Abstract
Introduction
This analysis aims to describe real-world clinical outcomes in US African American and Hispanic patients with systemic lupus erythematosus (SLE) receiving belimumab.
Methods
In this post hoc analysis of OBSErve US (GSK Study 117,295) data, patients received intravenous belimumab (10 mg/kg) over 24 months. Outcomes assessed every 6 months after belimumab initiation (index) included: physician-assessed overall clinical response (worse, no improvement, < 20%, 20–49%, 50–79%, ≥ 80% improvement), physician-assessed disease severity (mild, moderate, severe), oral corticosteroid (OCS) use and healthcare resource utilization (HCRU).
Results
Of 501 patients enrolled, 123 and 88 were African American and Hispanic respectively; 69 (56.1%) and 43 (48.8%) were receiving belimumab at 24 months. Of those, 88.4%/95.3% (African American/Hispanic) were female; mean (standard deviation [SD]) age was 41.6 (12.5)/42.2 (10.5) years. Within 6 months post-index, 91.3%/90.7% of patients still receiving belimumab had a ≥ 20% physician-assessed clinical improvement. Among 24 months completers, proportions of patients with severe SLE fell from 34.8%/25.6% at index to 2.9%/4.7% at Month 6 and 2.9%/0% at Month 24. The proportion of patients receiving OCS and mean (SD) daily OCS dose also decreased, from 82.6%/81.4% and 19.7 (12.8)/18.8 (10.0) mg/day at index to 50.7%/34.9% and 3.1 (3.2)/1.6 (2.4) mg/day at Month 24. Fewer patients were hospitalized or required ancillary care services at 18–24 months post-index versus 6 months pre-index.
Conclusion
Belimumab treatment for up to 2 years improved clinical outcomes, disease severity, mean OCS dose and HCRU in US African American and Hispanic patients with SLE, providing real-world evidence for enduring belimumab effectiveness in populations that are markedly impacted by SLE.
Similar content being viewed by others
Systemic lupus erythematosus (SLE) disproportionally impacts individuals of African American and Hispanic ancestry; as such an early diagnosis is important to help prevent SLE disease progression, flares, and organ damage whilst also helping to improve and/or maintain health-related quality of life. |
This analysis of observational data aimed to describe real-world clinical outcomes in US African American and Hispanic patients with SLE receiving belimumab as part of their routine care. |
Results shown here reflect the real-world effectiveness of belimumab, when used for 24 months, among African American and Hispanic patients with SLE. |
Over 24 months of belimumab treatment, marked improvements in patients’ clinical outcomes, disease severity, need for corticosteroid use, and healthcare resource utilization were noted. |
These findings support prolonged treatment with belimumab for the management of SLE in African American and Hispanic patients. |
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by a diverse range of clinical manifestations [1] with an estimated prevalence in the USA of between 70 and 150 cases per 100,000 people [2, 3]. SLE prevalence is approximately three times greater in African American patients and 1.5–2 times greater in Hispanic patients compared with Caucasians, regardless of sex [3]. Patients of non-Caucasian ethnicity, including Asian, African American and Hispanic patients, demonstrate more severe disease manifestations (including nephritis), more rapid accumulation of damage and a higher mortality rate, even at younger ages, than Caucasian patients [4,5,6,7]. Similarly, lupus nephritis (LN) is more common in patients of Asian, Black African or Hispanic backgrounds than in Caucasian patients [4, 8].
While clinical outcomes and prognoses are generally poorer in non-Caucasian than Caucasian patients with SLE, differences also exist between patients of Black African and Hispanic ancestry. Hispanic patients accrue more, and have faster progression of, SLE-related organ damage and are more likely to suffer from renal complications and end-stage kidney disease, while African American patients more often accrue integument damage [5, 9].
SLE leads to irreversible damage accrual in multiple organs, with increased age, corticosteroid use and Black African or Hispanic ancestry as risk factors [6, 10]. Current treat-to-target recommendations in SLE suggest disease remission or the lowest possible disease activity as well as damage prevention and limited corticosteroid exposure as important treatment goals [11].
The disease-modifying agent belimumab, a monoclonal antibody therapy that inhibits soluble B-lymphocyte stimulator, is approved in the USA for the treatment of active autoantibody-positive SLE in patients ≥ 5 years of age and for active LN in adults [12, 13]. Belimumab demonstrated consistent efficacy and safety, as well as a steroid-sparing effect, in four large Phase 3 trials [14,15,16,17]. In these trials, up to 50% of patients were of Hispanic ethnicity, but no more than 15% were of Black African ancestry, highlighting the need for further research in this population.
The EMBRACE trial (Belimumab in patients of Black African ancestry; NCT01632241) was the first trial that enrolled only patients with SLE who self-identified as being of black race (the study’s original terminology for patients of Black African ancestry). While EMBRACE did not meet its primary endpoint of SLE Responder Index-4 (SRI-4) response at Week 52 [18], the response rate in the belimumab arm was numerically higher than in the placebo arm and was consistent with the SRI-4 responses seen in previous Phase 3 belimumab trials [14,15,16,17]. There remains a lack of longitudinal real-world data for belimumab effectiveness in patients of either Black African ancestry (including African Americans) or Hispanic patients with SLE.
The OBSErve (evaluation Of use of Belimumab in clinical practice SEttings) program was conducted in various countries as individual observational, longitudinal studies to evaluate belimumab effectiveness in real-world populations [19,20,21,22,23,24]. A pooled analysis of the data from several OBSErve studies demonstrated that treatment with belimumab results in clinical improvements in patients with SLE [20]. Of the studies in the program, the OBSErve US study had the largest cohort of > 500 patients and included patients from a number of different ethnicities [19].
Given the higher prevalence and severity of SLE in patients of Black African ancestry and Hispanic ancestry, it is important to assess the effectiveness of belimumab in these populations. The aim of this post hoc analysis was to describe the clinical outcomes and overall patterns of healthcare for US patients of Black African ancestry (African Americans) and Hispanic patients, who received belimumab for up to 2 years as part of routine care for SLE in the USA.
Methods
Study Design
This post hoc analysis used data from the OBSErve US study (GSK Study 117295). Full details of the OBSErve US study have been published previously [19]; briefly, this non-interventional, retrospective, observational, real-world cohort study included the treatment history period (≥ 6 months prior to index [first belimumab infusion]) and the follow-up treatment period (up to 24 months post-index).
Compliance with Ethics Guidelines
The OBSErve US study was approved by the New England Institutional Review Board and conducted in accordance with the Declaration of Helsinki 2008 and the International Society for Pharmacoeconomics and Outcomes Research guidelines. Patient consent for the original OBSErve US study was not necessary because the study was non-interventional and data were de-identified.
Physicians and Patients
Data were collected from US rheumatology practices from February 2012 to May 2014. Eligible rheumatologists were required to have had ≥ 5 years of clinical practice experience and have treated ≥ 10 patients with SLE annually. Physicians identified medical charts of patients with SLE from their clinical practices who were ≥ 18 years of age, had received ≥ 8 infusions of belimumab (10 mg/kg intravenous; subcutaneous belimumab was approved in 2017 and was therefore not available at the time of the OBSErve US study) plus standard therapy and had ≥ 6 months and up to 24 months of medical history documented. Patients were proposed and selected for the study by their rheumatologists from their clinical practices. Each physician was limited to enroll no more than 25 patients. Patient data were collected via case report forms and all patient demographics, and disease and treatment characteristics were anonymized.
Study Objectives and Assessments
The primary objectives of this post hoc analysis were to examine clinical outcomes and describe overall patterns of care in patients with SLE who self-identified as being either African American or Hispanic and who received belimumab over the full 24-month period. The primary outcome was overall clinical response to belimumab at the end of a 24-month period, based on physician’s assessment over the last 6-month period (categorized as: worse, no improvement, < 20%, 20–49%, 50–79%, ≥ 80% improvement). Other clinical outcomes (assessed versus index at Month 6, 12, 18 and 24 post-index) included: physician-assessed SLE disease severity (categorized as: mild, moderate, severe), oral corticosteroid (OCS) use (dichotomized by use [≥ 7.5 mg/day vs. < 7.5 mg/day], no use or use as continuous outcome), disease activity (measured by Safety of Estrogen in Lupus Erythematosus National Assessment-SLE Disease Activity Index [SELENA-SLEDAI], Physician Global Assessment [PGA] and Patient Global Assessment [PtGA]), utilization of assessment tools and reasons for, and rate of, belimumab discontinuation. SELENA-SLEDAI scores were reported based on a scale range of 0–105, while PGA and PtGA scores were reported on a scale of 0–100; for all scales, higher scores indicating greater disease activity.
The secondary objective was to describe healthcare resource utilization (HCRU) in enrolled patients with SLE, including the number of rheumatologist and emergency room visits, hospitalizations and use of ancillary care services (e.g. physical or occupational therapy, home health care services).
Data Analysis and Other Considerations
Descriptive analyses were performed for all study objectives. OCS doses were converted to prednisone equivalent.
Results
Baseline Characteristics
In the OBSErve US study, 92 rheumatologists enrolled 501 patients with SLE; 123 (24.6%) were African American and 88 (17.6%) were Hispanic, of whom 69 (56.1%) and 43 (48.9%), respectively, continued to receive belimumab at Month 24. Of those patients who did not continue belimumab use over the 24 months, 26 (21.1%) African American and 23 (26.1%) Hispanic patients were lost to follow-up, and 28 (22.8%) and 22 (25.0%), respectively, discontinued belimumab (see Discontinuation of belimumab section below for more detail).
Baseline characteristics of the analyzed patients, including those who continued to receive belimumab at Month 24, are presented in Table 1. Our analyses focused on the 69 African American and 43 Hispanic patients who were receiving belimumab at 24 months. In the African American and Hispanic cohorts, most patients were female (88.4% and 95.3%) with mean (standard deviation [SD]) ages of 41.6 (12.5) and 42.2 (10.5) years, respectively (Table 1). Just under half of African American patients (n = 33, 47.8%) and over half of Hispanic patients (n = 25, 58.1%) had SLE for 1–5 years.
Among African American patients, none had mild SLE, 45 (65.2%) had moderate SLE, and 24 (34.8%) had severe disease at index. Among Hispanic patients, 1 (2.3%) had mild SLE, 31 (72.1%) had moderate disease, and 11 (25.6%) had severe SLE at index. In African American and Hispanic patients, mean (SD) assessment scores at index for PGA, SELENA-SLEDAI and PtGA were 75.4 (10.9) and 70.0 (16.2), 13.4 (3.2) and 11.3 (3.5), and 77.6 (11.7) and 72.5 (16.9), respectively. The most common clinical manifestation of SLE in African American patients at index was renal involvement/LN (data on biopsy-proven LN diagnosis/activity not collected) (n = 19, 27.5%), and the most common comorbidity was hypertension (n = 18, 26.1%) (Table 1). Among Hispanic patients at index, 14.0% (n = 6) had renal involvement/LN (data on biopsy-proven LN diagnosis/activity not collected), and the most common comorbidities were fibromyalgia (n = 10, 23.3%), osteoarthritis (n = 9, 20.9%) and hypertension (n = 9, 20.9%).
Reasons for Belimumab Initiation
The most common reasons for belimumab initiation for African American/Hispanic patients were lack of effectiveness of the previous treatment regimen (n = 54, 78.3%/n = 30, 69.8%), patient condition worsening (n = 46, 66.7%/n = 22, 51.2%) and to decrease corticosteroid use (n = 45, 65.2%/n = 30, 69.8%). Other reasons included poor tolerability of previous treatment regimen (n = 17, 24.6%/n = 16, 37.2%), patient request (n = 9, 13.0%/n = 6, 14.0%), previous treatment regimen being inconvenient (n = 1, 1.4%/n = 5, 11.6%) and drug-to-drug interactions with a previous medication (no patients/n = 2, 4.7%).
Overall Clinical Response
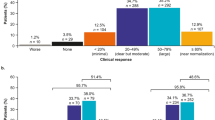
Among patients receiving belimumab at 24 months, 63 (91.3%) African American and 39 (90.7%) Hispanic patients had ≥ 20% improvement, and 12 (17.4%) and 5 (11.6%) patients, respectively, had ≥ 80% improvement in physician-assessed overall clinical response within the first 6 months post-index compared with 6 months pre-index. No patient experienced a lack of improvement (Fig. 1). The trend for improvement continued over the study period, with 81.2%/79.1%, 59.4%/86.0% and 44.9%/76.7% of African American/Hispanic patients having a ≥ 20% improvement and 7.2%/20.9%, 11.6%/18.6% and 10.1%/25.6% having a ≥ 80% improvement at 6–12, 12–18 and 18–24 months post-index, respectively (Fig. 1). Notably, a worse outcome was only reported for 0%/0%, 0%/2.3%, 2.9%/2.3% and 1.4%/0% of patients at 0–6, 6–12, 12–18 and 18–24 months post-index.
SLE Severity
The number of African American/Hispanic patients with severe disease, as assessed by physicians, decreased from 24 (34.8%)/11 (25.6%) at index to 2 (2.9%)/2 (4.7%) at Month 6, 1 (1.4%)/1 (2.3%) at Month 12, 1 (1.4%)/0 at Month 18 and 2 (2.9%)/0 at Month 24 (Fig. 2). Likewise, the number of patients with moderate disease decreased over the study period, with 45 (65.2%) and 31 (72.1%) African American and Hispanic patients respectively, having moderate SLE at index, falling to 15 (21.7%) and 8 (18.6%) patients at Month 24, while the number of patients with mild disease increased from none and 1 (2.3%) patient at index to 52 (75.4%) and 35 (81.4%) patients at Month 24 (Fig. 2).
SELENA-SLEDAI, PGA and PtGA
SELENA-SLEDAI, PGA and PtGA were the most frequently used tools to assess disease severity, evaluated at index in 13 (18.8%) and 18 (41.9%), 28 (40.6%) and 12 (27.9%), and 20 (29.0%) and 10 (23.3%) African American and Hispanic patients, respectively, who were receiving belimumab at 24 months (Table 1). Use of these instruments decreased over the study period to 8 (11.6%) and 16 (37.2%) patients for SELENA-SLEDAI, 21 (30.4%) and 8 (18.6%) patients for PGA and 18 (26.1%) and 7 (16.3%) patients for PtGA at Month 24. Physicians did not use any tools to assess disease severity in 33 (47.8%) and 18 (41.9%) patients at index and 35 (50.7%) and 19 (44.2%) African American and Hispanic patients, respectively, at Month 24.
In African American patients, there was an improvement in mean (SD) SELENA-SLEDAI score of 8.8 (5.2) points at Month 24 versus index in the 8 patients with a SELENA-SLEDAI assessment at both of these time points (mean [SD] scores: 13.5 [3.4] at index, 4.8 [4.0] at Month 24). For the 21 patients with a PGA assessment at Month 24, mean (SD) PGA score was 29.5 (24.0); this equated to an improvement in mean (SD) PGA score of 47.0 (24.9) points at Month 24 versus the index value of 75.0 (12.0) (analyzed in the 20 patients with PGA assessments at both index and Month 24). Mean (SD) PtGA score was 35.3 (26.2) at Month 24 (n = 18 patients with a PtGA assessment at this time point), representing a 52.6 (16.4) mean (SD) point improvement at Month 24 versus index (mean [SD] score: 75.8 [12.9], calculated in the 14 patients with a PtGA assessment at both time points).
In Hispanic patients with a SELENA-SLEDAI assessment at index and Month 24 (n = 16, 37.2%), there was a mean (SD) SELENA-SLEDAI score improvement of 8.4 (4.5) points at Month 24 versus index (mean [SD] score at Month 24: 3.1 [2.8]; mean score at index: 11.6 [3.6]). For patients with a PGA assessment at index and Month 24 (n = 5, 11.6%), the mean (SD) PGA score improved by 43.0 (25.4) points at Month 24 versus index (mean [SD] score at index: 72.0 [5.9]), and among those patients with a PtGA assessment at index and Month 24 (n = 4, 9.3%), the PtGA score improved by 55.0 (9.1) points at Month 24 versus index (mean [SD] score at index: 76.3 [4.8]).
OCS Use
As noted above, the ability to decrease corticosteroid use was among the most common reasons for belimumab initiation; indeed, from index to Month 24, the number of patients receiving OCS, including high-dose OCS, and the mean daily OCS dose progressively decreased. Among African American and Hispanic patients, 57 (82.6%) and 35 (81.4%) received OCS at index, falling to 35 (50.7%) and 15 (34.9%) patients at Month 24, respectively (Fig. 3a, b). Similarly, the number of patients receiving OCS at a dose of > 7.5 mg/day decreased from 52 (75.4%) and 32 (74.4%) at index to 7 (10.1%) and 1 (2.3%) at Month 24 (Fig. 3a, b), and the mean (SD) dose of OCS decreased from 19.7 (12.8) mg/day and 18.8 (10.0) mg/day at index to 3.1 (3.2) mg/day and 1.6 (2.4) mg/day at Month 24 (Fig. 3c, d).
HCRU
The number of African American patients who required at least one unscheduled rheumatologist visit at 6 months pre-index and 18–24 months post-index decreased from 42 (60.9%) to 22 (31.9%), as did the mean number of unscheduled visits (from 2.4 to 1.6 visits) (Table 2). The number of Hispanic patients requiring at least one unscheduled rheumatologist visit at 6 months pre-index and 18–24 months post-index fell from 29 (67.4%) to 8 (18.6%), respectively (Table 2). In this cohort, the mean number of visits remained relatively stable throughout the study except for the last 6 months, almost doubling from a mean of 1.6 visits at months 12–18 to 3.1 visits at months 18–24.
In African American patients, from 6 months pre-index to 18–24 months post-index, substantial reductions were observed in the number of patients with ≥ 1 emergency room visit (16 [23.2%] to 7 [10.1%]), hospitalizations (4 [5.8%] to 3 [4.3%]) and ancillary care services (10 [14.5%] to 3 [4.3%]), with mean numbers of emergency room visits and hospitalizations remaining relatively stable over the duration of the study (Table 2). In Hispanic patients, over the same period, there were reductions in the number of patients with ≥ 1 emergency room visit (3 [7.0%] to 2 [4.7%]) and ancillary care services (9 [20.9%] to 1 [2.3%]). One (2.3%) patient required a hospitalization at 0–6 months versus 2 (4.7%) patients at 6 months pre-index, with no patient hospitalized at 6–24 months post-index (Table 2).
Discontinuation of Belimumab
Over the study period, 28 (22.8%) African American and 22 (25.0%) Hispanic patients discontinued belimumab. The most common reasons for discontinuation in the former group were patient request (n = 13) and medication inefficacy (n = 7), and 2 patients discontinued belimumab due to an adverse event, while in the latter group, the most common reasons for discontinuation were loss to follow-up (n = 8) and loss of insurance or reimbursement (n = 7) (Table 3).
Discussion
This post hoc analysis of real-world data from African American and Hispanic patients with SLE in the OBSErve US study found substantial improvements in clinical outcomes, disease severity, the ability to taper or decrease OCS use and HCRU in patients with continued belimumab use over 24 months. Within 6 months, all patients experienced a physician-assessed clinical improvement (with approximately half showing an improvement of ≥ 50%), which continued throughout the study. In African American and Hispanic patients, OCS use fell by ~ 39% and ~ 57%, respectively, with fewer hospitalizations for SLE; in particular, no Hispanic patient was hospitalized for SLE from 6 months onwards. Despite only half of the participating providers performing regular SLE disease assessments, belimumab was associated with reductions in SELENA-SLEDAI, PGA and PtGA scores; disease severity thus showed a consistent decrease regardless of the use of a subjective or objective tool. Other real-world studies have shown a similar low use of SLE assessment tools in clinical practice [19, 24], which may in part be due to the complexity and time needed to perform these measures.
Overall, the results from the OBSErve US African American/Hispanic cohort are in line with previous findings [14,15,16,17] including those from the OBSErve program [19, 20]. Taken together, the results for African American and Hispanic patients presented here support previous findings in the BLISS clinical trials, the EMBRACE study and observational studies of belimumab, demonstrating associated clinical improvements, a corticosteroid-sparing effect and reduced HCRU over time [14,15,16,17,18].
Response to belimumab in African American patients was similar to that in the overall OBSErve US study population [19], for which a 6-month physician-assessed overall clinical improvement of ≥ 20% was observed in 88.4% of patients compared with 91.3% of African American patients in the current analysis. Enduring clinical responses in African American patients were also observed. Hispanic patients appeared to show a greater response to belimumab compared with African American patients, Caucasian patients and the total OBSErve US study population [19]. For example, 58.1% of Hispanic patients had an overall clinical improvement of ≥ 50% after 6 months of belimumab treatment compared with 47.8%, 46.8% and 48.7% of African American patients, Caucasian patients and the total OBSErve US population, respectively [19]. These marked improvements continued throughout the 24-month follow-up period for the Hispanic population, but not for African American, Caucasian or the total OBSErve US population, in which proportions decreased to 21.7%, 27.9% and 32.1% between Month 18 and 24, respectively [19]. Similarly, the proportion of Hispanic patients who required OCS after 24 months of belimumab treatment (34.9%) was lower than that among African American patients (50.7%), Caucasian patients (65.2%) and the total OBSErve US population (48.7%), while the mean daily OCS dose at Month 24 was 1.6 mg/day for Hispanic patients compared with 3.1, 5.6 and 6.1 mg/day for African American patients, Caucasian patients and the total OBSErve US population [19]. The reasons for these differences are unclear and may relate to physician practice differences, patient variability or other factors.
Our findings support the impact of belimumab on both reducing SLE disease severity in this population, with ~ 97% of African American patients and all Hispanic patients no longer having severe disease by 24 months, despite the former cohort having a substantially higher prevalence of severe disease at index (34.8 vs. 25.6% in Hispanic patients and 20.2% in the total OBSErve US population) [19]. Patients receiving belimumab were also able to reduce their daily OCS dose: African American patients by approximately six-fold (from a mean dose of 19.7 mg/day at index to 3.1 mg/day at Month 24) and Hispanic patients by approximately ten-fold (from a mean dose of 18.8 mg/day at index to 1.6 mg/day at Month 24). Treatment discontinuation rates among African American and Hispanic patients were similar to those reported for the overall OBSErve US study population (22.4%) [19].
Asian patients with SLE are recognized to have increased SLE prevalence and more severe clinical manifestations compared with Caucasian populations [4]. Although real-world data on the effectiveness of belimumab in Asian populations are lacking, a clinical trial in North East Asia and a recent open-label continuation study demonstrated favorable outcomes for Asian patients treated with belimumab [15, 25]. A continued collection and analysis of real-world evidence on the effectiveness of belimumab for all ethnicities is warranted in this highly heterogeneous disease and is particularly important for minority populations in which severe disease manifestations are known to be more common.
The OBSErve US study and the current analysis have a number of limitations, including patient dropout rate (although similar results were noted in the completer and intention-to-treat populations [data not shown]), small sample size, no control group, varied experience of participating clinicians with the clinical SLE tools, analysis-by-responder bias and the use of subjective, non-validated assessments. This analysis aimed to investigate the effectiveness of belimumab in African American and Hispanic patients who received continual belimumab treatment for 24 months; therefore, only patients who were receiving belimumab at 24 months post-index were included in the analysis. For this reason, statistical approaches to impute missing data such as last observation carried forward could not be employed. The OBSErve US study started in 2012 and physicians were allowed to assess SLE disease activity based on their personal practices at the time. As such, the SLE disease severity data are subjective to the physicians’ assessments conducted at that time. Furthermore, while different clinical assessment tools were used to evaluate disease severity, assessment tools were not applied in approximately half of cases and thus care should be taken when interpreting the present findings. The lack of a universally accepted instrument is an additional challenge for assessing SLE treatment. Despite these limitations, disease severity was similar in the OBSErve US study, irrespective of the different measures used.
Conclusions
This post hoc analysis of real-world patient data demonstrated sustained effectiveness of belimumab in African American and US Hispanic patients. The findings corroborate those from the wider patient population of the OBSErve US study and further support the efficacy of belimumab for the management of SLE in populations with higher prevalence of SLE and disease severity.
Change history
23 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40744-023-00614-5
References
Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis. 2006;1:6.
Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates using hospitalization data. Arthritis Rheum. 2007;56:2092.
Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the centers for disease control and prevention national lupus registries. Arthritis Rheumatol. 2021;73:991–6.
González L, Toloza S, McGwin G Jr, Alarcón G. Ethnicity in systemic lupus erythematosus (SLE): its influence on susceptibility and outcomes. Lupus. 2013;22:1214–24.
Uribe AG, Alarcón GS. Ethnic disparities in patients with systemic lupus erythematosus. Curr Rheumatol Rep. 2003;5:364–9.
Lewis MJ, Jawad AS. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology. 2017;56:i67–77.
Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39:257–68.
Bastian H, Roseman J, McGwin G Jr, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11:152–60.
Alarcón GS, McGwin G Jr, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: IX. Differences in damage accrual. Arthritis Rheum. 2001;44:2797–806.
Bruce IN, O’Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74:1706–13.
Van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. 2014;73:958–67.
GlaxoSmithKline. Benlysta Prescribing Information. 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125370s068,761043s008lbl.pdf. Accessed 19 Nov 2020.
Levy RA, Gonzalez-Rivera T, Khamashta M, et al. 10 years of belimumab experience: what have we learnt? Lupus. 2021;30:1705–21.
Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–30.
Zhang F, Bae S-C, Bass D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. 2018;77:355–63.
Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–31.
Stohl W, Schwarting A, Okada M, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two–week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69:1016–27.
Ginzler E, Guedes Barbosa LS, D’Cruz D, et al. Phase III/IV, randomized, fifty-two-week study of the efficacy and safety of belimumab in patients of black African ancestry with systemic lupus erythematosus. Arthritis Rheumatol. 2022;74:112–23.
Collins C, Dall’Era M, Kan H, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med. 2016;3: e000118.
Collins CE, Cortes-Hernández J, Garcia MA, et al. Real-world effectiveness of belimumab in the treatment of systemic lupus erythematosus: pooled analysis of multi-country data from the OBSErve studies. Rheumatol Ther. 2020;7:949–65.
Babini A, Cappuccio A, Caprarulo C, et al. Evaluation of belimumab treatment in patients with systemic lupus erythematosus in a clinical practice setting: results from a 24-month OBSErve study in Argentina. Lupus. 2020;29:1385–96.
Touma Z, Sayani A, Pineau CA, et al. Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada Study. Rheumatol Int. 2017;37:865–73.
Schwarting A, Schroeder JO, Alexander T, et al. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: results from the OBSErve Germany study. Rheumatol Ther. 2016;3:271–90.
von Kempis J, Duetsch S, Reuschling N, et al. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Med Wkly. 2019;149: w20022.
Tanaka Y, Bae SC, Bass D, et al. Long-term open-label continuation study of the safety and efficacy of belimumab for up to 7 years in patients with systemic lupus erythematosus from Japan and South Korea. RMD Open. 2021. https://doi.org/10.1136/rmdopen-2021-001629.
Acknowledgements
Funding
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The OBSErve US study (GSK Study 117295), the current analysis (GSK Study 208439), the Rapid Service Fee and Open Access fees were funded by GSK.
Medical Writing, Editorial and Other Assistance
Medical writing support was provided by Nicholas Thomas, PhD, of Fishawack Indicia Ltd., UK, part of Fishawack Health, and was funded by GSK.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Author Contributions
All authors contributed to the conception and design of this analysis and the data analysis or interpretation.
Prior Presentation
Parts of the data presented in the manuscript have previously been presented at the American College of Rheumatology (ACR) / Association of Rheumatology Health Professionals (ARHP) 2012 Annual Scientific Meeting (Collins C, et al. Arthritis Rheum. 2012;64(Suppl 10):2617), ACR/ARHP 2012 Annual Scientific Meeting (Collins C, et al. Arthritis Rheum. 2019;71(Suppl10):2524), the State of Texas Association of Rheumatologists 2020 Annual Meeting, the Rheumatology Winter Clinical Symposium 2020, the Association of Women in Rheumatology 2020 National Conference, the Congress of Clinical Rheumatology West (CCR-W) 2020 Annual Meeting, the Pan American League of Associations for Rheumatology 2020 Annual Meeting, the Florida Society of Rheumatology 2021 Annual Meeting, CCR-W 2021, and the Rheumatology Nurses Society 14th Annual Conference 2021.
Disclosures
Christopher F. Bell, Jake Chung, and Bernard Rubin are employees of GSK and hold stocks and shares in the company.
Compliance with Ethics Guidelines
The OBSErve US study was approved by the New England Institutional Review Board and conducted in accordance with the Declaration of Helsinki 2008 and the International Society for Pharmacoeconomics and Outcomes Research guidelines. Patient consent for the original OBSErve US study was not necessary because the study was non-interventional and data were de-identified.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available, but anonymized individual patient data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original online version of this article was revised to correct a value in a sentence.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bell, C.F., Chung, J. & Rubin, B. Real-World Clinical Outcomes in Belimumab-Treated US African American and Hispanic Patients with Systemic Lupus Erythematosus: A Retrospective, Observational Study. Rheumatol Ther 10, 447–462 (2023). https://doi.org/10.1007/s40744-022-00524-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00524-y