Abstract
Introduction
Non-specific chronic sialadenitis (NSCS) is a common pathology of labial salivary glands (LSGs), and NSCS with positive anti-SSA/SSB antibodies is common in clinical practice. Previous studies have evaluated the associations of high focus score (FS) with clinical manifestations in primary Sjögren’s syndrome (pSS) patients extensively, but the characteristics of pSS with NSCS have seldom been investigated. We here analyzed the characteristics of pSS patients with NSCS.
Methods
Among 425 patients who underwent LSG biopsies, 217 had pSS and 37 non-SS sicca patients had NSCS without other diseases (i.e., sicca controls). We categorized these 217 pSS patients into three groups based on the pathology of LSGs: FS ≥ 1 (n = 104), 0 ≤ FS < 1 (n = 76), and NSCS (n = 37). We then compared the three groups while focusing on the NSCS group. Multivariate logistic regression analysis was performed to identify variables that influenced NSCS.
Results
The mean age of pSS patients with NSCS (58.3 ± 11.0 years) was significantly higher than those with FS ≥ 1 (48.5 ± 14.9 years) and 0 ≤ FS < 1 (45.3 ± 13.7 years), but other clinical characteristics were similar. NSCS had a significant positive correlation with age (OR = 7.282, 95% CI 2.085–25.44 and OR = 13.130, 95% CI 3.368–51.189 for patients aged 45–64 years and > 65 years, respectively). Significantly higher levels of lymphocytic infiltration were found in the pSS NSCS group than in the sicca NSCS controls (48.6 vs. 10.8%, respectively).
Conclusions
The pSS patients with NSCS were older than corresponding non-NSCS pSS individuals, but they had similar clinical features. NSCS is associated with age and seldom occurred below the age of 45 years, regardless of the presence or absence of pSS. NSCS may be a subtype of pSS in elderly patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Non-specific chronic sialadenitis (NSCS) is a common pathology of the labial salivary gland. The pathology of NSCS with positive anti-SSA/SSB antibodies is common in clinical practice. |
We investigated the associations of NSCS with clinical characteristics in patients with primary Sjögren’s syndrome (pSS). |
The pSS patients with NSCS were older than those of non-NSCS pSS individuals, but they had similar clinical features. |
NSCS was associated with age and seldom occurred in patients under 45 years old, regardless of the presence or absence of pSS. |
Introduction
Primary Sjögren’s syndrome (pSS) is a common chronic, systemic autoimmune disease characterized by progressive lymphocytic infiltration that mainly affects the salivary and lachrymal glands, and leads to dryness of the mouth and eyes. Secondary Sjögren’s syndrome (sSS) refers to the disease when it occurs in association with another systemic autoimmune disease, such as rheumatoid arthritis, systemic lupus erythematosus, scleroderma, or dermatomyositis. Although pSS primarily affects the exocrine glands, it may also affect extra-glandular systems including musculoskeletal, cutaneous, renal, pulmonary, and neurological systems [1].
Focal lymphocytic sialadenitis (FLS) is vital for the diagnosis and classification of pSS; therefore, labial salivary gland (LSG) biopsy is commonly performed because it is a simple procedure with few risks [2]. Focus score (FS) refers to the mean number of mononuclear cell infiltrates containing at least 50 inflammatory cells per 4 mm2 of periductal or perivascular tissue adjacent to normal-appearing acini. Positive biopsies are defined as FS ≥ 1 [3]. The histopathological findings can be classified into FS ≥ 1 per 4 mm2 (FS ≥ 1), lymphocytic infiltration without focus or FS < 1 per 4 mm2 (0 < FS < 1), within normal limits (FS = 0), non-specific chronic sialadenitis (NSCS, including sclerosing chronic sialadenitis, which is advanced stage of NSCS), insufficient tissue for diagnosis, or other conditions [3,4,5,6]. NSCS is characterized by acinar atrophy, interstitial fibrosis, and duct dilation, and is often accompanied by lymphoid infiltrates [6]. The features of NSCS are relatively common and increase with age in the population, but these features may coexist with pSS, even presenting with aggregation-like FLS that do not have diagnostic value [4, 7]. Previous studies have extensively evaluated the associations of high FS with clinical manifestations in pSS patients [8,9,10], but the characteristics of pSS with NSCS were seldom investigated.
In the present study, we evaluated the associated features in pSS patients with NSCS in a large cohort mainly from northwest China who underwent LSG biopsies. In addition, we compared the pSS patients with NSCS to the controls with sicca symptoms and NSCS but no other manifestations (i.e., sicca NSCS controls).
Methods
Patients
We recruited 425 patients with suspected SS who underwent LSG biopsies at the First Affiliated Hospital of the Air Force Medical University (Shaanxi, China) between May 2020 and July 2021. The diagnosis of pSS was made according to the 2016 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria [11], which requires a positive anti-SSA/Ro or positive salivary gland biopsy. However, because the primary application of classification criteria is recruitment in clinical trials and studies, expert judgement is also very important in clinical work [11]. In our study, 15 biopsy-negative patients without anti-SSA had typical manifestations, high titer of anti-nuclear antibodies (ANA), positive anti-Ro52, and more lymphocytic infiltration, although not FS > 1 in LSGs. These patients were clinically diagnosed with pSS by an experienced rheumatologist in our research team. Positive anti-SSA antibodies require testing for the anti-Ro60 antibodies; isolated anti-Ro52 antibodies are not specific for SS [1]. In the present study, anti-SSA antibodies refer only to anti-Ro60 antibodies and not to anti-Ro52 antibodies. Patients were excluded if they had any other connective tissue disease, sarcoidosis, amyloidosis, lymphoma, viral hepatitis, human immunodeficiency virus, received anticholinergic drugs, IgG4-related disease, malignant tumor, or a history of cervical irradiation. All participants provided written informed consent, all procedures were performed in accordance with the Declaration of Helsinki, and the study was approved by the local ethics board at the First Affiliated Hospital of Air Force Medical University (KY20212091-C-1).
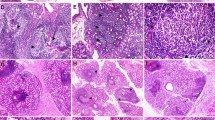
The patients included 255 SS patients, including 221 pSS and 34 sSS patients, as well as 170 non-SS patients comprising 57 with other connective tissue diseases (i.e., CTD patients) and 113 non-CTD patients. Based on the pathological diagnosis, 221 pSS patients were divided into four groups, namely FS ≥ 1 (n = 104), 0 ≤ FS < 1 (n = 76), NSCS (n = 37), and insufficient LSG tissues for pathological evaluation (n = 4). In the 113 non-SS and non-CTD patients, there were 37 sicca patients with NSCS who had dry mouth and/or dry eyes without other symptoms, labeled as sicca NSCS controls, and 76 patients with non-NSCS (Fig. 1). The representative hematoxylin and eosin (H&E)-stained images are shown in Fig. 2: A. A pSS patient with 0 < FS < 1; B. A pSS patient with FS of 5.3; C. A pSS patient with NSCS; and D. A sicca NSCS control.
LSG biopsies were performed in 425 patients including 255 SS (221 pSS and 34 sSS) and 170 non-SS patients (57 CTD and 113 non-CTD). Based on the pathological reports, the 221 pSS patients were divided into four groups: FS ≥ 1 (n = 104), 0 ≤ FS < 1 (n = 76), NSCS (n = 37), and insufficient LSG tissues for pathological evaluation (n = 4). The 113 non-SS and non-CTD patients comprised 37 sicca NSCS patients and 76 non-NSCS patients. pSS primary Sjögren’s syndrome, sSS secondary Sjögren’s syndrome, LSG labial salivary gland, CTD connective tissue disease, FS focus score, NSCS non-specific chronic sialadenitis
Hematoxylin and eosin-stained LSG tissues from pSS patients and a sicca NSCS control. A A pSS patient with 0 < FS < 1; B a pSS patient with FS of 5.3; C a pSS patient with NSCS and significant inflammatory cell infiltration; and D a sicca NSCS control with a small number of inflammatory cell infiltration. Left scale bars = 500 μm, right scale bars = 200 μm. LSG labial salivary gland, pSS primary Sjögren’s syndrome, FS focus score, NSCS non-specific chronic sialadenitis
We investigated the clinical and histopathological features of 217 pSS patients with sufficient LSG tissues for pathological evaluation, especially focusing on patients with NSCS. In addition, we compared the pSS patients with NSCS to sicca NSCS controls.
Clinical Assessment of Primary Sjögren’s Syndrome
The clinical data and medical records of pSS patients, including various clinical, laboratory, and treatment parameters, were collected on the day of LSG biopsy and during follow-up. Among the 217 cases, 24 (11.1%) received treatment over 1 month, including glucocorticoids, hydroxychloroquine, or other immunosuppressive agents. We used the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) to evaluate the disease activity [12]. Whole unstimulated salivary flow (WUSF, abnormal if ≤ 0.1 ml/min in 15 min) [13] and Schirmer’s test (abnormal if ≤ 5 mm wetting of filter paper in 5 min) [14] were performed in most patients.
Laboratory and Pathological Determination
ANA were detected using an indirect immunofluorescence assay (EUROIMMUN, Lübeck, Germany), and anti-extractable nuclear antigen antibodies, including anti-SSA, anti-Ro52, and anti-SSB/La antibodies, were measured using immunodotting assay (EUROIMMUN). In this study, most patients had positive ANA and ANA titers were recorded as four grades (1:100, 1:320, 1:1000, ≥ 1:3200). All patients underwent LSG biopsies. Sufficient LSGs require at least four minor salivary glands to ensure a minimum glandular surface area of 8 mm2, but if the minor salivary glands are small, this number rises to six. The oral pathologist then has sufficient sample to determine the focus score or the presence of NSCS [4]. H&E sections were assessed by the same experienced pathologist, and pathology reports were recorded according to the Chisholm and Mason grading system (grades 0–4) [15]. FS was calculated for each patient.
Statistical Analysis
The statistical analyses were performed using SPSS version 26.0 for Windows (IBM Corp., Armonk, NY, USA). Univariate analysis was used to compare the clinical data between pSS patients with NSCS and other groups and identify the associated variables. The univariate analysis included chi-square tests or Fisher’s exact test for categorical variables and t test or one-way ANOVA for continuous variables. The significant variables in univariate analysis and covariates considered clinically influential were analyzed by multivariate logistic regression to identify significant variables associated with NSCS in pSS patients. P < 0.05 was considered significant for two comparisons, and a P value of < 0.0125 was considered significant for multiple comparisons because three groups were compared against each other, thus requiring Bonferroni correction.
Results
Characteristics of pSS Patients with NSCS
The characteristics of 217 pSS patients with sufficient LSG tissues for pathological evaluation (including 104 with FS ≥ 1, 76 with 0 ≤ FS < 1, and 37 with NSCS) were analyzed. The mean age was 49.0 ± 14.5 years and the female/male ratio was 17.1:1. In the 76 pSS patients with 0 ≤ FS < 1, 25 patients had normal lymphocyte levels (FS = 0), 19 had FLS but FS of < 1 per 4 mm2, and 32 had more lymphocytic infiltration without focus.
We compared the clinical features and laboratory parameters among the three groups (FS ≥ 1, 0 ≤ FS < 1 and NSCS). We found that the mean age was significantly higher in the NSCS group (58.3 ± 11.0 years) than those in the FS ≥ 1 (48.5 ± 14.9 years) and 0 ≤ FS < 1 (45.3 ± 13.7 years) groups. There were only three (8.1%) patients aged 20–44 years in the NSCS group compared to 40 (38.5%) in the FS ≥ 1 and 37 (48.7%) in 0 ≤ FS < 1 (p < 0.001) groups. The percentage of patients aged > 65 years in the NSCS group (32.4%) was greater than those in the FS ≥ 1 (16.3%) and 0 ≤ FS < 1 (10.5%) groups, although the difference between the FS ≥ 1 and NSCS groups did not reach statistical significance (P = 0.038). Except for age, none of the other variables were significantly different between the NSCS group compared to the FS ≥ 1 and 0 ≤ FS < 1 groups. Therefore, sicca symptoms, visceral involvement, ESSDAI, and autoantibodies comprising anti-SSA, anti-Ro52, and anti-SSB were similar in pSS patients with NSCS compared to the non-NSCS pSS patients. In addition, we compared the parameters between the FS ≥ 1 and 0 ≤ FS < 1 groups and found that the mean ages and the percentages of patients in age subgroups (i.e., 20–44 years, 45–64 years, and > 65 years) were not significantly different. All other variables were similar between the two groups, except ANA titer ≥ 1:3200, anti-SSB, and erythrocyte sedimentation rate (ESR) (see Table 1).
Variables Associated with NSCS in pSS Patients
We used multivariate logistic regression analysis to identify the variables that influenced NSCS in pSS patients. Because the ages and clinical characteristics of pSS patients with FS ≥ 1 were similar to those of pSS patients with 0 ≤ FS < 1, we combined these two groups into a single non-NSCS group and performed regression analysis with the NSCS group. Three variables were analyzed, namely age, which was a significant variable in univariate analysis, and duration and ESSDAI, which were covariates that were considered clinically influential factors. NSCS in pSS patients was significantly associated with age of 45–64 years (OR = 7.282, 95% CI 2.085–25.44) and > 65 years (OR = 13.130, 95% CI 3.368–51.189) compared to 20–44 years. However, the duration and ESSDAI did not show significant results (Table 2).
Comparison of pSS Patients with NSCS and Sicca NSCS Controls
Although we aimed to evaluate the differences between pSS patients with NSCS and normal population with NSCS, we could not acquire the LSG of normal people, which is why we selected similar participants with only sicca symptoms and NSCS pathology without CTD or other diseases (i.e., sicca NSCS controls). There were 37 such cases among the 170 non-SS patients. We compared the clinical features and laboratory parameters between the two groups (Table 3). Interestingly, we found that pSS patients with NSCS and sicca NSCS controls had similar mean ages (58.3 ± 11.0 vs. 60.2 ± 12.0 years, respectively). Among the pSS patients with NSCS and sicca NSCS controls, there were three (8.1%) and one (2.7%) individuals aged 20–44 years, 22 (59.5%) and 28 (75.7%) individuals aged 45–64 years, and 12 (32.4%) and eight (21.6%) individuals aged > 65 years (all P > 0.05). Compared to pSS patients with NSCS, there were no differences in dry mouth, dry eyes, and positive Schirmer’s test, but there was a lower frequency of abnormal WUSF rate in the sicca NSCS controls. In sicca NSCS controls, there were 15 (40.5%) individuals with a low ANA titer of 1:100, probably because of their relatively older ages. The immunological features, including high ANA titer (≥ 1:1000), positive anti-SSA and anti-SSB, and high IgG levels, were predominantly seen in pSS patients with NSCS (P < 0.001).
Based on the pathological findings, we categorized the NSCS patients according to the levels of lymphocytic infiltration in LSG tissues into those with a low level of lymphocytic infiltration and a high level of lymphocytic infiltration. We found that the prevalence of a high level of lymphocytic infiltration was much higher in pSS patients with NSCS compared to sicca NSCS controls (18 [48.6%] vs. 4 [10.8%], respectively). In addition, in some pSS patients with NSCS, lymphocytic infiltrates were similar to foci but not adjacent to the normal-appearing acini (Fig. 2C).
Discussion
NSCS is a common pathology of LSG [6, 7]. In the current study, we investigated the characteristics of pSS patients with NSCS using LSG pathological reports from 425 patients with suspected SS who underwent LSG biopsy in which 217 confirmed pSS patients were analyzed. We categorized the 217 pSS patients into three groups: FS ≥ 1 (104 [47.9%]), 0 ≤ FS < 1 (76 [35.0%]), and NSCS (37 [17.1%]). We compared these three groups, with special emphasis on the NSCS group.
Our result showed that pSS patients with NSCS were much older than those non-NSCS pSS patients (58.3 ± 11.0 years in NSCS group vs. 48.5 ± 14.9 years in FS ≥ 1 group or 45.3 ± 13.7 years in 0 ≤ FS < 1 group). Chronic sialadenitis is a common finding in elderly populations [16, 17]. Daniels [17] studied 362 LSG specimens from patients with suspected SS and found that 17 had inflammation associated with acinar atrophy and interstitial fibrosis (indicating NSCS). In that study, the mean age was higher in the NSCS group (62.3 ± 9 years) compared to the FS < 1 group (50.3 ± 16 years) and FS ≥ 1 groups (44.6 ± 17 years to 55.8 ± 15 years); however, organ involvement and serological tests were not analyzed. Our result regarding age is consistent with the result of Daniels [17]. Interestingly, only three cases (8.1%) in the NSCS group were aged < 45 years compared to 40 (38.5%) in the FS ≥ 1 group and 37 (48.7%) in the 0 ≤ FS < 1 group in our study. In multivariate logistic regression analysis, we found that age was associated with NSCS, but duration and ESSDAI were not. Therefore, we concluded that NSCS in pSS was solely associated with age and seldom occurred under the age of 45 years.
We found that pSS patients with NSCS had similar clinical features to those of non-NSCS pSS individuals. We assessed the involved organs in pSS patients and calculated the ESSDAI. The manifestations and laboratory variables in the NSCS group were not significantly different between the NSCS group and FS ≥ 1 and 0 ≤ FS < 1 groups. Nearly one-third of the 217 pSS patients had hematological involvement, including leukocytopenia and thrombocytopenia. The frequency of lung, nerve, and kidney involvement was much lower, but their involvement has a significant impact on the prognosis. The prevalence of ESSDAI ≥ 10 in the FS ≥ 1 group (27.9%) was higher than that in the 0 ≤ FS < 1 group (19.7%) and NSCS group (13.5%), which indicated that patients with FS ≥ 1 may have a greater risk of severe conditions, although the differences did not reach statistical significance. Risselada et al. [8] found that cumulative ESSDAI was significantly correlated with LFS, and FS ≥ 3 was associated with lymphoma. In our study, we mainly focused on NSCS in pSS patients so we did not further analyze the patients with FS ≥ 1. Sharma et al. [18] investigated the differences between pSS patients with and without focal infiltration, and found that the two subgroups were not clinically different, except for the higher degree of corneal staining with Lissamine green, serum anti-La antibodies, and elevated IgG in patients with FS ≥ 1. In our study, patients with FS ≥ 1 also had no significant differences compared to the 0 ≤ FS < 1 group (which included 25 patients with FS = 0), except for ANA titer ≥ 1:3200, positive anti-La, and elevated ESR. In addition, the FS ≥ 1 group had an increased prevalence of increased IgG. These results were similar to those of Sharma et al. [18], who compared to FS ≥ 1 and FS = 0, while our study compared FS ≥ 1 and 0 ≤ FS < 1. In another study, Park et al. [19] found that positive histopathologic assessment in salivary glands had little impact on the clinical features of pSS when FS ≥ 1 patients were compared with FS < 1 patients. Therefore, we speculate that FS ≥ 1 may not be associated with clinical manifestations, such as systemic involvement, but a higher FS may be related to a more severe condition.
In the current pSS group, 37 (17.1%) had NSCS, of whom 31 (83.7%) had anti-SSA and 15 (40.5%) had anti-SSB antibodies. The diagnosis mainly depended on the clinical presentations and autoantibodies. Daniels et al. [6] investigated a large cohort from the database of the Sjögren’s International Collaborative Clinical Alliance and found that among 1726 participants with sialadenitis, 668 (38.7%) patients had NSCS/SCS, of whom 91 (14%) had positive serum anti-SSA/SSB. Therefore, the pathology of NSCS with anti-SSA/SSB is frequently encountered in clinical practice. Few studies have investigated the differences between pSS with NSCS and the general population with NSCS. To investigate this, we selected 37 individuals with NSCS, who only had sicca symptoms and were otherwise healthy, named sicca NSCS controls. As expected, there were no differences in mean ages in all individuals and subgroups between pSS patients with NSCS and sicca NSCS controls. Only one sicca NSCS control was aged 20–44 years compared to three NSCS pSS, which was in agreement with the fact that NSCS seldom occurs before 45 years of age, regardless of whether the patient has pSS. In these two groups, the most frequent age group was 45–65 years, followed by > 65 years, which may be because some individuals aged over 65 years have sicca symptoms but may not seek medical attention for it. Syrjänen et al. [20] studied 78 healthy individuals aged 19–87 years who underwent LSG biopsy and found that acinar atrophy was not present in individuals aged under 50 years; however, the prevalence of acinar atrophy progressively increased with age. In addition, the degree of fibrosis, ductal dilatation, and fatty infiltration increased with advancing age. Our findings are consistent with this report, suggesting that NSCS may increase with age.
Because sicca NSCS controls did not have organ involvement, most disease manifestations are not listed in Table 3, except for dry mouth, dry eyes, and rampant caries, which were similar between the groups. The aforementioned age-associated changes can result in reduced acinar capacity and may lead to decreased saliva production. However, immunological features comprising high ANA titer, positive anti-SSA, and anti-SSB, and high IgG were predominantly found in pSS patients with NSCS. Based on a combination of these features and clinical manifestations, the diagnosis of pSS was confirmed. In addition, we analyzed the levels of lymphocytic infiltration in LSG tissues of these two groups and found that the prevalence of a high level of infiltration was significantly greater in pSS with NSCS than sicca NSCS controls (48.6 vs. 10.8%, respectively). In addition, lymphocytic infiltrates in some pSS patients with NSCS may mimic foci, but are not located adjacent to normal-appearing acini. Therefore, we speculate that there is greater lymphocytic infiltration in pSS with NSCS compared to sicca NSCS controls. When pSS occurs in individuals with NSCS, a typical focus may be not present because a focus is defined as infiltration adjacent to normal-appearing acini [20]. When such patients with NSCS are considered to have pSS, the extent of lymphocytic infiltration should be determined. NSCS may be a pathological subtype of pSS in elderly patients, even though it is common in the general elderly population.
The presence of anti-SSA or a positive salivary gland biopsy is mandatory for the diagnosis of pSS, but the classification criteria are more suitable for use in clinical trials and epidemiologic studies to allow standardization and comparability of findings across studies [11, 21, 22]. The clinical manifestations of pSS are heterogeneous and the diagnosis must be based on the clinical assessment of all data, including clinical findings, laboratory tests, and pathological examination. The clinical diagnosis based on expert opinion is also important [22]. In this study, a small number of biopsy-negative patients (nine with 0 ≤ FS < 1 and six with NSCS) without anti-SSA were clinically diagnosed with pSS after evaluation by the experienced rheumatologist in our team based on the typical manifestations, high titer of ANA, and positive anti-Ro52. Some of these biopsy-negative tissues exhibited significant lymphocytic infiltration or had a focus, but did not achieve FS ≥ 1. Moreover, LSG biopsy had some limitations. Examination of multiple tissue sections in the same specimen may identify focal infiltration in some biopsy-negative patients because mild FLS foci are unevenly distributed [23]. Another possibility is that the biopsy may not obtain the damaged LSG, thereby not accurately reflecting the condition of the salivary glands [22].
There were some limitations in this study. This study included a relatively small number of pSS patients with NSCS and sicca NSCS controls; however, these patients were identified from among 425 LSG biopsy participants, which was a relatively large cohort. In future studies, a large cohort should be included to verify these results. Another limitation was that the pathological reports regarding the degree of lymphocytic infiltration in NSCS were subjective, but the evaluation was performed by the same experienced pathologist. In addition, we did not examine lymphoepithelial lesions and germinal centers of LSG tissues in pathological reports, which needs to be addressed in future studies.
Conclusions
In our study, pSS patients with NSCS had similar clinical features to those of non-NSCS pSS individuals. Furthermore, NSCS was associated with age, but not other variables of pSS. We found that NSCS seldom occurred before the age of 45 both in pSS and sicca NSCS controls who were otherwise healthy. The features of NSCS were acinar atrophy, interstitial fibrosis, and ductal dilation, which were often accompanied by some lymphocytic infiltration. These findings were relatively common and increased with age in both the general population and pSS patients. NSCS may be a subtype of pSS in elderly patients.
References
Mariette X, Criswell LA. Primary Sjögren’s syndrome. N Engl J Med. 2018;378:931–9.
Caporali R, Bonacci E, Epis O, Bobbio-Pallavicini F, Morbini P, Montecucco C. Safety and usefulness of minor salivary gland biopsy: retrospective analysis of 502 procedures performed at a single center. Arthritis Rheum. 2008;59:714–20.
Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjögren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–29.
Fisher BA, Jonsson R, Daniels T, et al. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjögren’s syndrome. Ann Rheum Dis. 2017;76:1161–8.
Fisher BA, Brown RM, Bowman SJ, Barone F. A review of salivary gland histopathology in primary Sjögren’s syndrome with a focus on its potential as a clinical trials biomarker. Ann Rheum Dis. 2015;74:1645–50.
Daniels TE, Cox D, Shiboski CH, et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren’s syndrome among 1,726 registry participants. Arthritis Rheum. 2011;63:2021–30.
Scott J. Qualitative and quantitative observations on the histology of human labial salivary glands obtained post mortem. J Biol Buccale. 1980;8:187–200.
Risselada AP, Kruize AA, Goldschmeding R, Lafeber FP, Bijlsma JW, van Roon JA. The prognostic value of routinely performed minor salivary gland assessments in primary Sjögren’s syndrome. Ann Rheum Dis. 2014;73:1537–40.
Kakugawa T, Sakamoto N, Ishimoto H, et al. Lymphocytic focus score is positively related to airway and interstitial lung diseases in primary Sjögren’s syndrome. Respir Med. 2018;137:95–102.
Carubbi F, Alunno A, Cipriani P, et al. A retrospective, multicenter study evaluating the prognostic value of minor salivary gland histology in a large cohort of patients with primary Sjögren’s syndrome. Lupus. 2015;24:315–20.
Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76:9–16.
Seror R, Ravaud P, Bowman SJ, et al. EULAR Sjogren’s syndrome disease activity index: Development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69:1103–9.
Speight PM, Kaul A, Melsom RD. Measurement of whole unstimulated salivary flow in the diagnosis of Sjögren’s syndrome. Ann Rheum Dis. 1992;51:499–502.
Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry. Am J Ophthalmol. 2010;149:405–15.
Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjögren’s disease. J Clin Pathol. 1968;21:656–60.
Drosos AA, Andonopoulos AP, Costopoulos JS, Papadimitriou CS. Moutsopoulos. Prevalence of primary Sjögren’s syndrome in an elderly population. Br J Rheumatol. 1988;27:123–7.
Daniels TE. Labial salivary gland biopsy in Sjögren’s syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984;27:147–56.
Sharma R, Chaudhari KS, Kurien BT, et al. Sjögren syndrome without focal lymphocytic infiltration of the salivary glands. J Rheumatol. 2020;47:394–9.
Park Y, Lee J, Koh JH, et al. Positive histopathologic assessment in salivary glands shows little impact on clinical features of established primary Sjögren’s syndrome in a Korean population. Clin Exp Rheumatol. 2020;38(Suppl 126):158–65.
Syrjänen S. Age-related changes in structure of labial minor salivary glands. Age Ageing. 1984;13:159–65.
Billings M, Amin HM, Alevizos I. Comparative analysis of the 2016 ACR-EULAR and the 2002 AECG classification criteria for Sjögren’s syndrome: findings from the NIH cohort. Oral Dis. 2018;24:184–90.
Kroese F, Haacke EA, Bombardieri M. The role of salivary gland histopathology in primary Sjögren’s syndrome: Promises and pitfalls. Clin Exp Rheumatol. 2018;36(Suppl 112):222–33.
Al-Hashimi I, Wright JM, Cooley CA, Nunn ME. Reproducibility of biopsy grade in Sjögren’s syndrome. J Oral Pathol Med. 2001;30:408–12.
Acknowledgements
Funding
Sponsorship for this study and the Rapid Service Fee were funded by National Natural Science Foundation Boost Program of Tangdu Hospital (Grant no. 2021ZTXM-030).
Editorial Assistance
We would like to thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Hong-Xia Li and Ya-Fei Wang: study design, methodology, collecting data, analysis and interpretation of the data, drafting and editing the manuscript. Ya-Xin Zhou and Yuan Feng: collecting data, data entry and data analysis. Zhen-Biao Wu: study design, supervising the analysis and interpretation of the data, revising the manuscript and project administration.
Disclosures
Hong-Xia Li, Ya-Fei Wang, Ya-Xin Zhou, Yuan Feng, and Zhen-Biao Wu have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the local ethics board at the First Affiliated Hospital of Air Force Medical University (KY20212091-C-1). All participants provided written informed consent and the study was performed in accordance with the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, HX., Wang, YF., Zhou, YX. et al. Characteristics of Patients with Primary Sjögren’s Syndrome and Non-specific Chronic Sialadenitis: A Subtype in Elderly Patients. Rheumatol Ther 9, 1347–1359 (2022). https://doi.org/10.1007/s40744-022-00476-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00476-3