Abstract
Objective
Patients with uncontrolled/refractory gout have heavy disease burden, but few treatment options. Pegloticase lowers serum urate (SU), but anti-drug antibodies can limit treatment efficacy. Evidence supports immunomodulator-pegloticase co-administration to increase sustained urate-lowering rates, but published cases are limited. This study investigated experience with pegloticase-immunomodulation co-therapy at two community rheumatology practices.
Methods
Patients initiating pegloticase with immunomodulation in 2017 or later were included. Patient/treatment characteristics and proportion of responders (≥ 12 pegloticase infusions, SU < 6 mg/dl at infusion-12) were examined. Patients on therapy at data collection with < 12 infusions were excluded from response analyses. eGFR before and after therapy was examined.
Results
Thirty-four patients (79% male, 62.4 ± 16.3 years) with uncontrolled gout (SU = 9.1 ± 2.0 mg/dl, 91% tophaceous) were included. Most-reported comorbidities were hypertension (76%), obesity (71%), osteoarthritis (68%), and CKD (47%). Pre-therapy eGFR was 65.4 ± 25.2 ml/min/1.73 m2 (41% eGFR < 60 ml/min/1.73 m2). All patients initiated immunomodulation before (5.3 ± 3.0 weeks, n = 32) or at (n = 2) first pegloticase infusion. Subcutaneous methotrexate (15.4 ± 4.9 mg/week, n = 20), oral methotrexate (15.3 ± 3.6 mg/week, n = 9), mycophenolate mofetil (1000 mg/day, n = 3), and azathioprine (100 mg/day, n = 2) were administered. Patients received 14.6 ± 7.1 infusions over 28.5 ± 14.9 weeks. Overall response rate was 89%, ranging among immunomodulators (subcutaneous methotrexate: 93%, oral methotrexate: 89%, mycophenolate mofetil: 100%, azathioprine: 50%). On average, eGFR increased during therapy (+ 10.3 ± 16.9 ml/min/1.73 m2), with CKD stability/improvement in 85%. Nineteen patients (56%) experienced gout flares. No infusion reactions or infections were noted. No new safety concerns were identified.
Conclusions
These real-world findings provide further support for increased pegloticase response rates when co-treatment with immunomodulating therapy is used.
Plain Language Summary
Patients with gout that does not respond to oral urate-lowering therapies have heavy disease burden and few treatment options. Pegloticase lowers serum urate levels (SU) and resolves tophi, but anti-drug antibodies can limit urate-lowering efficacy duration. Evidence increasingly supports co-administering an immunomodulator with pegloticase to increase the proportion of patients with sustained urate-lowering response. However, there are few published cases from real-world clinical practice. This study examined treatment with pegloticase + immunomodulation at two community rheumatology practices. Patients who began treatment with pegloticase and an immunomodulator in 2017 or later were included. The proportion of patients with sustained urate-lowering response (≥ 12 infusions received, SU < 6 mg/dl at infusion 12) was investigated. Renal function before and after therapy was also examined. Thirty-four patients were included. Before treatment, SU averaged 9.1 mg/dl and most-reported comorbidities were hypertension (76%), obesity (71%), osteoarthritis (68%), and chronic kidney disease (47%). All patients began using an immunomodulator before or at first pegloticase infusion (subcutaneous methotrexate [20 patients], oral methotrexate [9 patients], mycophenolate mofetil [3 patients], and azathioprine [2 patients]). On average, 14.6 infusions were administered over 28.5 weeks and overall response rate was 89%. Response rate varied among different immunomodulators: subcutaneous methotrexate: 93%, oral methotrexate: 89%, mycophenolate mofetil: 100%, azathioprine: 50%. On average, kidney function improved, with chronic kidney disease stage stability/improvement in 85% of patients. Nineteen patients (56%) experienced gout flares. No infusion reactions or infections were noted and no new safety concerns were identified. These real-world findings provide further support for administering immunomodulation as co-therapy to pegloticase.
Similar content being viewed by others
Why carry out this study? | |
Pegloticase lowers serum urate (SU) in patients with uncontrolled/refractory gout, but efficacy can be limited by anti-drug antibody development. | |
Co-administering pegloticase with an immunomodulating agent meaningfully increases the number of patients with sustained urate-lowering while on treatment but case data in the literature remain limited. | |
This study examined experience from two community rheumatology practices where a variety of immunomodulating agents are routinely co-prescribed with pegloticase therapy. | |
What was learned from the study? | |
This case series provides further real-world evidence supporting concomitant pegloticase and immunomodulator co-therapy. | |
In agreement with prior studies, the treatment responder rate observed here in the presence of immunomodulation co-therapy (89%) was markedly higher than the established pegloticase monotherapy response rate (42%). | |
Methotrexate has been most studied, but our findings suggest that co-therapy with other immunomodulators may also improve pegloticase response rates in uncontrolled gout patients. |
Introduction
Gout is a painful inflammatory arthritis caused by chronic hyperuricemia and has been associated with metabolic comorbidities [1, 2], cardiovascular disease [1,2,3,4,5], renal dysfunction [2, 6], lower quality of life (QOL) [1, 7], and increased mortality [1, 4, 8]. Patients with uncontrolled or refractory gout have even higher disease burden [9] and comorbidity rates [10], as well as more severe chronic kidney disease [10] and highly impacted QOL [11]. Unfortunately, these uncontrolled gout patients often have limited treatment options.
Pegloticase lowers serum urate (SU) in uncontrolled gout patients, but the durability of this effect can be limited in some patients largely because of anti-drug antibody development [12]. Evidence in the literature strongly supports the co-administration of pegloticase with an immunomodulating agent to increase treatment response rates. A systematic review of the literature showed an 83% pegloticase response rate with immunomodulator co-administration [13], compared to a 42% response rate of pegloticase monotherapy [12]. Further, a recently completed randomized, controlled trial (RCT) of pegloticase + methotrexate (MTX) co-therapy (MIRROR RCT; NCT03994731) showed a 71% response rate during month 6 (vs. 38% pegloticase + placebo) [14]. However, case data in the literature remain limited. The current study reports experience from two community rheumatology practices where immunomodulating therapy is routinely co-prescribed in patients undergoing pegloticase treatment. Treatment response rates, effect on kidney function, and safety signals were examined in uncontrolled gout patients co-treated with pegloticase and a single immunomodulatory agent.
Methods
This retrospective study of medical record data was reviewed and assigned exempt status by the wcg IRB (Puyallup, WA; IRB registration #: IRB00000533), waiving the requirement of informed consent. All study conduct adhered to the tenets of the Declaration of Helsinki of 1964 and its later amendments. Patients with uncontrolled gout who underwent pegloticase plus immunomodulation co-therapy and who received their first pegloticase infusion in 2017 or later were included. Medical record data were collected in March and May of 2021, including data through March 15, 2021. All patients were cared for at two community rheumatology practices and no exclusion criteria were applied.
Medical record data were extracted and de-identified by an independent party (The Lockwood Group, Stamford, CT) using a standardized data collection form. Examined parameters included patient demographics, gout characteristics, treatment parameters, and the proportion of patients who were considered to be pegloticase responders, defined as ≥ 12 pegloticase infusions received and SU < 6 mg/dl just prior to infusion 12. SU values that were below the quantitation limit (BQL) were entered as the quantitation limit to avoid artificially lowering mean SU values (e.g., < 1.5 mg/dl entered as 1.5 mg/dl). BQL varied between 0.2 and 1.5 mg/dl. Patients who remained on therapy at data collection, but had not yet reached infusion 12 were excluded from responder rate analyses. All patients had a target pegloticase administration schedule of 8 mg infusions every 2 weeks and received pre-infusion prophylaxis (125 mg IV methylprednisolone or 100 mg hydrocortisone, 180 mg oral fexofenadine or 25 mg IVP diphenhydramine). All patients were on a standard gout flare prophylaxis regimen during pegloticase + immunomodulation co-therapy. Estimated glomerular filtration rate (eGFR) was also examined before therapy and at last infusion to investigate change in kidney function during therapy. Adverse events (AEs) were categorized by the reporting physician as “flares,” “infusion reactions,” or “other.” To capture AEs not previously associated with pegloticase, further detail was provided for all “other” AEs. Data are presented as mean ± SD or as n (%) as appropriate. Changes in continuous variables over time were statistically examined using two-tailed, paired Student’s t tests. Statistical significance was defined as p < 0.05.
Results
Patient Characteristics
A total of 34 patients with uncontrolled gout were identified and included in the analyses. Most patients were male (79%) and Caucasian (74%) and mean age was 62.4 ± 16.3 years (Table 1). Comorbidities were common, with hypertension (76%), obesity (71%), osteoarthritis (68%), chronic kidney disease (CKD, 47%), and cardiovascular disease (35%) most frequently reported. Baseline eGFR averaged 65.4 ± 25.2 ml/min/1.73 m2 and 41% of patients had eGFR < 60 ml/min/1.73 m2. No kidney transplant or dialysis patients were included.
Gout duration averaged 14.7 ± 13.4 years and mean SU prior to pegloticase exposure was 9.1 ± 2.0 mg/dl. Subcutaneous (subQ) tophi were noted in 91% of patients and all had signs of severe gout, including chronic pain, frequent gout flares, and/or bone erosions on radiography. Thirty-one of 34 patients (91%) had documented oral ULT use prior to beginning pegloticase, with a history of allopurinol use in 28 patients (82%, mean dose: 270.8 ± 139.8 mg/day [n = 24]) and febuxostat use in three patients (9%, mean dose: 53.3 ± 23.1 mg/day [n = 3]). Of the three patients (9%) without oral ULT use noted, one patient was not on an oral ULT at first rheumatology office visit and two patients reported allopurinol intolerance.
Pegloticase Plus Immunomodulation Co-Therapy
A total of 498 pegloticase doses were administered (14.6 ± 7.1 infusions/patient) over a mean of 28.5 ± 14.9 weeks. Immunomodulator use included subQ methotrexate (MTX; 20 patients [59%], 10−25 mg/week), oral MTX (9 patients [26%], 7.5−20 mg/week), oral mycophenolate mofetil (MMF; 3 patients [9%], 1000 mg/day), and oral azathioprine (AZA; 2 patients [6%], 100 mg/day; Table 2). Immunomodulation co-therapy was initiated prior to pegloticase in 32 patients (94%) 5.3 ± 3.0 weeks before the first pegloticase infusion. The remaining two patients (6%) initiated immunomodulation (both subQ MTX) and pegloticase on the same day (Fig. 1).
Schematic of study design showing pegloticase and immunomodulation co-therapy. Patients were considered to have sustained urate-lowering efficacy (treatment responder) if they had received at least 12 pegloticase infusions and had SU < 6 mg/dl just prior to infusion 12. MTX methotrexate, LEF leflunomide, MMF mycophenolate mofetil, AZA azathioprine
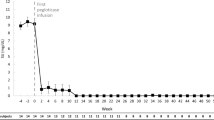
At data collection, 11 patients (32%) remained on therapy, 17 patients (50%) had met treatment goals and discontinued therapy, and six patients (18%) had prematurely discontinued therapy. Of the 11 patients who remained on therapy, six had not yet reached infusion 12 and were excluded from responder rate analyses. Premature therapy discontinuation occurred because of loss of follow-up (n = 2), urate-lowering efficacy loss (n = 1), patient choice (n = 1), COVID concerns (n = 1), and adverse event (n = 1; stroke [further described below]). Therefore, 28 patients were included in responder rate analyses. Twenty-five of 28 patients (89%) met treatment response criteria (Fig. 2). On average, SU fell to 1.0 ± 0.6 mg/dl following pegloticase infusion 1, remaining approximately 1 mg/dl for the remainder of therapy (Fig. 3). Of the 17 patients that had met treatment goals and discontinued pegloticase, 15/17 (88%) had tophi before beginning therapy. Of these 15, 13 (87%) no longer had visible tophi following pegloticase discontinuation. Ten of these 17 patients (59%) were administered allopurinol following pegloticase discontinuation at a mean dose of 360 mg/day (range, 100 to 600 mg/day).
Response rates for individual immunomodulators were 100% for MMF, 93% for subQ MTX, 89% for oral MTX, and 50% for AZA (Fig. 2). Of the three patients were considered non-responders, one met treatment goals after three pegloticase infusions and discontinued therapy, one had an SU of 11.3 mg/dl prior to infusion 12 but because of ongoing clinical improvement remained on therapy, without any reported infusion reactions, and one self-discontinued azathioprine 2 weeks prior to infusion 5 (SU rise after infusion 6 followed by pegloticase discontinuation).
Over the course of therapy, mean eGFR among pegloticase + immunomodulation co-treated patients improved from 65.4 ± 25.2 ml/min/1.73 m2 at baseline to 75.7 ± 30.8 ml/min/1.73 m2 at time of last pegloticase infusion (p = 0.001, N = 34). Mean eGFR change over pre-therapy values was + 10.3 ± 16.9 ml/min/1.73 m2 at last pegloticase infusion (N = 34 [all patients regardless of treatment duration or response]). Further, kidney disease remained stable or improved in 29 patients (85%), with CKD progression of one stage or less in the remaining five patients (15%). Of these, one patient moved from stage 1 to stage 2, two moved from stage 2 to stage 3a, and two moved from stage 3a to 3b. Patients with pre-therapy CKD (eGFR < 60 ml/min/1.73 m2, n = 14) were older (72.6 ± 10.1 vs. 55.2 ± 16.2 years, p < 0.001) and more often female (43 vs. 5%) than those without CKD (n = 20). Despite these differences, all findings were similar between patients with and without pre-therapy CKD, including the number of pegloticase infusions administered (CKD: 14.6 ± 8.3 vs. non-CKD: 14.7 ± 6.4 infusions), urate-lowering efficacy (91% [10/11] vs. 88% [15/17]), MTX usage (93% vs. 80%; mean dose: 14.2 ± 5.4 vs. 16.3 ± 3.4 mg/week), and mean change in eGFR (12.3 ± 21.5 vs. 8.9 ± 13.2 ml/min/1.73 m2).
Safety Observations
No new safety concerns were identified. Twenty patients (59%) experienced one or more adverse events during pegloticase plus immunomodulation co-therapy. The most common adverse event was acute gout flare, which was observed in 19 patients (56%). In these 19 patients, a mean of 1.8 ± 1.5 flares per patient were noted. Other adverse events included unrelated stroke in one patient (3%) after 15 pegloticase infusions (treatment discontinued) and new lower extremity pain (described as “other AE”) in three patients (9%). No infusion reactions or infections were noted. Clinical laboratory values were monitored in all patients as a precaution, including liver function tests (LFTs), with no new laboratory abnormalities emerging.
Discussion
These data re-inforce the potential of co-administering pegloticase plus immunomodulator to increase the proportion of patients who achieve sustained urate-lowering on therapy. This case series is important because of the larger number of included cases (N = 34) and the variety of immunomodulators examined. The overall treatment pegloticase with immunomodulation response rate was 89%, which represents a marked increase from the established 42% with pegloticase monotherapy [12]. The 89% response rate reported here is consistent with MIRROR RCT (71% response rate during month 6) [14], an open-label trial with oral MTX (79% response rate during month 6) [15], a randomized controlled trial with MMF (86% at 12 weeks) [16], two smaller case series with MTX (both n = 10, 80−100%) [17, 18], and a systematic literature review of all immunomodulators (83%) [13]. Another case series (n = 10) showed that leflunomide may also be an option for immunomodulatory co-therapy (70% response rate) [19]. The current study showed a response rate for MMF co-therapy of 100%, but the number of patients administered MMF as co-therapy was small (n = 3). Treatment response rate with MTX co-therapy was also high and similar between the oral and subQ routes of administration (89% and 93%, respectively). Lastly, patients co-treated with AZA had the lowest treatment response rate of 50%, but only two included patients had received AZA as co-therapy (one patient who self-discontinued AZA was a non-responder). No new safety concerns emerged for immunomodulation co-therapy as a whole or for any individual immunomodulator examined. These findings are important for treating uncontrolled gout patients, who frequently present substantial clinical challenges because of a high comorbidity burden [10, 20, 21] and limited treatment options. Additionally, the heterogeneity of uncontrolled gout patients means that some immunomodulating therapies may not be appropriate for some patients, emphasizing the importance of published data on a variety of immunomodulator use with pegloticase.
The association between gout and CKD is well known, with gout patients being three times more likely to develop CKD [6] and 2.4 times more likely to develop advanced CKD (stage 3–5) [22] than their non-gout counterparts. Interestingly, uncontrolled gout patients in the current case series had a mean eGFR improvement of 10.3 ml/min/1.73 m2 during pegloticase plus immunomodulation co-therapy (baseline: 65.4 ± 25.2 ml/min/1.73 m2, last infusion: 75.7 ± 30.8 ml/min/1.73 m2; p = 0.001). Additionally, 85% of patients had either stable or improved CKD stage (based on eGFR). This finding is in agreement with phase 3 pegloticase monotherapy trial data, which showed eGFR stability over 6 months of pegloticase treatment [23]. It is possible that eGFR could further improve with longer duration of pegloticase-induced SU-lowering and further study is needed. Longer-term (12-month) MIRROR RCT findings may provide further insight.
This study had several limitations. First, a selection bias may introduce errors into any retrospective study. To minimize the influence of such errors, all patients in both practices who initiated pegloticase with immunomodulation co-therapy in 2017 or later were included. Second, though this case series included more patients than prior pegloticase with immunomodulation case series, larger randomized controlled trials (RCTs) are needed to fully validate both efficacy and safety findings. Randomized controlled trials examining MMF co-therapy and MTX co-therapy have been completed, showing similar findings as reported here [14, 16].
Conclusions
In conclusion, this case series highlights experiences with pegloticase plus immunomodulation co-therapy in a real-world clinical setting, further supporting the use of immunomodulators to increase pegloticase treatment response rates. Importantly, several different immunomodulation agents may be effective for increasing treatment response rates and seem well tolerated by patients with uncontrolled gout. However, further study is needed, particularly with AZA and MMF.
References
Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2008;67:1310–6.
Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125(679–87): e1.
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Impact of gout on the risk of atrial fibrillation. Rheumatol (Oxf). 2016;55:721–8.
Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116:894–900.
Singh JA, Cleveland JD. Gout and the risk of incident atrial fibrillation in older adults: a study of US Medicare data. RMD Open. 2018;4: e000712.
Singh JA, Cleveland JD. Gout is associated with a higher risk of chronic renal disease in older adults: a retrospective cohort study of U.S. Medicare population. BMC Nephrol 2019;20:93.
Chandratre P, Roddy E, Clarson L, Richardson J, Hider SL, Mallen CD. Health-related quality of life in gout: a systematic review. Rheumatol (Oxf). 2013;52:2031–40.
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis. 2016;75:210–7.
Becker MA, Schumacher HR, Benjamin KL, et al. Quality of life and disability in patients with treatment-failure gout. J Rheumatol. 2009;36:1041–8.
Francis-Sedlak M, LaMoreaux B, Padnick-Silver L, Holt RJ, Bello AE. Characteristics, comorbidities, and potential consequences of uncontrolled gout: an insurance-claims database study. Rheumatol Ther. 2021;8:183–97.
Khanna PP, Nuki G, Bardin T, et al. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: results from a cross-sectional survey. Health Qual Life Outcomes. 2012;10:117.
Sundy JS, Baraf HS, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711–20.
Keenan RT, Botson JK, Masri KR, et al. The effect of immunomodulators on the efficacy and tolerability of pegloticase: a systematic review. Semin Arthritis Rheum. 2021;51:347–52.
Botson J, Saag K, Peternson J, et al. A randomized, double-blind, placebo-controlled, multicenter, study of methotrexate combined with pegloticase in patients with uncontrolled gout [abstract]. Ann Rheum Dis. 2022;81:112.
Botson JK, Tesser JRP, Bennett R, et al. Pegloticase in combination with methotrexate in patients with uncontrolled gout: a multicenter, open-label study (MIRROR). J Rheumatol. 2021;48:767–74.
Khanna PP, Khanna D, Cutter G, et al. Reducing immunogenicity of pegloticase with concomitant use of mycophenolate mofetil in patients with refractory gout: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2021;73:1523–32.
Albert JA, Hosey T, LaMoreaux B. Increased efficacy and tolerability of pegloticase in patients with uncontrolled gout co-treated with methotrexate: a retrospective study. Rheumatol Ther. 2020;7:639–48.
Botson JK, Peterson J. Pretreatment and coadministration with methotrexate improved durability of pegloticase response: an observational, proof-of-concept case series. J Clin Rheumatol 2020 [Epub ahead of print].
Masri K, Winterling K, LaMoreaux B. Effect of leflunomide on pegloticase response rate in patients with uncontrolled gout: a retrospective study. Rheumatol Ther. 2022;9:555–63.
Morlock R, Flores NM, Annunziata K, Chapnick J, Nuevo J. Economic burden of controlled gout, uncontrolled gout, and gout exacerbated by common comorbidities: results from the 2012–2013 National Health and Wellness Survey [abstract]. Value Health. 2015;18:A640–1.
Wu EQ, Forsythe A, Guérin A, Yu AP, Latremouille-Viau D, Tsaneva M. Comorbidity burden, healthcare resource utilization, and costs in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther. 2012;19:e157–66.
Roughley MJ, Belcher J, Mallen CD, Roddy E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther. 2015;17:90.
Yood RA, Ottery FD, Irish W, Wolfson M. Effect of pegloticase on renal function in patients with chronic kidney disease: a post hoc subgroup analysis of 2 randomized, placebo-controlled, phase 3 clinical trials. BMC Res Notes. 2014;7:54.
Acknowledgements
Funding
Funds provided by Horizon Therapeutics plc (Deerfield, IL, USA) were used for third-party data collection fees and the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
The manuscript was written/edited by the authors.
Author Contributions
Conceptualization: Aaron Broadwell, John Albert, and Brian LaMoreaux; Methodology: Brian LaMoreaux and Lissa Padnick-Silver; Formal analysis and investigation: all authors; Writing—original draft preparation: Lissa Padnick-Silver; Writing—review and editing: all authors; Funding acquisition: Brian LaMoreaux; Resources: all authors; Supervision: Brian LaMoreaux.
Disclosures
Dr. Aaron Broadwell is a speaker for and consultant to Horizon. Dr. John A. Albert is a speaker for and consultant to Horizon. Drs. Lissa Padnick-Silver and Brian LaMoreaux are employees of and stockholders in Horizon.
Compliance with Ethics Guidelines
This retrospective study of medical record data was reviewed and assigned exempt status by the wcg IRB (Puyallup, WA; IRB registration #: IRB00000533), waiving the requirement of informed consent. All study conduct adhered to the tenets of the Declaration of Helsinki of 1964 and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request; subject to approval by the study sponsor.
Prior Presentation
Preliminary findings of this study were presented at the 2021 annual meeting of the American College of Rheumatology (November 3–10, 2021; virtual meeting).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Broadwell, A., Albert, J.A., Padnick-Silver, L. et al. Community Practice Experiences with a Variety of Immunomodulatory Agents Co-Administered with Pegloticase for the Treatment of Uncontrolled Gout. Rheumatol Ther 9, 1549–1558 (2022). https://doi.org/10.1007/s40744-022-00492-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00492-3