Abstract
Introduction
Currently there is limited data to drive clinical decision making regarding the choice of biologic/targeted synthetic disease-modifying antirheumatic drugs (DMARD); thus, head-to-head comparisons are needed to help guide prescribing. In recent years, significant advancements have helped clarify the mechanistic basis of the clinical associations of autoantibodies in rheumatoid arthritis (RA). This study evaluated the effectiveness of abatacept versus tofacitinib in anti-cyclic citrullinated peptide (CCP+) patients with rheumatoid arthritis (RA).
Methods
CorEvitas (formerly known as CORRONA) Registry patients aged ≥ 18 years, who were CCP+ before initiating abatacept or tofacitinib (December 2012 onwards through October 2019), had 6-month follow-up data (baseline and 6-month Clinical Disease Activity Index [CDAI]), and were not in remission at index were included. Patients were frequency matched 1:1 by prior biologic use before propensity score matching (PSM). Primary (mean change [D] in CDAI) and secondary outcomes 6 months after index were compared using mixed-effects models adjusted for variables that remained unbalanced after PSM.
Results
Following PSM, most baseline characteristics for 291 patient pairs were well balanced between treatments, although fewer patients initiating abatacept versus tofacitinib received prior non-TNFi biologic DMARDs, and patients initiating abatacept versus tofacitinib had a higher physician global assessment score, patient-reported fatigue, and modified Health Assessment Questionnaire (mHAQ). In adjusted analyses, there were no significant differences in mean [D] from baseline in CDAI at 6 months with abatacept versus tofacitinib (P = 0.936). Patients naïve for b/tsDMARDs initiating abatacept had a numerically greater mean [D] in CDAI at 6 months versus tofacitinib, although this difference was not statistically significant (P = 0.662). There were no significant differences for any secondary outcomes.
Conclusions
In adjusted analyses, CCP+ patients with RA initiating treatment with abatacept versus tofacitinib did not show a statistically significant difference in reducing disease activity or improving patient-reported outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Head-to-head comparisons of biologic/targeted synthetic DMARDsare needed to help guide rheumatology provider prescribing. |
To evaluate the effectiveness of abatacept versus tofacitinib in CCP+ (a surrogate for anti-cyclic citrullinated peptide [ACPA]) patients with rheumatoid arthritis. |
What was learned from this study? |
In adjusted analyses, CCP+ patients with RA initiating treatment with abatacept did not show a statistically significant difference in reducing disease activity or improving patient-reported outcomes compared with CCP+ patients initiating treatment with tofacitinib. |
To achieve a highly individualized and tailored approach to patient management, physicians will need to consider factors including comorbidity burden, patient preference, disease progression, and the risk–benefit ratio for each agent when selecting medications. |
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory arthritis associated with joint inflammation, impaired quality of life, and increased rates of disability [1]. Over the last two decades, advances in our knowledge of RA have increased significantly. Knowledge such as recognizing that RA starts early and damage can develop before clinical symptoms present has led to early diagnosis and instituting prompt treatment within the window of opportunity [1, 2]. Another notable advancement is a better understanding of the disease pathogenesis, which has led to the development of targeted therapeutics that allow for reasonable control of signs and symptoms, thus improving the overall outcome of the disease [3]. To further help patients achieve sustained remission or low disease activity (LDA), the identification of specific serum biomarkers indicative of factors such as poor prognosis, worse outcomes, erosive disease, and less responsiveness to therapies would be beneficial when developing a personalized treatment approach for patients [4].
Rheumatoid arthritis patients may respond differently to medications with different modes of action based on their underlying serology. In recent years, significant advancements have helped clarify the mechanistic basis of the clinical associations of autoantibodies in RA [5]. One of the hallmarks of RA is the production of autoantibodies, including anti-citrullinated protein antibodies (ACPA). ACPAs are included in the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) diagnostic criteria) [6]. ACPAs are highly specific serum biomarkers often presenting in serum before the onset of disease [7]. The presence of ACPAs suggests a poor prognosis including rapid disease progression, more severe joint damage, and increased disability [8, 9]. Likewise, RA patients who are positive for anti-cyclic citrullinated peptide (CCP+, a surrogate for ACPA) antibodies are more likely to develop severe, erosive disease than those who are anti-CCP-negative (CCP–) [8,9,10].
The wide array of biologic disease-modifying antirheumatic drugs (bDMARDs) has led to tremendous advancements in the treatment of RA. Although there are well-established prognostic factors at the group level (i.e., high disease activity, the early presence of erosive changes, extra-articular disease, previous biologic failure), they do not constitute specific predictors of response to a particular drug; thus, we are still unable to predict outcomes at the patient level by treatment. Hence, health care providers are left to implement the trial-and-error approach within the permitted treatments of insurance coverage to prescribing advanced therapies [11].
Prior work has demonstrated that response to RA therapies may vary based on CCP± status. Specifically, patients who were CCP+ had a more significant clinical response to treatment with abatacept than those who were CCP–. However, this relationship was not seen with tumor necrosis factor α inhibitors (TNFi) [12, 13]. A better understanding of the effectiveness of well-established b/tsDMARDs s for treating RA in particular subgroups of patients based on serologic biomarkers will enable more individualized care. To that end, in this current study, we used propensity score matching (PSM) to compare the effectiveness of abatacept versus tofacitinib in a large real-world cohort of CCP+ patients.
Methods
Study Sample
Data Source
The CorEvitas (formerly known as Corrona) RA Registry is an independent, prospective, national, observational cohort. Patients were recruited from 207 private practices and academic sites with 880 participating rheumatologists across 42 states in the US. Treatment and outcomes data for patients with RA are collected and analyzed [14]. As of March 31, 2022, the registry included 217 private and academic active clinical sites with 931 physicians throughout 42 states in the US. Data on 458,982 patient visits and approximately 228,871 patient-years of follow-up observation time have been collected. The mean duration of patient follow-up was 4.8 years (median 3.4 years).
Population
This study selected patients from the CorEvitas Registry who met the following inclusion criteria: RA confirmed by a rheumatologist; ≥ 18 years of age; initiation of abatacept or tofacitinib between December 2012 (when the FDA approved tofacitinib) and October 2019; CCP + (titer ≥ 20 U/ml) any time before starting or at the start of treatment; had a follow-up visit at 6 months (± 3 months) after starting treatment; a Clinical Disease Activity Index (CDAI) score at initiation or prior to treatment initiation and the 6-month follow-up visit; was not in remission based on CDAI at treatment initiation. There were 369 CCP+ abatacept and 475 CCP+ tofacitinib patients who had a 6-month follow-up visit; CDAI at initiation and at 6 months; and were not in remission at the index visit.
Propensity Score Matching
This study performed 1:1 frequency matching by the number of prior biologics (0, 1, or 2+ prior biologics) between abatacept and tofacitinib CCP+ patients. Once matched based on prior biologic use, we assessed the level of imbalance between the abatacept and tofacitinib CCP+ patients using standardized differences.
Standardized differences provide a measure of clinically important difference even if there is no statistically significant difference between the treatment groups [15, 16]. Therefore, we used standardized differences > 0.10 as suggesting meaningful differences between groups. Variables with standardized differences > 0.10 between the groups were considered to have remained potentially unbalanced. After considering standardized differences based on the frequency matching, a propensity score model for treatment (abatacept versus tofacitinib) [16] was fit using the following variables in the final propensity score model: gender, duration of RA, college education, number of prior conventional synthetic (cs) DMARDs (csDMARDs), number of prior bDMARDs, targeted synthetic (ts) DMARDs, follow-up months, baseline CDAI, history of cancer, history of cardiovascular disease (CVD), body mass index (BMI), monotherapy, and prednisone dose > 7.5 at index visit.
We matched the treatment groups and compared characteristics between matched groups using the propensity score. For patients who switched agents during the 6 months of follow-up, we used the outcome measure at the time of switch (rather than at 6 months) for continuous outcomes. For dichotomous outcomes, we imputed them as non-responders (e.g., did not achieve remission for patients that switched prior to their 6-month follow-up visit). For patients who discontinued their medication prior to the follow-up visit without switching to another b/tsDMARD, their outcomes at the follow-up visit were used.
Measures and Data Collection
Data were collected during the study period from physician assessment and patient questionnaires completed during routine clinical visits. These forms were used to gather information on disease severity and activity (including serologic markers) and components of ACR response criteria); comorbidities; use of medications including steroids, csDMARDs, tsDMARDs and bDMARDs; and adverse events. As a strictly observational registry that reflects typical clinical practice, the CorEvitas registry does not mandate that laboratory data, including serologic markers and acute-phase reactants, be performed, however asks participating investigators to submit these results if they were performed.
Data on demographics, insurance status, comorbid conditions, RA disease characteristics, and RA medications were available for > 97% of patients. Data disease activity elements collected included: CDAI; swollen joint count in 28 joints; tender joint count in 28 joints; Physician Global Assessment (PGA) and Patient Global Assessment (PtGA); modified ACR 20, 50, and 70% response criteria (mACR20, mACR50, and mACR70). The mACR is based on achieving the swollen and tender joint responses, as well as two out of four additional measures, including the modified Health Assessment Questionnaire (mHAQ) to assess physical function, patient pain, patient fatigue, and the Patient Global Assessment (PtGA); it does not include erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP).
Study Outcomes
The primary outcome was mean improvement in CDAI score over 6 months (± 3 months) following treatment initiation among anti-CCP+ patients treated with abatacept versus tofacitinib. The secondary outcomes compared the response to therapy among CCP+ patients who were treated with abatacept versus tofacitinib at 6 months based on the following outcomes: the achievement of CDAI remission (CDAI score ≤ 2.8); the achievement of CDAI LDA (CDAI score ≤ 10) among those not in remission or LDA at initiation; and achievement of modified ACR20, ACR50, ACR70 responses which included changes from baseline for the modified HAQ (mHAQ), patient pain, patient fatigue, and PtGA. The proportion of patients in each group who discontinued or switched therapies over the study period was assessed.
Statistical Analysis
Patient demographic and disease characteristics at initiation for the two treatment groups were reported with calculated standardized differences. The outcome of response at 6 months was modeled using mixed-effects models. The primary fixed effect of interest was the treatment group (using tofacitinib initiators as the reference group), and site identification was the random effect. After PSM, remaining unbalanced variables, based on standardized differences > 0.10, were included as covariates in the adjusted multivariable models. Covariates included in the multivariable model were age, gender, race, work status, Medicare, blood pressure (systolic), history of non-TNFi DMARD use, baseline CDAI, and mHAQ at index visit.
Compliance with Ethics Guidelines
The study was performed in accordance with the Declaration of Helsinki and the Guidelines for Good Pharmacoepidemiology Practice (GPP). All participating investigators were required to obtain full board approval for conducting noninterventional research involving human subjects with a limited dataset. Sponsor approval and continuing review was obtained through a central Institutional Review Board (IRB), the New England Independent Review Board (NEIRB; no. 120160610). For academic investigative sites that did not receive authorization to use the central IRB, full board approval was obtained from their respective governing IRBs, and documentation of approval was submitted to CorEvitas, LLC before the site’s participation and initiation of any study procedures. All patients in the registry were required to provide written informed consent and authorization before participating.
Results
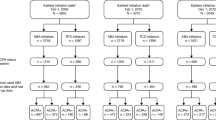
A total of 369 CCP+ abatacept patients and 475 CCP+ tofacitinib patients met the inclusion criteria. After 1:1 frequency matching based on the number of previous biologics, there were 369 patients in each group (Fig. 1). The baseline characteristics in the frequency-matched cohort before PSM showed that the two treatment groups differed (absolute value of the standardized differences > 0.10) in terms of gender, race, education, BMI, working status, comorbidities, duration of RA, physician global assessment, and prior treatment of RA. After considering standardized differences based on the frequency matching, a propensity score model for treatment (abatacept versus tofacitinib) was fit. After PSM, the overall population included 291 patients in each group (Fig. 1). In the overall PS matched population, most patients were female, 61 years old, with a RA duration of 12 years, and the age of RA onset at 48 years (Table 1). Age, gender, race, and baseline CDAI were specified a priori to be included as covariates in adjusted models. Additional baseline characteristics that remained unbalanced (standardized difference > 0.10) between treatments and were included as covariates in adjusted models included: systolic blood pressure, work status, Medicare, mHAQ, and history of non-TNFi DMARD use. While the mean change from baseline CDAI to 6-month CDAI was numerically greater in the abatacept group than in the tofacitinib group, the difference between the groups was not significant.
Patient flow chart. *Propensity score matching was based on gender, duration of RA, college education, number of prior cDMARDs, number of prior b/ts DMARDs, follow-up months, baseline CDAI, history of cancer, history of CVD, BMI, monotherapy, and prednisone dose > 7.5 at index visit. cDMARD conventional disease-modifying antirheumatic drugs; b/ts DMARD biologic/targeted synthetic DMARD; CCP cyclic citrullinated peptide
After adjusting for covariates that were unbalanced between the abatacept and tofacitinib anti-CCP+ patients after PSM, there was no significant difference in change from baseline CDAI to 6-month CDAI between the two groups (abatacept 4.59 [95% CI 3.62, 5.55] vs. tofacitinib 4.10 [95% CI: 3.07, 5.13]; [P = 0.936]) (Table 2). There was also no significant difference between the percent of patients who remained on the drug at the 6-month visit; percent who discontinued initiated drug and did not start another biologic at/before 6 months; and the percent who switched initiated drug to another biologic at/before the 6-month visit (P = 0.265) (Table 2). In exploratory analyses, there were no significant differences when looking separately by line of therapy (0, 1, and ≥ 2 prior b/tsDMARDs) (Supplementary Table 1).
After adjusting for remaining unbalanced covariates between the abatacept CCP+ and tofacitinib CCP+ patients after PSM, there was a numerical advantage in terms of improvement of patient-reported outcomes with treatment with abatacept as compared to tofacitinib, but none were significant: change from baseline to 6-month patient pain (P = 0.682), change from baseline to 6-month patient fatigue (P = 0.531), or change from baseline to 6-month PtGA (0.726) [Fig. 2]. There was no difference with respect to change from baseline to 6-month mHAQ (P = 0.817) between drug groups. In terms of the adjusted odds ratios for binary secondary outcomes, there was a numerical advantage for abatacept with respect to achievement of remission and mACR20 and a numerical advantage for tofacitinib with respect to achievement of LDA, amACR50, mACR70). Overall, there were no significant differences between abatacept and tofacitinib CCP+ patients for these binary secondary outcomes [Fig. 3].
Secondary continuous outcomes measured at 6 months in CCP+ initiators of abatacept vs. tofacitinib. aAdjusted for covariates since CCP+ initiators of abatacept vs. tofacitinib were unbalanced after propensity score matching. Covariates include age, gender, race, work status, Medicare, blood pressure (systolic), history of non-TNFi DMARD use, baseline CDAI and modified Health Assessment Questionnaire (mHAQ) at index visit. bThe P value tests the difference between the CCP+ abatacept and CCP+ tofacitinib initiators according to the secondary outcome
Secondary binary outcomes measured at 6 months in CCP+ initiators of abatacept vs. tofacitinib. aAdjusted for covariates since CCP+ initiators of abatacept vs. tofacitinib were unbalanced after propensity score matching. Covariates include age, gender, race, work status, Medicare, blood pressure (systolic), history of non-TNFi DMARD use, baseline CDAI and modified Health Assessment Questionnaire (mHAQ) at index visit. bThe P value tests the difference between the CCP+ abatacept and CCP+ tofacitinib initiators according to the secondary outcome. cCDAI ≤ 10 among those with moderate or high disease activity. dCDAI ≤ 2.8 among those with low disease activity or more severe. eModified ACR: based on two out of four measures (not using erythrocyte sedimentation rate [ESR] or c-reactive protein [CRP])
Discussion
Using data from CorEvitas, a large US-based RA registry, we conducted a comparative effectiveness study comparing the effectiveness of abatacept to tofacitinib in a population of patients who were CCP+ . In adjusted analyses, CCP+ patients with RA initiating treatment with abatacept did not show a statistically significant difference in reducing disease activity or patient-reported outcomes compared with patients initiating treatment with tofacitinib.
Findings from clinical trials and real-world studies have also found no differences in abatacept compared with tofacitinib. Vieira et al. [17] compared tofacitinib versus abatacept, golimumab, rituximab, and tocilizumab in a network meta-analysis consisting of five trials based on 2136 patients. When comparing tofacitinib and abatacept efficacy outcomes (e.g., ACR20, ACR50, and ACR70) at weeks 12 and 24, risk estimates of ACR response rates for tofacitinib 5 mg BID were comparable to abatacept. In a real-world observational study of tofacitinib using prospectively collected data from an Israeli registry, Shouval et al. [18] focused on event-free survival (EFS), defined as the time from treatment initiation to treatment failure from any cause. They found that EFS with tofacitinib was similar to abatacept in a univariate (hazard ratio [HZ] = 1.05, 95% CI 0.74–1.49, P value: 0.791) and multivariable analysis (HZ = 1.24, 95% CI 0.85–1.81, P value: 0.269). Hirose et al. [19] used propensity score matching (PSM) in a multi-center, retrospective, observational study in 12 hospitals and clinics for RA in Japan. Patients included 70 PS matched pairs of tofacitinib-abatacept patients. At week 24, there were no significant differences in improvement in DAS28-ESR, CDAI, and SDAI and achievement of DAS-ESR remission in the two treatment groups.
We designed this observational study as a head-to-head comparison using PSM among CCP+ patients so each therapy has a fair and equal chance to succeed. Our prior work has demonstrated that response to RA therapies may vary based on CCP+ status. Specifically, in preliminary analyses, we found that patients who were CCP+ had a more significant clinical response to treatment with abatacept than those who were CCP–. We did not find a relationship between CCP+ and response to tofacitinib [12]. However, a post hoc pooled analysis of five phase III randomized clinical trials and a pooled analysis of long-term extension studies found that patients who were CCP+ /rheumatoid factor (RF)+ were more likely to achieve response with tofacitinib compared with those who were CCP−/RF− [20, 21]. For this reason, our comparison was in the CCP+ populations of abatacept and tofacitinib initiators.
This study has several strengths. The CorEvitas RA Registry is a US-based registry that includes a large cohort of patients with RA with physician-validated outcome measures. Previous analyses have compared Medicare patients with RA enrolled within the RA Registry to those not enrolled and found similar demographic and comorbidity characteristics, supporting the generalizability of data from the CorEvitas RA Registry [22]. The study is methodologically robust to address channeling of medications as PSM was used to identify patients with similar characteristics within each line of therapy to offset potential selection bias. Furthermore, the clinician can benefit from this study, as it provides real-world clinically relevant evidence that may assist them in making more informed treatment decisions. This study also has limitations. In real-world settings, patients are not randomized to treatment, but physicians select medications based on specific individual patient profiles and thus can lead to bias. We performed PSM to try an offset selection bias by matching patients with similar characteristics, including disease activity, severity, and line of therapy. The sample size was small, and the duration of follow-up (6 months) was short for both treatments. Given the person-time available for the patient groups included in the manuscript, we were not powered to adequately compare safety in the two groups. In addition, our patient cohort was a majority White population with health insurance and thus results may not be generalizable to other patient populations.
Conclusions
In adjusted analyses, CCP+ patients with RA initiating treatment with abatacept did not show a statistically significant difference in reducing disease activity or improving patient-reported outcomes compared with CCP+ patients initiating treatment with tofacitinib. Additional studies with longer follow-up times would be of interest to explore these findings in a larger population, particularly in b/tsDMARD-naive patients. According to EULAR treatment guidelines, the goal of RA treatment is to achieve sustained remission or low disease activity [23]. When selecting medications, physicians will need to consider factors including comorbidity burden, patient preference, disease progression, and the risk–benefit ratio for each agent in order to achieve a highly individualized and tailored approach to patient management.
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001.
Liao KP, Costenbader KH. Getting them even earlier: Identifying individuals before clinical presentation with rheumatoid arthritis. Arthritis Rheum. 2009;61(12):1620–2.
Nam JL, Takase-Minegishi K, Ramiro S, Chatzidionysiou K, Smolen JS, van der Heijde D, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1113–36.
Puentes-Osorio Y, Amariles P, Calleja MA, Merino V, Diaz-Coronado JC, Taborda D. Potential clinical biomarkers in rheumatoid arthritis with an omic approach. Auto Immun Highlights. 2021;12(1):9.
Bugatti S, Manzo A, Montecucco C, Caporali R. The clinical value of autoantibodies in rheumatoid arthritis. Front Med (Lausanne). 2018;5:339.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
van de Stadt LA, de Koning MH, van de Stadt RJ, Wolbink G, Dijkmans BA, Hamann D, et al. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum. 2011;63(11):3226–33.
van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7(5):R949–58.
Hecht C, Englbrecht M, Rech J, Schmidt S, Araujo E, Engelke K, et al. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann Rheum Dis. 2015;74(12):2151–6.
Scherer HU, Haupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110: 102400.
Romao VC, Fonseca JE. Major challenges in rheumatology: will we ever treat smarter, instead of just harder? Front Med (Lausanne). 2019;6:144.
Harrold LR, Litman HJ, Connolly SE, Kelly S, Hua W, Alemao E, et al. Effect of anticitrullinated protein antibody status on response to abatacept or antitumor necrosis factor-alpha therapy in patients with rheumatoid arthritis: A US national observational study. J Rheumatol. 2018;45(1):32–9.
Harrold LR, Litman HJ, Connolly SE, Alemao E, Kelly S, Rebello S, et al. Comparative effectiveness of abatacept versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis who are anti-CCP positive in the United States Corrona Registry. Rheumatol Ther. 2019;6(2):217–30.
Kremer JM. The Corrona US registry of rheumatic and autoimmune diseases. Clin Exp Rheumatol. 2016;34(5 101):S96–9.
Cohen J. Statistical power analysis for the behavioral sciences. Routledge Academic, New York 1988.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
Vieira MC, Zwillich SH, Jansen JP, Smiechowski B, Spurden D, Wallenstein GV. Tofacitinib versus biologic treatments in patients with active rheumatoid arthritis who have had an inadequate response to tumor necrosis factor inhibitors: results from a network meta-analysis. Clin Ther. 2016;38(12):2628-41e5.
Shouval A, Lidar M, Reitblat T, Zisman D, Balbir-Gurman A, Mashiach T, et al. Real-world effectiveness of tofacitinib in patients with rheumatoid arthritis: a prospective observational study. Clin Exp Rheumatol. 2021;39(6):1378–84.
Hirose W, Harigai M, Amano K, Hidaka T, Itoh K, Aoki K, et al. Impact of the HLA-DRB1 shared epitope on responses to treatment with tofacitinib or abatacept in patients with rheumatoid arthritis. Arthritis Res Ther. 2021;23(1):228.
Bird P, Hall S, Nash P, Connell CA, Kwok K, Witcombe D, et al. Treatment outcomes in patients with seropositive versus seronegative rheumatoid arthritis in phase III randomised clinical trials of tofacitinib. RMD Open. 2019;5(1): e000742.
Pope JE, Keystone E, Jamal S, Wang L, Fallon L, Woolcott J, et al. Persistence of tofacitinib in the treatment of rheumatoid arthritis in open-label, long-term extension studies up to 9.5 years. ACR Open Rheumatol. 2019;1(2):73–82.
Curtis JR, Chen L, Bharat A, Delzell E, Greenberg JD, Harrold L, et al. Linkage of a de-identified United States rheumatoid arthritis registry with administrative data to facilitate comparative effectiveness research. Arthritis Care Res (Hoboken). 2014;66(12):1790–8.
Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were sponsored by CorEvitas, LLC. CorEvitas has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Inc., Arena, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly and Company, Genentech, Gilead Sciences, Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Inc., LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Inc., Regeneron Pharmaceuticals, Inc., Sanofi, Sun Pharmaceutical Industries Ltd., and UCB S.A.
Medical Writing and Editorial Assistance
Support for third-party writing assistance was furnished by Michelle D. Karpman, PhD, and editorial assistance by Kristen Westerhoff and Leslie A. Whitehead of CorEvitas, LLC and funded by Bristol Myers Squibb.
Author Contributions
Leslie R. Harrold, Keith Wittstock, Sheila Kelly, Vadim Khaychuk, and Xu Han contributed to the concept and design of the study, the statistical analysis plan, report development and review, publication development and review, and are accountable for the final version of the manuscript. Ying Shan, Page C. Moore, and Lin Gua contributed to the concept and design of the study, the statistical analysis plan, report development and review, the statistical analysis, publication development and review, and are accountable for the final version of the manuscript.
Prior Presentation
A portion of this data was presented at ACR Convergence 2021, Virtual, November 5–9, 2021.
Disclosures
Leslie Harrold: employee, shareholder: CorEvitas, LLC; consulting fees: AbbVie, Bristol Myers Squibb, Roche; Speakers Bureau: Bristol Myers Squibb. Keith Wittstock, Sheila Kelly and Vadim Khaychuk: employee or employee/stockholder of Bristol Myers Squibb; Xue Han, former employee of Bristol Myers Squibb; Ying Shan, Page Moore and Lin Guo: employee: CorEvitas, LLC.
Compliance with Ethics Guidelines
The study was performed in accordance with the Declaration of Helsinki and the Guidelines for Good Pharmacoepidemiology Practice (GPP). All participating investigators were required to obtain full board approval for conducting noninterventional research involving human subjects with a limited dataset. Sponsor approval and continuing review was obtained through a central Institutional Review Board (IRB), the New England Independent Review Board (NEIRB; no. 120160610). For academic investigative sites that did not receive authorization to use the central IRB, full board approval was obtained from their respective governing IRBs, and documentation of approval was submitted to CorEvitas, LLC before the site’s participation and initiation of any study procedures. All patients in the registry were required to provide written informed consent and authorization before participating.
Data Availability
Data are available from CorEvitas, LLC through a commercial subscription agreement and are not publicly available. No additional data are available from the authors.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Harrold, L.R., Wittstock, K., Kelly, S. et al. Comparative Effectiveness of Abatacept vs. Tofacitinib in Rheumatoid Arthritis Patients who are CCP+. Rheumatol Ther 10, 575–587 (2023). https://doi.org/10.1007/s40744-022-00523-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00523-z