Abstract
Introduction
We conducted a post hoc analysis of efficacy and safety of filgotinib stratified by estimated radiographic progression rate before baseline (BL) in patients with rheumatoid arthritis (RA) who had inadequate response to methotrexate (MTX; FINCH 1; NCT02889796) or were naïve to it (FINCH 3; NCT02886728).
Methods
Radiographic progression rate was BL-Modified Total Sharp Score (mTSS) divided by RA duration (BL mTSS/year); estimated rapid radiographic progression (e-RRP) was BL change in mTSS/year ≥ 5; and estimated nonrapid radiographic progression (e-NRRP) was BL mTSS/year < 5. Efficacy and safety were compared between subgroups. All p-values are nominal.
Results
In FINCH 1 and FINCH 3, 558/1755 (31.8%) and 787/1249 (63.0%) patients, respectively, had BL e-RRP. BL characteristics were generally similar between subgroups within each trial. At week (W) 24, in FINCH 1, proportions achieving a Disease Activity Score 28 for rheumatoid arthritis with C-reactive protein < 2.6 were significantly greater with filgotinib 200 (FIL200) and 100 mg (FIL100) versus placebo among e-RRP and e-NRRP subgroups. In each study, proportions of FIL-treated patients achieving Clinical Disease Activity Index ≤ 2.8 and Simple Disease Activity Index ≤ 3.3 were similar between subgroups. In FINCH 3, disease activity measures were at least numerically improved among patients receiving FIL versus MTX monotherapy. At W24, mTSS changes from BL (CFB) were greater among patients with e-RRP in FINCH 1 and FINCH 3 versus e-NRRP (0.81 versus 0.19, p = 0.001; 0.67 versus 0.25, p = 0.31, respectively). At W52, in FINCH 1, mTSS CFBs were smaller among e-RRP patients treated with FIL200 (0.40; p < 0.001) and FIL100 (0.77; p = 0.024) versus adalimumab (ADA; 1.46). In FINCH 3 at W52, mTSS CFBs were significantly smaller with FIL200 versus MTX among e-RRP patients. Rates of treatment-emergent adverse events (AEs) were comparable between subgroups and across treatment arms.
Conclusions
Patients with previous e-RRP who received standard care tended to progress radiographically. FIL200 demonstrated persistent, consistent benefit for disease activity control among e-RRP and e-NRRP subgroups, and AE profiles were similar between subgroups. Although filgotinib efficacy was somewhat reduced among patients with e-RRP, filgotinib treatment slowed radiographic progression in both subgroups.
Trial Registration
Clinicaltrials.gov NCT02889796, NCT02886728.
Similar content being viewed by others
Why carry out this study? |
Several randomized clinical trials and real-world publications have confirmed that rapid radiographic progression within the first year of rheumatoid arthritis (RA) strongly predicted subsequent radiographic progression and functional impairment. |
In FINCH 1, filgotinib 200 mg (FIL200) and filgotinib 100 mg (FIL100) plus methotrexate (MTX) were more efficacious than placebo plus MTX in patients with inadequate response (IR) to MTX. FIL200 showed comparable efficacy and safety to the tumor necrosis factor inhibitor adalimumab (ADA). In FINCH 3, FIL200 or FIL100 plus MTX and FIL200 monotherapy were associated with significant improvements in disease activity versus MTX alone in an MTX-naïve population. |
What was learned from this study? |
The Modified Total Sharp Score (mTSS) change from baseline (BL) at week 24 among patients who received MTX alone in both the filgotinib MTX-IR and MTX-naïve trials was approximately 2.5–4 times greater among patients with estimated rapid radiographic progression (e-RRP; BL mTSS/year ≥ 5) versus patients with estimated nonrapid radiographic progression (e-NRRP; BL mTSS/year < 5). Only FIL200 plus MTX showed consistent suppression of mTSS progression over 52 weeks in both subgroups. |
RA patients with previous rapid radiographic progression had higher risk of structural progression in subsequent treatment phases versus patients who had no or less previous rapid progression; status of previous rapid progression may need to be considered when recommending treatment. |
Filgotinib 200 mg once daily may provide consistent and sustained benefit for clinical outcomes and freedom from radiographic progression in both e-RRP and e-NRRP subgroups, while demonstrating a consistent safety and tolerability profile. |
Introduction
Treatment goals for rheumatoid arthritis (RA) include achievement of clinical remission and prevention of structural damage. The 2019 European Alliance of Associations for Rheumatology (EULAR) RA management guidelines [1] indicate that treatment decisions should be based on considerations of both efficacy, such as disease activity and progression of structural damage, and safety, including comorbidities and risk of adverse events. For patients who fail initial treatment with methotrexate (MTX) or other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), treatment decisions around addition of an advanced therapy should consider the presence of risk factors for poor prognosis (poor prognostic factors, PPF) of long-term joint damage. Several risk factors related to radiographic progression of joint damage have been identified for early onset of RA, including seropositive status for rheumatoid factor (RF) or anti-cyclic citrullinated antibodies (anti-CCP), persistently high disease activity, high acute reactants (such as C-reactive protein, CRP), high swollen joint count, and presence of radiographic bone erosion in the early phase of disease onset [1,2,3].
In the most recent drug development programs of advanced therapies for RA, including biologic agents (bDMARDs) and targeted synthetic DMARDs [tsDMARDs, i.e., Janus kinase (JAK) inhibitors], patients with moderate-to-severe RA who had at least one of the defined PPFs were enrolled for testing both clinical outcomes and radiographic structural endpoints [4,5,6,7]. The Phase 3 clinical program for filgotinib, an oral JAK1-preferential inhibitor, studied populations of RA patients who were naïve to MTX (MTX-naïve, FINCH 3: NCT02886728) or who had inadequate response to MTX (MTX-IR, FINCH 1: NCT02889796) and who had at least one of the major PPFs defined above. In FINCH 1, filgotinib 200 mg (FIL200) and filgotinib 100 mg (FIL100) were evaluated as add-on therapy to stable background MTX, and both FIL200 and FIL100 were more efficacious than placebo (PBO) plus MTX [8]. In FINCH 3, FIL200 or FIL100 in combination with MTX and FIL200 monotherapy were associated with significant improvements in disease activity versus MTX alone [9]. Subsequent post hoc analyses confirmed these findings among patients with all four PPFs and with < four PPFs in both the MTX-naïve [10] and MTX-IR (submitted) populations.
Several publications, from randomized clinical trials and real-world data, have confirmed that having rapid radiographic progression (RRP) within the first year of RA was a strong predictor of subsequent radiographic progression and functional impairment [11,12,13]. Therefore, it is important to determine whether patients with rapidly progressing joint damage with first-line treatment are likely to continue to rapidly progress despite subsequent treatment. We conducted post hoc analyses FINCH 1 (MTX-IR) and FINCH 3 (MTX-naive) to determine whether treatment with filgotinib was associated with reduced radiographic progression among patients who showed previous rapid radiographic progression.
Methods
Study Design and Patient Population
The study designs of FINCH 1 and FINCH 3 were described in detail previously [8, 9] (also Supplementary Fig. 1). In brief, in FINCH 1, RA patients with moderate-to-severe disease activity who were inadequately responding to MTX were randomized to oral FIL200 or FIL100 once daily, subcutaneous adalimumab (ADA) 40 mg every other week, or PBO, 3:3:2:3. All patients took stable doses of MTX at study entry. At week 24, patients who received PBO were rerandomized to FIL200 or FIL100 and continued up to week 52 in blinded fashion. The key inclusion criteria were as follows: (1) seropositivity for RF or anti-CCP antibodies; (2) ≥ one joint erosion on hand/wrist or foot radiographs or ≥ 3 erosions if seronegative for anti-CCP antibodies or RF; or (3) serum high-sensitivity CRP (hsCRP) ≥ 6 mg/L. In FINCH 3, patients who were naïve to MTX (doses of MTX up to 3 weeks were allowed) were randomized 2:1:1:2 to receive oral FIL200 once daily plus MTX, FIL100 once daily plus MTX, FIL200 monotherapy once daily, or oral MTX monotherapy, for up to 52 weeks. The key inclusion criteria were as follows: (1) seropositivity for RF or anti-CCP antibodies; (2) serum hsCRP ≥ 4 mg/L; or (3) ≥ one documented joint erosion on radiographs of the hands, wrists, or feet.
The study protocols were reviewed and approved by all local institutional review boards or ethics committees of participating institutions. The studies were carried out in accordance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. Informed consent to participate was provided by all participants.
Endpoints and Assessments
The primary and key secondary efficacy endpoints of FINCH 1 and FINCH 3 were described previously [8, 9]. In brief, the primary endpoint was American College of Rheumatology 20% improvement (ACR20) response at week 12 for FINCH 1 and at week 24 for FINCH 3. Key secondary endpoints included proportions of patients with Disease Activity Score in 28 joints with CRP (DAS28[CRP]) < 2.6 at week 24 and radiographic assessment of joint structure by change from baseline (BL) van der Heijde Modified Total Sharp Score (mTSS CFB) [14] at week 24. Primary and key secondary endpoints were also measured up to week 52.
Safety was assessed from records of treatment-emergent adverse events (TEAEs) coded according to the Medical Dictionary for Regulatory Activities V.22.0 and graded using the Common Terminology Criteria for Adverse Events V.4.03. AEs of special interest, including adjudicated major adverse cardiac events (MACE) and venous thromboembolic events (VTE), malignancy, serious infections, and opportunistic infections were collected and summarized.
The definition of rapid radiographic progression applied in this post hoc analysis was estimated yearly change in mTSS ≥ 5 [15]. The yearly radiographic progression rate was estimated by dividing BL mTSS by duration of RA in years (BL mTSS/year). Thus, estimated rapid radiographic progression (e-RRP) was ≥ 5, while estimated nonrapid radiographic progression (e-NRRP) was < 5.
Efficacy outcomes included proportions of patients achieving DAS28(CRP) < 2.6, Clinical Disease Activity Index (CDAI) ≤ 2.8 or Simple Disease Activity Index (SDAI) ≤ 3.3 at week 24. Radiographic progression was evaluated by mean mTSS CFB at weeks 24 and 52 for both trials. Proportions of patients who showed no progression, i.e., mean mTSS CFB ≤ 0.5 at weeks 24 and 52, were determined. For those patients who received PBO in FINCH 1 up to week 24 and switched to FIL100 or FIL200, the mean mTSS CFB and proportions who were progression free were also evaluated at week 52.
Statistical Analysis
For efficacy and safety analyses, the full analysis set (FAS) was used, which comprised all patients who were randomized and received at least one dose of study drug [8, 9]. For this post hoc analysis, no predefined hypothesis was tested to compare the efficacy and safety of patients with e-RRP and e-NRRP or between treatment arms. All comparisons are descriptive, and all p-values presented are nominal. Comparison of the proportions of clinical remissions, mean mTSS CFB, and proportions free of radiographic progression were performed between treatment arms, within the e-RRP and e-NRRP subgroups. Analyses of binary endpoints, i.e., proportions of clinical remission and proportions free of radiographic progression, were performed using Fisher’s exact test, and patients with missing data were imputed as nonresponders. For the continuous endpoint, i.e., mean mTSS CFB, missing data were not imputed, and a mixed model for repeated measures with treatment, visit, treatment-by-visit interaction, and BL value included as fixed effects, and patient as a random effect was used. Covariate analysis to identify the associations between BL factors and previous e-RRP was also conducted, referring to the unadjusted analyses and criteria previously reported [16, 17].
Results
Patient Characteristics
Of 1755 MTX-IR (FINCH 1 study) and 1249 MTX-naïve patients (FINCH 3 study) who were randomized and received study drugs, 588 (31.8%) and 787 (63.0%) had BL mTSS/year ≥ 5, representing the e-RRP subgroup. Tables 1 and 2 present BL patient and disease characteristics of the two subgroups in the MTX-IR and MTX-naïve populations. Except for the mTSS-related parameters, which were used for stratification, BL characteristics—including age, sex, DAS28(CRP), CDAI, SDAI, RF positivity, proportion of high hsCRP, and components of American College of Rheumatology core set—were generally similar between e-RRP and e-NRRP subgroups in both MTX-IR and MTX-naïve populations. Body weight (BW) and body mass index (BMI) tended to be slightly lower in the e-RRP subgroup among the MTX-IR population, while RA duration, Health Assessment Questionnaire–Disability Index (HAQ-DI), and proportion of nonsmokers tended to be slightly lower for the same subgroup among the MTX-naïve population. There were no differences in the mean dose of concurrent corticosteroids between e-RRP and e-NRRP in either trial population, and the mean concomitant MTX dose was similar in the MTX-IR population. Mean yearly mTSS were 17.57–23.09 in the e-RRP subgroups and 1.46–1.61 in the e-NRRP subgroups for MTX-IR and 55.87–123.93 and 1.73–2.04 for MTX-naïve; median yearly mTSS in e-RRP and e-NRRP subgroups were, respectively, 10.34 and 1.53 in the MTX-IR trial and 24.73 and 1.57 in the MTX-naïve population.
Efficacy: Disease Activity and Remission Measures
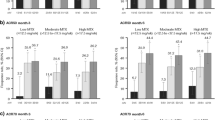
For patients from FINCH 1 and FINCH 3 within the e-RRP or e-NRRP subgroup, achievement of DAS28(CRP) < 2.6 at week 24 was more prevalent among filgotinib plus MTX regimens compared with MTX treatment (p < 0.05) with numerically greater rates in the FIL200 plus MTX arms (Fig. 1). Similar trends were confirmed with remission criteria in both subgroups in both trials, with the exception of CDAI remission for patients with e-RRP in the FIL200 and FIL100 arms (p = 0.077 and 0.14, respectively) and SDAI remission in the FIL100 arm (p = 0.13) in the MTX-IR trial.
DAS28(CRP) < 2.6 and CDAI and SDAI remission rates at week 24. Error bars represent the 95% CI. p-Values are nominal versus PBO or MTX. BL baseline, CDAI Clinical Disease Activity Index, DAS28(CRP) Disease Activity Score in 28 joints with C-reactive protein, e-NRRP estimated nonrapid radiographic progression, e-RRP estimated rapid radiographic progression, FIL filgotinib, MTX methotrexate, SDAI Simplified Disease Activity Index
In both FINCH 1 and FINCH 3 populations, there were no significant differences in proportions achieving clinical responses between the e-RRP and e-NRRP subgroups within each treatment arm, except for patients who received ADA (Tables 3, 4). In the MTX-IR trial, the proportions of patients between the e-RRP and e-NRRP subgroups who achieved DAS28(CRP) < 2.6, CDAI ≤ 2.8, and SDAI ≤ 3.3 were not significantly different in FIL200 (55.3% versus 55.5%, 19.3% versus 27.1%, 23.0% versus 28.8%, among e-RRP and e-NRRP subgroups, respectively) and FIL100 (36.5% versus 42.2%, 18.2% versus 22.7%, 17.8% versus 21.4%). Lower rates of DAS28(CRP) < 2.6 achievement and of CDAI and SDAI remission were seen in e-RRP versus e-NRRP for patients who received ADA (32.6% versus 45.5%, p = 0.052; 8.6% versus 24.7%, p = 0.001; 9.8% versus 24.3%, p = 0.004, respectively; Supplementary Figure 2). In the MTX-naïve trial, there were no significant differences between e-RRP and e-NRRP subgroups for clinical response rates at week 24 across all treatment arms (Tables 3, 4).
Efficacy: Radiographic Progression
In both trials, radiographic progression as measured by mTSS CFB at week 24 was numerically greater in e-RRP subgroups across all the treatment arms compared with e-NRRP subgroups of the same treatment (e.g., 0.81 versus 0.19, p < 0.001, in MTX-IR patients taking PBO plus MTX and 0.67 versus 0.25, p = 0.310, in MTX-naïve patients taking MTX) (Tables 3, 4). In MTX-naïve patients receiving MTX alone at week 52, mTSS CFB was approximately 1.0 in patients with e-RRP and below 0.5 in patients with e-NRRP (Fig. 2, Table 4). No significant difference was observed (p = 0.30). However, the proportion with no radiographic progression was lower in the e-RRP subgroup compared with that in the e-NRRP subgroup.
mTSS change from baseline at weeks 24 and 52 in e-RRP/e-NRRP subgroups. A FINCH 1 (MTX-IR). B FINCH 3 (MTX-naïve). All p-values are nominal versus PBO or ADA at W24 and versus ADA at W52 in FINCH 1 and versus MTX mono in FINCH 3. ADA at W24 is out of scope for statistical calculation. ADA adalimumab, BL baseline, CFB change from baseline, e-NRRP estimated nonrapid radiographic progression, e-RRP estimated rapid radiographic progression, FIL100 filgotinib 100 mg, FIL200 filgotinib 200 mg, IR inadequate response, LS least-squares, mono monotherapy, mTSS Modified Total Sharp Score, MTX methotrexate, PBO placebo, W week
In the MTX-IR population, both the FIL200 and FIL100 groups showed significant suppression of radiographic progression compared with PBO at week 24 in the e-RRP subgroup (p = 0.007 and 0.01, respectively) and in the e-NRRP subgroup (p = 0.006 and 0.025, respectively, for FIL200 versus PBO and for FIL100 versus PBO, Fig. 2). At week 52, mTSS CFB was significantly smaller in both the FIL200 and FIL100 groups compared with ADA among the e-RRP subgroup (0.40, 0.77, and 1.46 for FIL200, FIL100, and ADA, respectively, p < 0.001 and = 0.024 for FIL200 and FIL100 versus ADA, respectively), while changes were comparable between treatments among the e-NRRP subgroup. Among patients rerandomized from PBO to FIL200 or FIL100, those in the e-RRP subgroups had greater radiographic progression (1.63 or 1.58) than did patients in the e-NRRP subgroups (0.25 or 0.69; p < 0.001 or = 0.022, respectively; Fig. 2 and Table 3).
In the MTX-naïve population, only the FIL200 monotherapy group among patients with e-RRP showed significantly reduced mTSS CFB at week 24 versus the MTX monotherapy group (p = 0.015). Consistent with findings for the full study population [9], at week 52, all filgotinib treatment groups showed significantly lower mTSS CFB in patients with e-RRP compared with MTX monotherapy (p < 0.01, = 0.043, and = 0.011 for FIL200 plus MTX, FIL100 plus MTX, and FIL200 monotherapy, respectively). The results among patients with e-NRRP were mixed, partially due to relatively small changes in mTSS even among patients who were receiving MTX monotherapy (Fig. 2).
The proportions of patients with no radiographic progression in treatment arms were compared with PBO/MTX arms, and the differences were examined between e-RRP and e-NRRP subgroups of each treatment at weeks 24 and 52 (Figs. 3, 4, Tables 3, 4). In the MTX-IR population, the proportion with no radiographic progression of PBO plus MTX was significantly lower among patients with e-RRP compared with those with e-NRRP at week 24. The patients with e-RRP who were receiving FIL200 or FIL100 showed greater proportions with no radiographic progression (mTSS CFB ≤ 0.5) compared with patients who were receiving PBO at week 24 (87.7%, 88.5%, 76.2%, respectively; p = 0.024 and 0.014, respectively, for FIL200 plus MTX and FIL100 plus MTX versus PBO plus MTX; Fig. 3A). For patients with e-NRRP, numerically greater proportions with no radiographic progression were observed in all treatment arms, including PBO plus MTX, but only FIL200 showed significantly greater proportions with no radiographic progression versus PBO (97.0% versus 91.6%, p = 0.012). At week 52, FIL200 plus MTX sustained the proportion of e-RRP patients with no progression, while with FIL100 plus MTX it decreased from 88.5% to 76.8%. For those with e-NRRP, proportions with no progression were sustained in all treatment arms, including FIL100 plus MTX. The proportions of both switching arms in e-RRP subgroup were reduced from 76.2% to 65.3% or 69.1%, respectively, for PBO plus MTX to FIL200 plus MTX or to FIL100 plus MTX, while those in the e-NRRP subgroup were sustained. Among MTX-naïve patients, those with e-RRP receiving MTX showed lower proportions of no radiographic progression compared with those with e-NRRP at weeks 24 and 52 (p = 0.032 and 0.029, respectively; Table 4). Only those receiving FIL200 (with or without MTX) in the e-RRP subgroup showed greater proportions of no radiographic progression at week 24 compared with those receiving MTX (Fig. 3B). While those receiving FIL200 plus MTX showed lower proportions of no radiographic progression in e-RRP subgroup compared with those with e-NRRP at week 24 (86.9% versus 94.0%, p = 0.033), no significant difference was observed at week 52 (86.0% versus 91.9%, p = 0.12, Table 4). The proportion of no radiographic progression at week 24 in all treatment arms, except FIL200 monotherapy, were sustained at week 52. In Supplementary Fig. 3, proportions with no mTSS worsening (≤ 0.0 points) are compared; at week 24, proportions with ≤ 0.0 change were higher in the FIL200 plus MTX group compared with PBO plus MTX in MTX-IR patients and in the FIL200 plus MTX group compared with MTX in MTX-naïve patients. The difference between FIL200 plus MTX and MTX alone was maintained at week 52 in MTX-naïve patients.
Ratio of no radiographic progression in e-RRP and e-NRRP subgroups. A Ratio of no radiographic progression in FINCH 1 (MTX-IR). B Ratio of no radiographic progression in FINCH 3 (MTX-naïve). Full analysis set includes subjects who were randomized and received ≥ 1 dose of study drug. The Fisher’s exact test was used for comparisons between treatment groups. Observed case: only observed values were used for analysis. No missing data imputation was performed. Odds ratio was calculated using 2 × 2 table and the 95% exact confidence limits were provided. *Nominal p < 0.05. For FINCH 1 (MTX-IR), Week 24 ORs are FIL versus PBO + MTX; Week 52 ORs are FIL versus ADA + MTX. For FINCH 3 (MTX-naïve), Week 24 and 52 ORs are FIL versus MTX. ADA adalimumab, e-NRRP estimated nonrapid radiographic progression, e-RRP estimated rapid radiographic progression, MTX methotrexate, OR odds ratio, PBO placebo, RRP rapid radiographic progression
Cumulative percentile of mTSS change from baseline, and proportions without radiographic progression, at W24 and W52 by e-RRP/e-NRRP subgroup in A FINCH 1 (MTX-IR) and B FINCH 3 (MTX-naïve). Cumulative probability plot of mTSS change from BL at W24 and W52 of each treatment arm for e-RRP and e-NRRP subgroups. CFB in mTSS was analyzed using an analysis of covariance model in which actual scores were converted to rank scores, using the treatment group as a factor and BL rank score as a covariate. Rate of mTSS nonprogression (mTSS CFB ≤ 0.5) was compared using Fisher’s exact test. ADA adalimumab, BL baseline, CFB change from BL, e-NRRP estimated nonrapid radiographic progression, e-RRP estimated rapid radiographic progression, FIL100 filgotinib 100 mg, FIL200 filgotinib 200 mg, IR inadequate response, mono monotherapy, mTSS Modified Total Sharp Score, MTX methotrexate, PBO placebo, W week
Association Factors
We conducted univariate and multivariate analyses for BL characteristics to understand the factors associated with e-RRP (Supplementary Tables 1 and 2). For univariate analysis, in the MTX-IR population, RA duration, anti-CCP antibody positivity, body mass index, hsCRP ≥ 30 mg/L, Physician Global Assessment (PhGA), and mTSS-related parameters were significantly associated with e-RRP (Supplementary Table 1). In the MTX-naïve population, age, RA duration, hsCRP ≥ 30 mg/L (with reference group of hsCRP < 10 mg/L), CDAI, HAQ-DI, Patient Global Assessment (PaGA), pain score, SF-36 physical component scores, smoking status, and mTSS-related parameters were associated with e-RRP. For multivariate analysis, among the MTX-IR population, low BMI, hsCRP ≥ 30 mg/L versus < 10 mg/L, and PhGA were confirmed to be associated with e-RRP, while age, CDAI, SDAI, and SF-36 physical component score were associated with e-RRP in the MTX-naïve population (Supplementary Table 2).
Safety
In both MTX-IR and MTX-naïve populations, TEAEs among patients in the e-RRP and e-NRRP subgroups—including rates of all TEAEs, serious TEAEs, TEAEs leading to permanent discontinuation, and death—were generally similar across treatment arms (Tables 5, 6). In FINCH 1, FIL200 plus MTX and the switching arm from PBO to FIL200 with e-RRP showed no relevant difference in incidence of TEAEs leading to premature discontinuation of study drug compared with those in the e-NRRP subgroup (Table 5). This was also true of the ADA group. The switching arm from PBO to FIL100 with e-RRP showed a numerically higher rate of infections compared with that with e-NRRP; rates in e-RRP patients who switched from PBO to FIL100 from week 24 to 52 (24.6%, n = 14, 28 weeks) were comparable to those in the PBO arm observed in the initial 24 weeks (20.7%, n = 29). However, infection rates among patients who switched from PBO to FIL200 were numerically higher among the e-NRRP subgroup than in the e-RRP subgroup. In FINCH 3, FIL100 plus MTX patients with e-RRP showed higher rates of TEAEs, including TEAEs leading to temporary interruption of study drug; TEAEs of grade 3 or higher; and TEAEs leading to premature discontinuation of study drug, compared with patients with e-NRRP (Table 6). All treatment arms with e-RRP, except FIL200 monotherapy, showed higher rates of infections compared with those with e-NRRP. There were no evident trends for AEs of special interest, including infections, serious infections, opportunistic infections, herpes zoster, MACE, VTE, and malignancy across treatment groups or between e-RRP and e-NRRP subgroups.
Discussion
The purpose of this post hoc analysis of two randomized controlled trials of filgotinib is to understand whether the previous rate of radiographic progression may affect clinical remission and structural progression.
From this post hoc analysis, we learned that patients who had progressed rapidly early in their disease may also progress faster during subsequent treatment. The mTSS CFB at week 24 in patients in the e-RRP subgroups was approximately 2.5–4 times that in patients in the e-NRRP subgroup in the MTX treatment arms from both MTX-IR and MTX-naïve trials, indicating that previously rapidly progressing patients tended to continue progressing faster even on treatment, with the caveat that the differences may not be clinically meaningful. Only FIL200 plus MTX showed consistent suppression of mTSS progression over 52 weeks. FIL100 plus MTX and ADA plus MTX did not suppress mTSS progression over 52 weeks: The differences in mTSS CFB at W52 were not significant, although among patients with e-RRP receiving ADA, CFB mTSS was numerically higher than that in e-NRRP (1.27 versus 0.23). Among the possible explanations of the relatively larger mean mTSS CFB in ADA-treated patients is the contribution of five outliers with extremely rapid radiographic progression (14.0–39.5 CFB mTSS) who had been randomized to the ADA arm (Fig. 4). Of note, the proportions of the e-RRP and e-NRRP patients with no radiographic progression were equivalent between the FIL200 and the ADA arms. This suggests that the numbers needed to treat to prevent one radiographic progression would be similar between ADA and FIL200 and that the power to “stop” structural progression might be equivalent between these two modes of action [18, 19], but we do not know if there is any difference between them for “slowing down” structural progression, as it was not possible to confirm this in the current analysis owing to the small proportions of patients who progressed during the trial period. One hypothesis to explain why JAK inhibition may exert stronger effects on radiographic progression is suppression of multiple targets of cytokine pathways without elevating serum interleukin (IL)-17 [which is considered to be linked to joint erosion and was elevated by blocking the tumor necrosis factor (TNF) pathway]. To confirm this, further investigation including a prospective study focusing on populations of patients with rapid radiographic progression may be needed.
Distinct from the MTX-IR population, patients who were MTX-naïve showed similar drug responses regardless of the rapidity of previous structural damage. This may be explained, at least in part, by the relative timing of intervention between the MTX-IR population—which represented patients with more established disease—and the MTX-naïve population—which represented patients who were earlier in their RA course. Also, there was an evident discrepancy between clinical response and inhibition of mTSS progression. For all treatment arms, the proportions of patients who achieved clinical responses were numerically similar between the e-RRP and e-NRRP subgroups, yet, mTSS progression was greater among patients with e-RRP versus those with e-NRRP in the MTX only arm.
In the MTX-naïve patient population, MTX was associated with no radiographic progression in about 79% of the e-RRP subgroup, while 88% of patients in the e-NRRP subgroup showed no progression. FIL200 with or without MTX may lead to no progression in 87–90% of the e-RRP subgroup, similar to the rate with MTX for patients in the e-NRRP subgroup. For those patients who have high risk of structural progression or who have already rapidly progressed, adding FIL200 to established MTX treatment may be beneficial.
Baseline demographics and disease characteristics were generally similar between patients in the e-RRP and e-NRRP subgroups. From the multivariate analysis results, seropositivity was not associated with e-RRP, both among MTX-IR and -naïve populations. hsCRP ≥ 30 mg/L at BL was associated with e-RRP in the MTX-IR population but not in the MTX-naïve population. Disease activity, including CDAI and SDAI, was associated with e-RRP in the MTX-naïve population but not in the MTX-IR. Other previously identified factors, including BL erosion score and swollen joint count, were not associated with e-RRP in either MTX-naïve or MTX-IR populations.
There are several possible explanations for the lack of consistency of this analysis with previous risk-factor analyses. First, the association factors identified in this analysis were retrospective, namely, they did not predict future rapid radiographic progression but confirmed factors associated with previous rapid progression. Second, owing to the selection bias of the two clinical trials, which included only patients with at least one risk factor per inclusion criteria [8, 9], patients enrolled in FINCH 1 and FINCH 3 may not reflect the general RA population. We still consider this type of analysis useful and informative. The lack of association between previous risk factors and e-RRP may mean that e-RRP might be an independent factor for radiographic progression. It seems that patients with previous rapid radiographic progression are still at high risk for continued rapid progression under subsequent treatment regimens.
Low BMI was associated with e-RRP in the MTX-IR population. A reverse correlation between BMI and radiographic progression has been published previously [16]. The reasons why leaner patients tend to have higher risk of structural progression may include the presence of rheumatoid cachexia, which can be caused by a lack of adequate control of inflammatory status [20, 21]. This risk factor should also be considered when selecting advanced therapy. An interesting finding from this analysis is the association of PhGA, but not PaGA or pain score, with e-RRP in the MTX-IR population. A publication by Movahedi et al. [22] showed that modified-DAS28(CRP) (calculated with only CRP, swollen joint count 28, and PhGA components)[23] was a stronger predictor of RRP in RA patients treated with MTX. This may mean that physician judgment is another important factor of predicting radiographic progression.
This post hoc analysis has several limitations. First, the subgroup analyses were limited to small patient numbers that were not powered to confirm any hypothesis, and the nominal statistically significant differences might have happened by chance. Second, the so-called “rapid radiographic progression” by yearly mTSS progression ≥ 5 was arbitrarily calculated by dividing BL mTSS by disease duration, with the understanding that the structural progression of RA is not linear over the entire disease course. Third, the BL factors associated with rapid radiographic progression might be biased by the skewed population of the clinical trials, which may not reflect the general, real-world patient population. Fourth, the MTX-naïve population may not be truly naïve, because per the inclusion criteria, patients with < 4 doses of MTX were permitted to participate. And last, some patients may not have truly been “early” in their RA disease course; some had disease duration > 5 years, without being treated with MTX.
Conclusions
From this post hoc analysis of MTX-IR and MTX-naïve populations from filgotinib clinical trials, we learned that RA patients with previous rapid radiographic progression are at higher risk of structural progression in subsequent phases of treatment compared with patients who had no or less previous rapid progression. Status of previous rapid progression may need to be considered when recommending drug treatment and dosage. Filgotinib 200 mg once daily gave consistent and sustained benefit for both clinical outcomes and freedom from radiographic progression in both e-RRP and e-NRRP subgroups, while demonstrating a consistent safety and tolerability profile across subgroups. Although filgotinib efficacy was somewhat reduced among patients with e-RRP, treatment with filgotinib slowed radiographic progression in both subgroups.
References
Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Bird P, Nicholls D, Barrett R, de Jager J, Griffiths H, Roberts L, et al. Longitudinal study of clinical prognostic factors in patients with early rheumatoid arthritis: the PREDICT study. Int J Rheum Dis. 2017;20(4):460–8.
Lindqvist E, Eberhardt K, Bendtzen K, Heinegard D, Saxne T. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2005;64(2):196–201.
van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65(3):559–70.
Huizinga TW, Fleischmann RM, Jasson M, Radin AR, van Adelsberg J, Fiore S, et al. Sarilumab, a fully human monoclonal antibody against IL-6Ralpha in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. 2014;73(9):1626–34.
Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen ML, Reyes Gonzaga J, et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2017;376(7):652–62.
Fleischmann R, Mysler E, Bessette L, Peterfy CG, Durez P, Tanaka Y, et al. Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: results through 3 years from the SELECT-COMPARE study. RMD Open. 2022;8(1):e002012.
Combe B, Kivitz A, Tanaka Y, van der Heijde D, Simon JA, Baraf HSB, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis. 2021;80(7):848–58.
Westhovens R, Rigby WFC, van der Heijde D, Ching DWT, Stohl W, Kay J, et al. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann Rheum Dis. 2021;80(6):727–738.
Aletaha D, Westhovens R, Gaujoux-Viala C, Adami G, Matsumoto A, Bird P, et al. Efficacy and safety of filgotinib in methotrexate-naive patients with rheumatoid arthritis with poor prognostic factors: post hoc analysis of FINCH 3. RMD Open. 2021;7(2):e001621.
Ten Klooster PM, Versteeg LGA, Oude Voshaar MAH, de la Torre I, De Leonardis F, Fakhouri W, et al. Radiographic progression can still occur in individual patients with low or moderate disease activity in the current treat-to-target paradigm: real-world data from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Arthritis Res Ther. 2019;21(1):237.
Versteeg GA, Steunebrink L, Vonkeman HE, Ten Klooster PM, Van Der Bijl AE, Van De Laar M. Early radiological progression remains associated with long-term joint damage in real-world rheumatoid arthritis patients treated to the target of remission. Scand J Rheumatol. 2022;51(2):87–96.
van den Broek M, Dirven L, de Vries-Bouwstra JK, Dehpoor AJ, Goekoop-Ruiterman YP, Gerards AH, et al. Rapid radiological progression in the first year of early rheumatoid arthritis is predictive of disability and joint damage progression during 8 years of follow-up. Ann Rheum Dis. 2012;71(9):1530–3.
van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27(1):261–3.
Vastesaeger N, Xu S, Aletaha D, St Clair EW, Smolen JS. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology (Oxford). 2009;48(9):1114–21.
Rydell E, Forslind K, Nilsson JA, Jacobsson LTH, Turesson C. Smoking, body mass index, disease activity, and the risk of rapid radiographic progression in patients with early rheumatoid arthritis. Arthritis Res Ther. 2018;20(1):82.
Vanier A, Smolen JS, Allaart CF, Van Vollenhoven R, Verschueren P, Vastesaeger N, et al. An updated matrix to predict rapid radiographic progression of early rheumatoid arthritis patients: pooled analyses from several databases. Rheumatology (Oxford). 2020;59(8):1842–52.
Emery P, Durez P, Hueber AJ, de la Torre I, Larsson E, Holzkamper T, et al. Baricitinib inhibits structural joint damage progression in patients with rheumatoid arthritis-a comprehensive review. Arthritis Res Ther. 2021;23(1):3.
Tanaka Y, Luo Y, O’Shea JJ, Nakayamada S. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18(3):133–45.
Platzer A, Alasti F, Smolen JS, Aletaha D, Radner H, Bluml S. Trajectory clusters of radiographic progression in patients with rheumatoid arthritis: associations with clinical variables. Ann Rheum Dis. 2022;81(2):175–83.
Rajbhandary R, Khezri A, Panush RS. Rheumatoid cachexia: what is it and why is it important? J Rheumatol. 2011;38(3):406–8.
Movahedi M, Weber D, Akhavan P, Keystone EC. Modified disease activity score at 3 months is a significant predictor for rapid radiographic progression at 12 months compared with other measures in patients with rheumatoid arthritis. ACR Open Rheumatol. 2020;2(3):188–94.
Baker JF, Conaghan PG, Smolen JS, Aletaha D, Shults J, Emery P, et al. Development and validation of modified disease activity scores in rheumatoid arthritis: superior correlation with magnetic resonance imaging-detected synovitis and radiographic progression. Arthritis Rheumatol. 2014;66(4):794–802.
Acknowledgements
Funding
Funding for this analysis and the Rapid Service Fee was provided by Eisai Co., Ltd. and Gilead Sciences K.K. The sponsors participated in the planning, execution, and interpretation of the research.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Rob Coover, MPH, and Gregory Bezkorovainy, MA, of AlphaScientia, LLC, San Francisco, CA; and funded by Eisai Co., Ltd. and Gilead Sciences K.K. Zhaoyu Yin, ex-colleague of Gilead Sciences Inc, supported biostatistical analyses. Chen Chi and her teams, Gilead Sciences Inc., helped with programming support of biostatistical analyses.
Author Contributions
Y.T., T.T., D.A., B.B., K.E., S.K., and V.R. were involved in the conception and design of the study/analyses. L.H. performed the data and statistical analyses. Y.T., T.A., D.A., B.B., A.P., and T.T. contributed to the interpretation of the data. All authors critically revised the manuscript for important intellectual content and provided final approval for publication.
Prior Presentation
This work was presented as a poster at the EULAR European Congress of Rheumatology 1–4 June 2022. Ann Rheumatic Dis. 2022;81(suppl 1):604. POS0664.
Disclosures
Yoshiya Tanaka has received speaking fees and/or honoraria from AbbVie, Amgen, Astellas, AstraZeneca, Behringer-Ingelheim, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Gilead, Mitsubishi-Tanabe, and YL Biologics, and has received research grants from AbbVie, Asahi-Kasei, Boehringer-Ingelheim, Chugai, Corrona, Daiichi Sankyo, Eisai, Kowa, Mitsubishi-Tanabe, and Takeda. Tatsuya Atsumi has accepted research grants and/or honoraria for meetings from Gilead Sciences, Inc.; Mitsubishi Tanabe; Chugai; Astellas Pharma; Takeda; Pfizer; AbbVie: Eisai; Daiichi Sankyo; Bristol-Myers Squibb; UCB Japan Co. Ltd.; Eli Lilly Japan K.K., Otsuka Pharmaceutical Co., Ltd.; and Alexion Inc. Daniel Aletaha reports grants or research support from AbbVie, Merck Sharp & Dohme, Novartis, and Roche; serving as a consultant for Janssen; serving on a speaker’s bureau for Bristol-Myers Squibb, Merck Sharp & Dohme, and UCB; and serving as a consultant and on a speaker’s bureau for AbbVie; Amgen; Celgene; Eli Lilly; Medac; Merck; Novartis; Pfizer; Roche; Sandoz; and Sanofi/Genzyme. Beatrix Bartok, Alena Pechonkina, and Ling Han are employees and shareholders of Gilead Sciences, Inc. Kahaku Emoto is a former employee of Gilead Sciences K.K. and shareholder of Gilead Sciences, Inc. Shungo Kano is an employee of Gilead Sciences K.K. and shareholder of Gilead Sciences, Inc. Vijay Rajendran is an employee and shareholder of Galapagos BV. Tsutomu Takeuchi reports receiving grant/research support from AbbVie, Asahi Kasei, Astellas, Chugai, Daiichi Sankyo, Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, and UCB Japan; serving as a consultant for Astellas, Chugai, and Eli Lilly Japan; and serving on a speakers’ bureau for AbbVie, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Eli Lilly Japan, Mitsubishi-Tanabe, Novartis, Pfizer Japan, Sanofi, and Gilead Sciences, Inc.
Compliance with Ethics Guidelines
The study protocols were reviewed and approved by all local institutional review boards or ethics committees of participating institutions. The studies were carried out in accordance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. Informed consent to participate was provided by all participants. FINCH 1 was approved by the Advarra Central Institutional Review Board (reference 00000971) and FINCH 3 was approved by Ethics Committee Research UZ/KU Leuven (reference S59627).
Data Availability
Anonymized individual patient data will be shared upon request for research purposes, dependent upon the nature of the request, the merit of the proposed research, the availability of the data, and its intended use. The full data sharing policy for Gilead Sciences, Inc., can be found at https://www.gilead.com/science-and-medicine/research/clinical-trials-transparency-and-data-sharing-policy.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tanaka, Y., Atsumi, T., Aletaha, D. et al. Benefit of Filgotinib, a JAK1 Preferential Inhibitor, in Rheumatoid Arthritis Patients with Previous Rapid Radiographic Progression: Post Hoc Analysis of Two Trials. Rheumatol Ther 10, 161–185 (2023). https://doi.org/10.1007/s40744-022-00503-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00503-3