Abstract
Introduction
The association between diabetes mellitus (DM) and risk of osteoarthritis (OA) is inconsistent based on published observational studies. This study aimed to conduct a two-sample Mendelian randomization (MR) analysis to explore the causal link between glycated hemoglobin (HbA1c) level and OA risk.
Methods
Genome-wide association studies (GWAS) summary statistics were obtained from the publicly available Integrative Epidemiology Unit (IEU) OpenGWAS database. A series of screening processes were performed to select qualified instrumental single-nucleotide polymorphisms (SNPs) strongly related to exposure. The inverse-variance-weighted method, weighted-median method, and MR–Egger method were performed to ensure robust and reliable results. The MR–Egger intercept test, Cochran’s Q test, and the leave-one-out sensitivity analysis were utilized to assess the horizontal pleiotropy, heterogeneities, and stability of these genetic variants for OA. Odds ratio (OR) and 95% confidence interval (CI) were calculated.
Results
MR analyses found a robust causal association of genetically determined HbA1c with knee OA (OR = 1.561; 95% CI 1.110–2.197; P = 0.011), but not with hip OA (OR = 1.073; 95% CI 0.674–1.710; P = 0.766) or overall OA (OR = 1.141; 95% CI 0.904–1.441; P = 0.804). Sensitivity analyses showed that there was a strong association between SNPs and HbA1c (F = 21.138), no evidence of heterogeneity (Q = 150.625, P = 0.402), and no potential SNPs affecting the causal link.
Conclusion
Our MR study supported a causal effect of genetically increased HbA1c on knee OA risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Analyses of HbA1c levels are more consistent with the long-term effects of exposure on outcomes in MR studies. This study aimed to conduct a two-sample MR study to access the potential effect of genetically predicted levels of HbA1c on OA |
What was learned from the study? |
This study investigated the causal association between HbA1c and OA risk using a two-sample MR analysis based on a large GWAS summary statistics database. The current study supported a causal effect of genetically increased HbA1c on knee OA risk. Our study suggested that clinicians should timely manage the HbA1c levels in DM patients to avoid the negative effects of long-term high HbA1c levels on their joints |
Introduction
Osteoarthritis (OA) is one of the most important chronic diseases worldwide, which is the leading cause of disability and negatively affects the quality of life in patients [1]. According to a systematic analysis of the Global Burden of Disease Study, more than 303 million people reported limited mobility due to OA, of which 263 million had knee OA [2]. The risk of OA is related to multiple factors. Older age, female gender, obesity, previous joint injury (e.g., ankle fracture or knee ligament tear), and abnormal joint anatomy (e.g., shallow acetabulum) are all associated with an increased risk of OA [1]. Furthermore, previous studies have reported that some chronic diseases such as diabetes mellitus (DM) are also linked to a higher risk of OA [3,4,5].
The negative effects of DM on OA may be related to the increase of oxidative stress and proinflammatory cytokines, as well as exposure to chronic high glucose concentrations [6]. Several studies have investigated the relationship between DM or hyperglycemia and OA, but no consistent conclusions have been reached [7,8,9,10]. A meta-analysis demonstrated that type 2 DM may be significantly associated with the development of OA [11]. However, an updated meta-analysis showed that DM was not an independent risk factor for OA, which may be related to body mass index (BMI) being the most important confounder [12]. Inconsistent results may be influenced by many factors, such as different study design types (cohort or cross-sectional), adjustment for confounders, location of OA (knee, hip, or hand), and diagnostic indicators (fasting blood glucose, postprandial 2 h blood glucose, or glycated hemoglobin). Existing observational studies cannot address confounding by various biases and reverse causality inference, and are limited by small sample sizes.
Mendelian randomization (MR) uses genetic variants strongly associated with exposure to determine the causal relationship between exposure and outcome, and it avoids reverse causality inference, reflecting the long-term effect of exposure on outcome [13]. A previous MR study between DM and OA did not find a causal relationship between genetically increased fasting and 2-h postprandial blood glucose levels with hip and knee OA risk [14]. However, their study did not analyze glycated hemoglobin (HbA1c) as exposure to OA. HbA1c can better reflect blood glucose levels over longer periods compared with fasting blood glucose and 2-h postprandial blood glucose [15]. Therefore, analyses of HbA1c levels are more consistent with the long-term effects of exposure on outcomes in MR studies. This study aimed to conduct a two-sample MR study to access the potential effect of genetically predicted levels of HbA1c on OA.
Methods
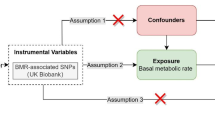
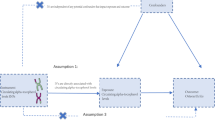
Study Design and Data Source
The study design is shown in Fig. 1. This study utilized a two-sample MR method to explore the causal effect of HbA1C on OA. Three assumptions are to be tested in this MR design: (1) the selected genetic variants are related to HbA1C levels; (2) these genetic variants are not associated with confounders (BMI [16], bone mineral density (BMD) [17], and tobacco smoking [18]); (3) these genetic variants are related to OA only via HbA1C. Summary single-nucleotide polymorphism (SNP)-phenotype association data were obtained from genome-wide association studies (GWAS) in the Integrative Epidemiology Unit (IEU) OpenGWAS database (https://gwas.mrcieu.ac.uk/datasets/). The OpenGWAS database is an open-access cloud-based data infrastructure that currently contains 126 billion genetic associations from 14,582 complete GWAS datasets, representing a diverse range of human phenotypes in diverse populations and disease outcomes [19]. HbA1c was measured from fasting or nonfasting whole blood using National Glycohemoglobin Standardization Program (CGSP)-certified methods [20]. The identification of OA patients was based on the patients who have been diagnosed with OA through the hospital as recorded in the database. [21]. Each study was approved by the respective institutional ethics review board and informed consent of the participants was obtained. Therefore, this study was exempt from institutional review board approval of the Wuhan No.1 Hospital because this study was based on a secondary analysis of the original study. All study conduct adhered to the tenets of the Declaration of Helsinki of 1964 and its later amendments.
Instrument Variables
SNPs associated with exposure (HbA1c), outcome (OA), and confounders (BMI, BMD, and tobacco smoking) are summarized in Table 1. SNPs related to HbA1C are obtained from a larger meta-analysis of GWAS including a total of 46,368 participants [20]. OA was divided into overall OA, knee OA, and hip OA, and the SNPs associated with them were derived from the same GWAS study based on UK Biobank data [21]. Confounders in this study including BMI, heel BMD, forearm BMD, and tobacco smoking, and the SNPs related to them were obtained from the IEU OpenGWAS database. A series of quality control steps were performed to select instrumental variables. First, SNPs with genome-wide significant (P < 0.00000005) were selected. Then, SNPs without linkage disequilibrium (defined by r2 < 0.001 and clump distance > 10,000 kb) were used as instrument variables. Finally, SNPs with minor allele frequency (MAF) < 0.01 and palindromic SNPs were excluded.
Pleiotropy Analysis
For the instrument variables selected, pleiotropy needs to be removed and the exclusion restriction assumptions (assumptions 2 and 3). Genetic variants should be strongly associated with exposure, independent of confounders associated with exposure and outcome, and influence outcome only through exposure. SNPs that were directly related to the OA [P < 0.017 = 0.05/3 (one exposure and three outcomes)] and not through HbA1c were excluded (assumption 3). SNPs associated with confounders (BMI, heel BMD, forearm BMD, and tobacco smoking) were excluded (assumption 2). The intercept and P-value of MR–Egger regression were calculated to test for pleiotropic bias. MR–PRESSO analysis was utilized to conform to the horizontal pleiotropic outliers [22].
Statistical Analysis
Two-sample MR analysis was performed to estimate the effect of HbA1c on OA using summarized data of SNP-HbA1c and SNP-OA associations. The inverse-variance-weighted (IVW) method based on the fixed-effects model is the main analytical method used in this study to generate a causal effect estimate. The IVW method is based on the Wald ratio method to obtain an unconfounded estimate of the exposure of genetic prediction outcomes [23]. The weighted-median method provided robust and consistent estimates of outcomes, even though nearly 50% of the genetic variants were invalid instruments [24]. The MR–Egger method used weighted linear regression to detect broad horizontal pleiotropy. However, MR–Egger results often exhibit low precision and may be affected by abnormal genetic variation. The leave-one-out analysis was performed to assess the robust association of SNPs with HbA1c levels, and the symmetry in the resulting figure represents no pleiotropy. For testing the results, Cochran’s Q-test was conducted to evaluate the statistical heterogeneity between SNPs in the IVW method, with P < 0.05 considered significantly heterogeneous. Furthermore, we calculated the F-statistics and variance explained (R2) for each exposure was calculated to assess the instrument variable strength, and F-statistics > 10 were considered to imply adequate instrument strength [25]. An online tool, mRnd, was used to calculate the power to detect causal effect. The equation of the mRnd tool used an approximate linear model applied to the binary outcomes, and the parameters calculated by the equation included the sample size, the effect size of exposure on outcome at the epidemiological level, the standard deviation of exposure and outcome, and the proportion of phenotypic variation explained by instrument variable SNPs [26]. Power calculation demonstrated that the sample provided sufficient statistical power (≥ 88%) for causal analysis of HbA1c on knee and overall OA (Supplementary Table 1). All statistical analyses were completed in R 4.1.1 software (TwoSampleMR package), and the observed two-sided P < 0.017 was considered statistically significant.
Results
Instrument Variables Selection and Pleiotropy
A total of 244 SNPs associated with HbA1c were selected with genome-wide significance (P < 5 × 10–8) and no linkage disequilibrium. Table 2 presents the results of the pleiotropy analysis of the SNPs. A total of 221 SNPs overlapped between SNP-HbA1c and SNP-OA. After excluding palindromic SNPs and SNPs with MAF, 175 SNPs were retained. After removing SNPs that directly affect OA but not through HbA1c (assumption 3), the numbers of SNPs associated with knee OA, hip OA, and overall OA were 169, 168, and 166, respectively. SNPs associated with confounders were excluded (assumption 2), and the numbers of SNPs associated with knee OA, hip OA, and overall OA through HbA1c remained 148, 147, and 146, respectively. The MR–Egger analysis demonstrated no pleiotropic bias in the remaining SNPs.
Two-sample MR Analysis for Causal Link of HbA1c with OA
Figure 2 presents the MR estimates for different methods to assess the causal effect of HbA1c on OA. The inverse-variance-weighted indicated that genetically predicted HbA1c was related to an increased risk of knee OA [odds ratio (OR) = 1.561; 95% confidence interval (CI), 1.110–2.197; P = 0.011]. The MR–Egger analysis demonstrated that genetically predicted HbA1c was suggestively associated with an increased risk of knee OA (OR = 3.175; 95% CI 1.080–9.315; P = 0.037). However, the associations of HbA1c with the hip OA (OR = 1.073; 95% CI 0.674–1.710; P = 0.766) and overall OA (OR = 1.141; 95% CI 0.904–1.441; P = 0.804) were not found.
Table 3 presents the results of the instrumental variable strength and heterogeneity test. There was a strong association between SNPs and HbA1c (F = 21.138), and no evidence of heterogeneity on the inverse-variance-weighted analysis (Q = 150.625, P = 0.402). Furthermore, the leave-one-out analysis showed that there were no potential SNPs affecting the causal link and the results were stable (Supplement Fig. 1).
Discussion
The association between DM and OA is controversial in the studies that have been reported. This study used summary statistics from GWASs to explore the causal association between HbA1c and OA. The results found that HbA1c causally increased knee OA, whereas the estimated effect of HbA1c on hip OA and overall OA were not significant, suggesting that HbA1c had different causal effects on OA at different sites.
Previous studies have demonstrated that type 2 DM was associated with an increased risk of OA [3, 4]. Dubey et al. found that type 1 and type 2 DM were closely related to knee OA [8]. Eymard et al. suggested that type 2 DM was significantly linked to reduced joint space in men with knee OA after adjusting for confounders [9]. A cohort study showed that high levels of HbA1c and fasting serum glucose were positively related to a higher risk of knee OA [5]. However, the conclusions of two meta-analyses on the relationship between DM and OA were inconsistent [11, 12]. The meta-analysis of Williams et al. found that DM was associated with the development of OA [11], whereas Khor et al. indicated that DM was not a risk factor for OA [12]. Previous observational and interventional studies on the association of HbA1c with OA risk may be limited to drawing a causal relationship. The current study explored the causal relationship between HbA1c and OA risk using MR analysis. Our results suggested a causal relationship between high HbA1c levels and the risk of knee OA. However, a previous MR study found no significant causal effects of genetically increased type 2 DM risk, fasting glucose, and 2-h postprandial glucose on hip OA and knee OA [14]. A possible reason for the inconsistency between our results and the previous study was that the study by Cui et al. focused on fasting glucose and 2-h postprandial glucose, while our study focused on HbA1c. Compared with fasting glucose and 2-h postprandial glucose, HbA1c can better reflect blood glucose levels in a relatively long period of time [15]. Furthermore, some confounders have been reported that may affect the accuracy of the results. Hartley et al. found a causal effect between high BMD levels and an increased risk of OA [17]. A large MR study based on the UK Biobank demonstrated that BMI had a major causal effect on OA risk at weight-bearing joints [16]. Our study excluded some SNPs related to confounders such as BMI, BMD, and tobacco smoking, which ensured the reliability of the results.
OA is related to a local and systemic low-grade inflammation state [6]. Some studies have reported the possible mechanisms between blood glucose and OA. The effect of type 2 DM on OA may be through the following pathways: high blood glucose exposure shows local toxicity to joint tissue, increasing local oxidative stress, cytokine, proteolytic enzyme production, and advanced glycation end products (AGEs) accumulation [6, 27, 28]. High intra-articular glucose levels promote cartilage degradation by increasing the expression of cyclooxygenase-2 (COX-2), and matrix metalloproteinase 13 (MMP-13), while reducing collagen type II and peroxisome proliferator-activated receptor gamma (PPARγ) synthesis [4]. High-glucose environment inhibited autophagy in chondrocytes and the activity of hemooxygenase-1, which has important antioxidant properties [29]. This results in increased oxidative stress and cartilage damage, and promotes the secretion of potent proinflammatory mediators such as prostaglandin E2 (PGE2), interleukin-1β (IL-1β) IL-6, IL-10, and tumor necrosis factor-α (TNF-α) [30]. Furthermore, the accumulation of AGEs can adversely affect cartilage turnover and mechanical properties [31].
In this study, we selected instrument variables to examine the causal relationship between HbA1c and OA through a series of quality control steps, such as selecting SNPs with the genome-wide association and independent inheritance without any LD, and excluding SNPs related to confounders. To ensure that the results were robust and reliable, sensitive analyses were performed and F-statistics were used to assess the strength of the association between the SNPs and exposure. However, some limitations of our study need to be considered. First, although some of the reported confounders such as BMI, BMD, and tobacco smoking were excluded from our study, there may be some potential confounders that could have influenced the results. Second, we were unable to estimate the degree of overlap of participants in the studies, since the exposure and outcome studies used in two-sample MR analysis should not involve overlapping participants. However, sample overlap bias can be minimized by strong instrumental variables. Third, due to database limitations, this study did not analyze the relationship between HbA1c and OA, and the relationship between HbA1c levels and non-weight-bearing joint OA needs to be explored.
Conclusions
This study investigated the causal association between HbA1c and OA risk using a two-sample MR analysis based on a large GWAS summary statistics database. The current study supported a causal effect of genetically increased HbA1c on knee OA risk. Our study suggested that clinicians should timely manage the HbA1c levels in DM patients to avoid the negative effects of long-term high HbA1c levels on their joints.
References
Yue L, Berman J. What is osteoarthritis? JAMA. 2022;327:1300.
Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392:1789–858.
Veronese N, Cooper C, Reginster JY, et al. Type 2 diabetes mellitus and osteoarthritis. Semin Arthritis Rheum. 2019;49:9–19.
Cannata F, Vadalà G, Ambrosio L, et al. Osteoarthritis and type 2 diabetes: from pathogenetic factors to therapeutic intervention. Diabetes Metab Res Rev. 2020;36: e3254.
Zheng J, Huang X, Huang J, et al. Associations of diabetes status and hyperglycemia with symptomatic knee osteoarthritis. Arthritis Care Res. 2022. https://doi.org/10.1002/acr.24872.
Courties A, Sellam J. Osteoarthritis and type 2 diabetes mellitus: What are the links? Diabetes Res Clin Pract. 2016;122:198–206.
Rogers-Soeder TS, Lane NE, Walimbe M, et al. Association of diabetes mellitus and biomarkers of abnormal glucose metabolism with incident radiographic knee osteoarthritis. Arthritis Care Res. 2020;72:98–106.
Dubey NK, Ningrum DNA, Dubey R, et al. Correlation between diabetes mellitus and knee osteoarthritis: a dry-to-wet lab approach. Int J Mol Sci. 2018;19:3021.
Eymard F, Parsons C, Edwards MH, et al. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthri Cartil. 2015;23:851–9.
Nieves-Plaza M, Castro-Santana LE, Font YM, Mayor AM, Vilá LM. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2013;19:1–6.
Williams MF, London DA, Husni EM, Navaneethan S, Kashyap SR. Type 2 diabetes and osteoarthritis: a systematic review and meta-analysis. J Diabetes Complicat. 2016;30:944–50.
Khor A, Ma CA, Hong C, Hui LL, Leung YY. Diabetes mellitus is not a risk factor for osteoarthritis. RMD Open. 2020;6:e001030.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318:1925–6.
Cui Z, Feng H, He B, Xing Y, Liu Z, Tian Y. Type 2 diabetes and glycemic traits are not causal factors of osteoarthritis: a two-sample mendelian randomization analysis. Front Genet. 2020;11: 597876.
Ho-Pham LT, Nguyen UDT, Tran TX, Nguyen TV. Discordance in the diagnosis of diabetes: comparison between HbA1c and fasting plasma glucose. PLoS One. 2017;12: e0182192.
Funck-Brentano T, Nethander M, Movérare-Skrtic S, Richette P, Ohlsson C. Causal factors for knee, hip, and hand osteoarthritis: a Mendelian randomization study in the UK biobank. Arthrit Rheumatol (Hoboken, NJ). 2019;71:1634–41.
Hartley A, Sanderson E, Granell R, et al. Using multivariable Mendelian randomization to estimate the causal effect of bone mineral density on osteoarthritis risk, independently of body mass index. Int J Epidemiol. 2022;51:1254–67.
Ni J, Wang P, Yin KJ, et al. Does smoking protect against developing osteoarthritis? Evidence from a genetically informed perspective. Semin Arthritis Rheum. 2022;55: 152013.
Elsworth B, Lyon M, Alexander T, et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv. 2020;08: 244293.
Soranzo N, Sanna S, Wheeler E, et al. Common variants at 10 genomic loci influence hemoglobin A1(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59:3229–39.
Zengini E, Hatzikotoulas K, Tachmazidou I, et al. Genome-wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat Genet. 2018;50:549–58.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Hartwig FP, Davies NM, Hemani G, Davey SG. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45:1717–26.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64.
Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–501.
Laiguillon MC, Courties A, Houard X, et al. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthr Cartil. 2015;23:1513–22.
Rosa SC, Rufino AT, Judas FM, Tenreiro CM, Lopes MC, Mendes AF. Role of glucose as a modulator of anabolic and catabolic gene expression in normal and osteoarthritic human chondrocytes. J Cell Biochem. 2011;112:2813–24.
Ribeiro M, López de Figueroa P, Blanco FJ, Mendes AF, Caramés B. Insulin decreases autophagy and leads to cartilage degradation. Osteoarthr Cartil. 2016;24:731–9.
Vaamonde-Garcia C, Courties A, Pigenet A, et al. The nuclear factor-erythroid 2-related factor/heme oxygenase-1 axis is critical for the inflammatory features of type 2 diabetes-associated osteoarthritis. J Biol Chem. 2017;292:14505–15.
Yang Q, Chen C, Wu S, Zhang Y, Mao X, Wang W. Advanced glycation end products downregulates peroxisome proliferator-activated receptor γ expression in cultured rabbit chondrocyte through MAPK pathway. Eur J Pharmacol. 2010;649:108–14.
Acknowledgements
Funding
The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Lirong Chen and Huiqin Yang designed the study. Lirong Chen wrote the manuscript. Lirong Chen and Chao Jia collected, analyzed, and interpreted the data. Huiqin Yang critically reviewed, edited, and approved the manuscript. All authors read and approved the final manuscript.
Disclosures
Lirong Chen, Chao Jia, and Huiqin Yang have nothing to disclose.
Compliance with Ethics Guidelines
Each study was approved by the respective institutional ethics review board and informed consent of the participants was obtained. Therefore, this study was exempt from institutional review board approval of the Wuhan No.1 Hospital because this study was based on a secondary analysis of the original study. All study conduct adhered to the tenets of the Declaration of Helsinki of 1964 and its later amendments.
Data Availability
The original data for this study were obtained from the OpenGWAS database, https://gwas.mrcieu.ac.uk/datasets/. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, L., Jia, C. & Yang, H. Causal Effect of Higher Glycated Hemoglobin (HbA1c) Levels on Knee Osteoarthritis Risk: A Mendelian Randomization Study. Rheumatol Ther 10, 239–247 (2023). https://doi.org/10.1007/s40744-022-00510-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00510-4