1):22–32.
Kawabe A, Nakano K, Kubo S, Asakawa T, Tanaka Y. Differential long-term retention of biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis by age group from the FIRST registry. Arthritis Res Ther. 2020;22(1):136.
Souto A, Maneiro JR, Gómez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford). 2016;55(3):523–34.
Cho SK, Sung YK, Kim D, et al. Drug retention and safety of TNF inhibitors in elderly patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2016;17:333.
Atsumi T, Yamamoto K, Takeuchi T, et al. The first double-blind, randomised, parallel-group certolizumab pegol study in methotrexate-naive early rheumatoid arthritis patients with poor prognostic factors, C-OPERA, shows inhibition of radiographic progression. Ann Rheum Dis. 2016;75(1):75–83.
Bi L, Li Y, He L, et al. Efficacy and safety of certolizumab pegol in combination with methotrexate in methotrexate-inadequate responder Chinese patients with active rheumatoid arthritis: 24-week results from a randomised, double-blind, placebo-controlled phase 3 study. Clin Exp Rheumatol. 2019;37(2):227–34.
Keystone E, Heijde DVD, Mason D Jr, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two–week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58(11):3319–29.
Smolen J, Landewé RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68(6):797–804.
Yamamoto K, Takeuchi T, Yamanaka H, et al. Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: the J-RAPID randomized, placebo-controlled trial. Mod Rheumatol. 2014;24(5):715–24.
European Medicines Agency. Cimzia European product assessment report. February 11, 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/cimzia. Accessed Sep 2021.
Keystone E, Landewé R, van Vollenhoven R, et al. Long-term safety and efficacy of certolizumab pegol in combination with methotrexate in the treatment of rheumatoid arthritis: 5-year results from the RAPID 1 trial and open-label extension. Ann Rheum Dis. 2014;73(12):2094–100.
Smolen JS, van Vollenhoven R, Kavanaugh A, et al. Certolizumab pegol plus methotrexate 5-year results from the rheumatoid arthritis prevention of structural damage (RAPID) 2 randomized controlled trial and long-term extension in rheumatoid arthritis patients. Arthritis Res Ther. 2015;17(1):245.
Bykerk V, Gottlieb AB, Reich K, et al. FRI0087 Durability of certolizumab pegol in patients with rheumatoid arthritis or psoriasis over three years: an analysis of pooled clinical trial data. Ann Rheum Dis. 2020;79(Suppl 1):621.
Bykerk VP, Blauvelt A, Curtis JR, et al. Associations between safety of certolizumab pegol, disease activity, and patient characteristics, including corticosteroid use and body mass index. ACR Open Rheumatol. 2021;3(8):501–11.
Curtis JR, Winthrop K, O’Brien C, Ndlovu MN, de Longueville M, Haraoui B. Use of a baseline risk score to identify the risk of serious infectious events in patients with rheumatoid arthritis during certolizumab pegol treatment. Arthritis Res Ther. 2017;19(1):276.
Curtis JR, Mariette X, Gaujoux-Viala C, et al. Long-term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn’s disease: a pooled analysis of 11,317 patients across clinical trials. RMD Open. 2019;5(1): e000942.
Burmester GR, Matucci-Cerinic M, Mariette X, et al. Safety and effectiveness of adalimumab in patients with rheumatoid arthritis over 5 years of therapy in a phase 3b and subsequent postmarketing observational study. Arthritis Res Ther. 2014;16(1):R24.
Genovese MC, Schiff M, Luggen M, et al. Long-term safety and efficacy of abatacept through 5 years of treatment in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor inhibitor therapy. J Rheumatol. 2012;39(8):1546–54.
Klareskog L, Gaubitz M, Rodríguez-Valverde V, Malaise M, Dougados M, Wajdula J. Assessment of long-term safety and efficacy of etanercept in a 5-year extension study in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2011;29(2):238–47.
Smolen JS, Kay J, Landewé RB, et al. Golimumab in patients with active rheumatoid arthritis who have previous experience with tumour necrosis factor inhibitors: results of a long-term extension of the randomised, double-blind, placebo-controlled GO-AFTER study through week 160. Ann Rheum Dis. 2012;71(10):1671–9.
Weinblatt ME, Bathon JM, Kremer JM, et al. Safety and efficacy of etanercept beyond 10 years of therapy in North American patients with early and longstanding rheumatoid arthritis. Arthritis Care Res (Hoboken). 2011;63(3):373–82.
Ishchenko A, Lories RJ. Safety and efficacy of biological disease-modifying antirheumatic drugs in older rheumatoid arthritis patients: staying the distance. Drugs Aging. 2016;33(6):387–98.
Lindstrom TM, Robinson WH. Rheumatoid arthritis: a role for immunosenescence? J Am Geriatr Soc. 2010;58(8):1565–75.
van Onna M, Boonen A. The challenging interplay between rheumatoid arthritis, ageing and comorbidities. BMC Musculoskelet Disord. 2016;17(1):184.
Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15.
Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7(1): e135.
Owczarczyk-Saczonek A, Owczarek W, Osmola-Mańkowska A, Adamski Z, Placek W, Rakowska A. Secondary failure of TNF-α inhibitors in clinical practice. Dermatol Ther. 2019;32(1): e12760.
Emery P, Bingham CO, Burmester GR, et al. Certolizumab pegol in combination with dose-optimised methotrexate in DMARD-naïve patients with early, active rheumatoid arthritis with poor prognostic factors: 1-year results from C-EARLY, a randomised, double-blind, placebo-controlled phase III study. Ann Rheum Dis. 2017;76(1):96–104.
Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3–15.
Yu Z, Lu B, Agosti J, et al. Implementation of treat-to-target for rheumatoid arthritis in the US: analysis of baseline data from a randomized controlled trial. Arthritis Care Res (Hoboken). 2018;70(5):801–6.
Furst DE, Shaikh SA, Greenwald M, et al. Two dosing regimens of certolizumab pegol in patients with active rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67(2):151–60.
Smolen JS, Burmester GR, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388(10061):2763–74.
Neovius M, Arkema EV, Olsson H, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 2015;74(2):354–60.
Acknowledgements
Funding
Sponsorship for this study and Rapid Service Fee were funded by UCB Pharma. Support for third-party writing assistance for this article, provided by Paige Foo Jia-Qi, MPharm, Yi Ling Teo, PhD, Costello Medical, Singapore, and Emma Phillips, PhD, Costello Medical, Cambridge, UK, was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Medical Writing, Editorial, and Other Assistance
The authors thank the patients, the investigators, and their teams who took part in the original clinical studies. The authors also acknowledge Irina Mountian, MD, PhD, and Keyla Brooks, PharmD from UCB Pharma for publication coordination, Paige Foo Jia-Qi, MPharm (Costello Medical, Singapore), Yi Ling Teo, PhD (Costello Medical, Singapore), and Emma Phillips, PhD (Costello Medical, Cambridge, UK) for medical writing and editorial assistance based on the authors’ input and direction. This study was funded by UCB Pharma.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Substantial contributions to study conception and design: Vivian P. Bykerk, Peter Nash, David Nicholls, Yoshiya Tanaka, Kevin Winthrop, Christina Popova, Nicola Tilt, Derek Haaland; substantial contributions to analysis and interpretation of the data: Vivian P. Bykerk, Peter Nash, David Nicholls, Yoshiya Tanaka, Kevin Winthrop, Christina Popova, Nicola Tilt, Derek Haaland; drafting the article or revising it critically for important intellectual content: Vivian P. Bykerk, Peter Nash, David Nicholls, Yoshiya Tanaka, Kevin Winthrop, Christina Popova, Nicola Tilt, Derek Haaland; final approval of the version of the article to be published: Vivian P. Bykerk, Peter Nash, David Nicholls, Yoshiya Tanaka, Kevin Winthrop, Christina Popova, Nicola Tilt, Derek Haaland.
Disclosures
Vivian P. Bykerk: Consulting fees from AbbVie, Bristol Myers Squibb, Genentech, Pfizer, Regeneron, and UCB Pharma. Peter Nash: Grants for research and clinical trials and honoraria for advice and lectures on behalf of AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Gilead/Galapagos, GSK, Janssen, Novartis, Pfizer, Samsung, Sanofi, and UCB Pharma; Funding for research, clinical trials, and honoraria for advice and lectures on behalf of AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos/Gilead, GSK, Janssen, Novartis, Pfizer, Samsung, and UCB Pharma. David Nicholls: Grants for clinical trials and honoraria for advice and lectures on behalf of AbbVie, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Gilead/Galapagos, GSK, Janssen, Novartis, Pfizer, Sanofi Aventis, Servatus, and UCB Pharma. Yoshiya Tanaka: Speaking fees and/or honoraria from AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, GSK, Mitsubishi-Tanabe, and Pfizer; Research grants from Asahi-Kasei, AbbVie, Boehringer Ingelheim, Chugai, Daiichi-Sankyo, Eisai, and Takeda. Kevin Winthrop: Consultant fees from AbbVie, Bristol Myers Squibb, Eli Lilly, Gilead, Pfizer, Roche, and UCB Pharma. Christina Popova: Employee and stockholder of UCB Pharma. Nicola Tilt: Employee and stockholder of UCB Pharma. Derek Haaland: Advisory boards/consulting, conducted research, and/or received grants from Abbott, AbbVie, Amgen, AstraZeneca, Adiga Life Sciences, Bristol Myers Squibb, Celgene, Circassia, Eli Lilly, GSK, Janssen, Novartis, Pfizer, Roche, Sanofi Genzyme, Takeda, and UCB Pharma.
Compliance with Ethics Guidelines
Ethics approval was not required for this study as this was a post-hoc analysis. The original studies were conducted with patients’ informed consent and in accordance with the Declaration of Helsinki (1964) and its later amendments.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bykerk, V.P., Nash, P., Nicholls, D. et al. Long-Term Durability of Certolizumab Pegol in Patients with Rheumatoid Arthritis Over 5 Years: An Analysis of Pooled Clinical Trial Data. Rheumatol Ther 10, 693–706 (2023). https://doi.org/10.1007/s40744-023-00541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00541-5