Abstract
Introduction
Rheumatoid arthritis (RA) clinical guidelines do not provide strong recommendations for the choice of disease-modifying anti-rheumatic drugs (DMARD) in patients with an inadequate response to methotrexate (MTX), and only limited evidence is available on factors influencing rheumatologist treatment decisions. We aimed to describe therapeutic preferences after the failure of a first-line strategy of MTX in simulated cases of patients with RA.
Methods
Fictional but realistic case-vignettes (n = 64) of patients with RA and an inadequate response to MTX were developed with a combination of RA-poor prognostic factors and comorbidities. Physicians were presented with eight vignettes and chose the most and least appropriate therapeutic option from the following six options randomly proposed 3 by 3: (1) replacing MTX with another csDMARD; (2) combining MTX with one or more csDMARDs; (3) adding a bDMARD of either TNF inhibitors (TNFi), tocilizumab (TCZ), abatacept (ABA), or rituximab (RTZ). A total of 1605 complete case vignettes were produced and randomly assigned to a representative sample of French rheumatologists. For each vignette, whenever a treatment was preferred, one point was incremented for this treatment; if this treatment was the least desired, one point was removed. Preferences were elicited using a normalized best–worst score.
Results
Two hundred and four French rheumatologists participated in the study with each vignette being assessed 20–28 times for a completion rate of 94%. TNFi was the first-choice strategy (80% of vignettes), except in cases with a history of infection and pulmonary comorbidity, where ABA was the first preference (85%). TCZ came third in 83% of the cases. Other options were never preferred and repeatedly yielded negative scores.
Conclusions
We observed a conservative trend with TNFi as the main therapeutic choice for patients with RA and inadequate response to MTX. Preference for bDMARD-based strategies increased with the number of RA-poor prognosis factors, whereas an increase in the number of comorbidities resulted in an increased preference for ABA. Understanding clinical decision-making will be particularly important as the therapeutic landscape for RA continues to evolve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There are limited data available on therapeutic decision-making for the treatment of rheumatoid arthritis (RA) following the failure of first-line therapy. |
Multiple factors influence therapeutic decision-making including those related to the disease (prognostic factors), patient (comorbidities, socio-demographics), and physician (experience, conditions of practice, frequency of consultation). |
The purpose of the study was to describe the therapeutic preferences of physicians involved in the management of RA, according to predefined profiles of fictitious RA patients, in cases of inadequate response to a first-line strategy of methotrexate (MTX). |
What was learned from the study? |
We observed a conservative trend with TNF inhibitors (TNFi) being the main therapeutic choice and abatacept (ABA) a choice for patients with pulmonary involvement and a history of serious or recurrent infection. |
We conclude that prescribing physicians are typically conservative in their approach to treating RA patients with an inadequate response to MTX and that factors related to the physician (years and mode of practice, number of RA patients seen per week) have little or no impact on therapeutic decisions. |
We also found that the presence of comorbidities resulted in a decreased preference for adding TNFi and tocilizumab (TCZ) and an increased preference of ABA and rituximab (RTX), as well as the other strategies. |
Digital Features
This article is published with digital features, including a summary slide to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14423927.
Introduction
Rheumatoid arthritis (RA), a chronic inflammatory condition, results in persistent and symmetrical inflammation of joints and affects about 1% of the global adult population [1]. The disease can lead to major joint destruction, resulting in disability, impaired quality of life, and reduced life expectancy. The therapeutic management of RA is primarily based on the use of disease-modifying anti-rheumatic drugs (DMARDs). According to the international guidelines for the management of RA, conventional synthetic (cs) DMARDs, as monotherapy or in combination, are the recommended first-line strategy for patients with active RA. Methotrexate (MTX) should be prescribed unless contraindicated [2, 3]. In cases of therapeutic failure, the next strategy should be guided by the presence of poor prognostic factors such as structural progression, high clinical, and/or biological activity and autoantibodies, namely rheumatoid factor (RF) and/or anti-citrullinated protein antibodies (ACPA) [4]. The addition of biological (b) DMARDs, or targeted synthetic (ts) DMARDs, is recommended in cases with poor prognostic factors.
Therapeutic decisions remain relatively complex in RA, particularly after MTX failure, which occurs in a significant proportion of patients [5]. There are several DMARDs available and several possible uses including replacing or in conjunction with MTX. Up to 2017, several bDMARDs were approved for use following failure of first-line therapy, these include compounds with five different mechanisms of action: tumor necrosis factor inhibition (TNFi), interleukin-6 receptor inhibition, B-cell depletion, and T-cell co-stimulation blockade [6]. However, only a few published face-to-face randomized controlled trials and network meta-analyses are available to inform therapeutic decision-making [7, 8]. All failed to identify superiority of one of these agents over the others when used in combination with MTX or a csDMARD equivalent. This resulted in clinical practice guidelines positioning all these compounds at the same level for RA patients with inadequate response to MTX or another csDMARD and failing to propose a hierarchy of these agents or a therapeutic sequence for their use in daily practice [2].
Besides RA characteristics and prognosis, patient characteristics (e.g., age, comorbidities, lifestyle, social issues) and factors related to the physician (e.g., experience, conditions of practice) and health system (e.g., prescription conditions) may have an influence on therapeutic decisions (including route of administration) [9]. However, these topics are only marginally addressed by clinical practice guidelines, which provide a general framework for therapeutic decisions but only limited guidance on the best course of treatment in a specific clinical situation. There is growing interest in studying clinical decisions with several approaches available based on real-life situations (trained actors) or fictional simulations of clinical cases in the form of case-vignettes [10].
Data on therapeutic decisions for the management of RA remain limited, particularly for second-line strategies. Fautrel et al. described rheumatologists’ therapeutic decisions in patients with RA receiving a first-line strategy and the criteria associated with these decisions based on simulated clinical cases [11]. A more recent study by Hifinger et al. examined rheumatologists’ preferences regarding the attributes of a hypothetical DMARD (efficacy, safety, patient preference, cost-effectiveness, and overall cost) in several European countries [12]. Rheumatologists were willing to reduce efficacy in favor of other attributes, albeit with variations between countries.
In this context, a deeper understanding of rheumatologists’ prescription preferences is required to better understand how targeted DMARDs are used in daily practice. Preference elicitation for such complex situation is always a challenge since direct and concise questions rarely cover the full spectrum of possible clinical situations or therapeutic options. Thus, the discrete choice experiment (DCE) methodology, based on the Thurstone pairwise method, has been proposed to facilitate preference elicitation in difficult decision-making processes [13,14,15]. This approach disentangles complex clinical situations with a series of simplified scenarios in which different attributes reflect the patient and disease characteristics, thus producing multiple variants of one single scenario. The therapeutic choice options are then presented against one another, i.e., in pairs. Unlike other expert opinion approaches, such as the RAND Appropriateness Method [16] in which respondents are asked to rate the appropriateness of a specific option, the pairwise method does not require any direct or absolute rating (or ranking) of the proposed options. An alternative approach is to present more than 2 therapeutic options and to ask respondents to identify what they would consider the best and the worst therapeutic choices for each scenario. The compilation of the expert responses enables the ranking of the different options, which expresses respondents’ overall preferences for each clinical situation [17].
Using a set of formatted clinical case-vignettes and associated questionnaires, adopting the discrete choice experiment (DCE) approach, we conducted a study to describe the therapeutic preferences of French rheumatologists when selecting a second therapeutic strategy after the failure of MTX in simulated patients with RA. This work also aimed at evaluating the impact of selected factors related to RA, the patient, and physician on the decision-making process.
Methods
Study Design
This was a non-interventional multicenter study that took place from May 15, 2018 to December 21, 2018. The study methodology was based on the presentation to physicians of case-vignettes, i.e., fictional but realistic and formatted clinical cases in which patient and disease characteristics were randomly varied to cover a wide spectrum of clinical possibilities [10]. Cases were associated with questionnaires on therapeutic decisions using the DCE approach [13,14,15]. Answers reported by physicians were anonymous. Physicians were remunerated for their participation; this study was conducted in accordance with French regulatory requirements. The protocol and all administrative documents, including the financial agreement, were approved by the National Medical Council (Conseil National de l’Ordre des Médecins [CNOM]). The database was declared to the National Data Protection Authority (Commission Nationale de l’Informatique et des Libertés [CNIL]). Submission to an ethics committee was not required under French law.
Selection of Participants
We wanted to conduct the study in a representative sample of rheumatologists who were hospital-based, office-based, or with a mixed practice (i.e., both hospital and office-based). Contacted physicians were required to be members of the French medical council (CNOM) with no limits in terms of age or number of years of practice. Practicing rheumatologists were identified in a database provided by IQVIA. It included 828 French rheumatologists who were offered to participate in the study. The goal of participant recruitment was to obtain a sample representative of the geographic distribution of French rheumatologists. A socio-demographic questionnaire was presented at the beginning of the study. The questionnaire collected data related to the physician and his/her usual clinical practice. It also requested information regarding mode of practice, sex, age category, number of years of clinical practice in the current specialty, frequency of patients with RA seen in consultation, referral hospital, and whether they were initiating bDMARD or csDMARD treatments for patients with RA.
Case-Vignettes Development
Case-vignettes were developed and pretested in collaboration with two experts in the treatment of RA (ES and BF). The basic structure of a vignette briefly described a patient living with RA, stable over the past 5 years thanks to an initial therapeutic strategy (optimized MTX 20 mg weekly and 5 mg of prednisone daily) and presenting with a recent increase in disease activity (see example Figure S1a in the electronic supplementary material). The medical history and examination included six specific clinical characteristics selected from a review of the literature (Table 1). These study variables were either poor prognostic factors (3) or comorbidities (3) (Table 1). The three poor prognostic factors have been consistently used for guiding treatment decisions in RA, namely disease activity according to Disease Activity Score in 28 Joints (DAS28), presence of structural damage, and presence of autoantibodies [2, 3]. The selected comorbidities were chosen as variables of interest because of their frequency in patients with RA [18]. None of the comorbidities were associated with an absolute or relative contraindication for any therapeutic option.
The set of vignettes was developed using a random combination of the six predefined study variables [19]. Each variable had two categories, and the number of possible combinations was 26 = 64. An exploratory analysis was performed on disease activity; for each category (high and moderate), two additional sub-categories were defined according to the predominance of subjective (tender joint [TJ]; patient global assessment on a 0–100 VAS [Global VAS]) or objective (swollen joint [SJ]; C-reactive protein [CRP]) components of the DAS28. The final drafting of vignettes was conducted in a way as to obtain clinical cases that were realistic and as close as possible to the current practice of physicians while maintaining a strong homogeneity. To avoid the participants being confronted with only the variables of interest, and to ensure that the vignettes would present patient complexity similar to actual practice, each vignette also included information regarding the patient’s age (ranging from 41 to 59 years), sex (male-to-female ratio 1:3), occupation, and comorbidity without impact on RA management (see Figure S1b in the electronic supplementary material for details) [10].
Physician Preference Assessment
The assessment of the physicians’ therapeutic preferences was based on the DCE approach, a quantitative technique for revealing individual preferences in complex decision situations when direct questioning is not possible [13,14,15]. All participants were asked to complete eight vignettes via an online platform. Upon the presentation of a vignette, each participant had to assess the six following therapeutic options, randomly proposed 3 by 3: replacing MTX by another csDMARD (csDMARD switch), adding one or more csDMARD to MTX (csDMARDs combination), adding a TNFi, adding tocilizumab (TCZ), adding abatacept (ABA), or adding rituximab (RTX). For each vignette, ten different combinations of three therapeutic options were assessed. For each combination of three therapeutic options, the participants had to select the most and least appropriate therapeutic option (best–worst [BW] scaling method) [20].
Statistical Analysis
Sample size calculations revealed that 216 participants were needed to conduct this cross-sectional study based on the following elements: (1) a Balanced Incomplete Block Design was used to randomly distribute the 64 vignettes (each rheumatologist needed to evaluate the same number of vignettes); (2) each vignette had to be evaluated the same number of times by 25–30 different rheumatologists to obtain precise estimates; and (3) each rheumatologist needed to evaluate a maximum of ten vignettes [21].
Analyses were mainly descriptive, as the study was observational. Therapeutic option preference was expressed using a score. A bonus of 1 point was attributed to a therapeutic option when it was considered as the preferred choice, and a penalty of − 1 point was attributed when it was considered as the worst option. Therapeutic option scores were calculated as the difference between the number of bonus points and penalty points, and they ranged from − 5 to + 5 for each vignette. A normalized BW score (BWS) was then computed for each therapeutic option ranging from − 1 (worst option) to + 1 (best option); this normalized score was used to rank the therapeutic options separately in each vignette and then to rank them globally. The multiplication of vignettes and therapeutic options associated with the score-based ranking enabled the analysis of physician preferences. Statistical analysis was carried out using SAS software Version 9.4, except graphs for which the R software Version 3.5.1 were used.
Results
Participants
A total of 211 French rheumatologists took part in the study (signed a contract and received a center number), 204 (97%) assessed at least one vignette, and 199 (94%) of the investigators who started the study evaluated all eight vignettes (Fig. 1). A detailed description of the participants is found in Table 2. Investigators were predominantly women (56.9 vs. 43.1%), 46.9% were hospital-based, 26.5% office-based, and the remaining 26.5% had a mixed practice. The average practice duration was 16.8 years, and most participants (84.4%) reported seeing more than one patient with RA per week. Only 2.8% reported seeing a patient with RA less than once per month. The majority of participants reported initiating both csDMARDs and bDMARDs (80.1%), while 19.9% reported initiating csDMARDs only. The geographical distribution of respondents was representative of the rheumatologist distribution in mainland France (data not shown).
Vignettes
A total of 1605 vignettes were assessed in this study with each of the 64 individual vignettes being assessed between 20 and 28 times. Of the 1605 vignettes, 87% included at least one poor prognostic factor while 12.5% included all three poor prognostic factors. Additionally, 87.5% of the vignettes included at least one comorbidity of interest and 12.5% included the three comorbidities of interest.
Therapeutic Preferences
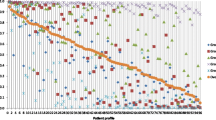
A full algorithm with the ranking of all therapeutic options for each vignette is presented in Fig. 2. A TNFi was the preferred strategy in 80% of the vignettes and ABA was the first option in the remaining cases. In all the cases where ABA was the first option, there was a history of infection and, in most (85%), a pulmonary involvement. Abatacept was the most frequently selected second option in 75% of the vignettes, while TNFi was selected in 20% and TCZ in 5%. Tocilizumab was chosen as the third strategy in most cases (83%). Tumor necrosis factor inhibitor, ABA, and TCZ were ranked in this order in 70% of the vignettes. The average normalized BW score was computed globally for each strategy and showed that TNFi, ABA, and TCZ were associated with a positive BW score while all other strategies, including RTX, were associated with a negative BW score (Fig. 3).
Summary of standardized BW scores (six study variables, six preferred options) for all 64 case-vignettes. 1 + csDMARD conventional synthetic disease-modifying anti-rheumatic drug combination, ABA abatacept, ACPA anti-citrullinated protein antibodies, csDMARD Sw conventional synthetic disease-modifying anti-rheumatic drug switch, RF rheumatoid factor, RTX rituximab, TCZ tocilizumab, TNFI tumor necrosis inhibitors
The analysis of the BW score according to the presence of each disease variable of interest yielded similar findings to the one based on the global normalized BW score (Fig. 4). An increase in the number of poor prognostic factors increased physician preference for a strategy based on the addition of a bDMARD, i.e., TNFi, ABA, and TCZ (more positive score), as well as RTX (less negative score, i.e., less rejected strategy). Inversely, an increase in the number of poor prognostic factors decreased physician preference for csDMARD-based strategies, i.e., csDMARD replacement or combination (Fig. 5a). An increase in the number of comorbidities resulted in a decreased preference for adding TNFi and TCZ, and in an increased preference for ABA and RTX, as well as for the csDMARD-based strategies (Fig. 5b). An exploratory analysis by disease activity with four categories (high objective, high subjective, moderate objective, and moderate subjective) revealed a marked increase in preference for TCZ in cases where patients presented with high disease activity and predominance of DAS28 objective components (SJ and CRP) (data not shown). However, TCZ remained the third preferred option even in such patients. Factors related to the prescribing physician appeared to have either a limited or no impact on therapeutic decisions (see Figure S2 in the electronic supplementary material for details)..
Standardized BW scores by a disease activity (DAS28), b presence of autoantibodies (RF/ACPA), c structural progression, d history of infection, e cardiovascular comorbidity, and f pulmonary comorbidity. ABA abatacept, ACPA anti-citrullinated protein antibodies, BWS best–worst scores, csDMARDs conventional synthetic disease-modifying anti-rheumatic drug, RF rheumatoid factor, RTX rituximab, TCZ tocilizumab, TNFI tumor necrosis inhibitors
Discussion
This study aimed to describe therapeutic preferences in RA after failure of MTX and to evaluate the impact of selected factors related to RA, the patient, and physician on the decision-making process. It is important to point out that French rheumatologists have no consistent constraint in their prescription of targeted therapies in RA patients with inadequate response to MTX; all six proposed options are accepted by the health authorities and reimbursed for all patients by the national public health insurance. This substantial drug prescription freedom led to reasonable use of targeted therapies, which are used in approximately 31% of RA patients [22].
We developed 64 fictional, but realistic, clinical case-vignettes to elicit the therapeutic preferences of 211 French rheumatologists. In the current study, case-vignettes were associated with questionnaires on therapeutic decisions using the well-established DCE approach. The multiplication of vignettes and therapeutic options associated with a score-based ranking enabled the assessment of physician preferences.
Our study demonstrates an overall conservative trend among physicians, with TNFi being the strategy of choice in 80% in these simulated patients with RA. This is aligned with the results of several publications on treatment pattern in real patients [23,24,25]. The exception to this trend was found in patients with a history of infection and pulmonary comorbidity, where ABA was the preferred option. This result was not unexpected, as some guidelines have proposed to favor ABA over TNFi in patients with history of serious infections based on very low-quality evidence [3]. On the other hand, there is only limited evidence to support the use of ABA in interstitial lung disease associated with RA and no evidence to support its use in the case of rheumatoid lung nodules [26]. Rituximab was consistently ranked after the other bDMARDs strategies; this could be explained by the instructions-for-use on its label, which describes RTX as being indicated for use in patients with RA who display inadequate response or intolerance to one or more TNFi.
Poor prognostic factors have been identified as an important decision-criteria for treatment intensification with bDMARDs [2, 27]. This was reflected in our work, as an increase in the number of poor prognostic factors had a positive impact on the preference of all bDMARD-based strategies, i.e., TNFi, ABA, and TCZ (more positive score), as well as RTX (less negative score). Reliability of DAS28 in the individual patient has been questioned as the presence of subjective components (i.e., TJ and VAS) can lead to misclassification of disease activity and potentially overtreatment [28]. Some authors have argued that patient global assessment should be removed from composite measures of disease activity [29]. While the added value of subjective components to guide treatment intensification is still debated, the impact on therapeutic decision after MTX failure is not known. In our study, an analysis performed according to the predominance of objective or subjective components of DAS28 showed mainly an increased preference for TCZ in case of high disease activity with predominance of objective components. The effect on the BW score of the other strategies was more limited.
Guidelines for RA invite physicians to include comorbidities in treatment decisions [2, 18]. We found that an increase in the number of comorbidities resulted in a decreased preference for adding TNFi and TCZ, and an increased preference for ABA and RTX, as well as for the csDMARD-based strategies. The influence of comorbidities on therapeutic decisions in RA is not well understood. Previous studies have shown a higher prevalence of comorbidities in patients on csDMARDs in comparison to patients on bDMARDs [24, 30, 31]. Frisell et al. observed substantial differences in baseline characteristics of patients at the start of a first bDMARD with patients starting on ABA or RTX more likely to have a history of comorbidity (e.g., serious infection, pulmonary disease) than patients starting a TNFi or TCZ [25]. The comorbidities selected as variables of interest in this study were neither associated with an absolute nor relative contraindication, nor with a recommendation for any therapeutic option. This was done to limit the weight that such comorbidities would have had on the therapeutic decision.
There is limited and conflicting evidence regarding the impact of prescribers’ demographic characteristics and mode of practice on treatment decisions in RA [32, 33]. Results may vary according to geographic location. For instance, in France, bDMARDs used to treat RA should only be initiated by rheumatologists who are hospital-based, and those with a mixed practice (both private office- and hospital-based). Office-based rheumatologists will usually refer patients with RA to hospital clinics to receive their first bDMARD prescription, while renewal of bDMARD prescriptions may be done in the office-setting thereafter. In our study, the mode of practice (office-, hospital-, or mixed) did not appear to influence physician preference. Other factors related to the prescribing physician, such as years of practice and number of RA patients seen per week also appeared to have either a limited or no impact on physician preference.
Physician preference has been identified in previous research as a significant determinant of treatment decisions in RA [34, 35]. With an increasing number of therapeutic options available for patients in RA, it is important for physicians to better understand treatment decisions in clinical practice. Preference for a given therapeutic option may lead to a non-random allocation of treatment. This decision can be motivated by scientific evidence, prescribing experience, or a mere misconception about the therapy [25, 36]. Evaluating medical practices and behaviors is essential to improving the quality of care. The methods used must be accurate, valid, and closely mimic cases encountered in daily practice. There are several methods available including the audit of clinical practices, trained actors to present as patients, or the use of case-vignettes. Case-vignettes have been increasingly used in various chronic conditions to assess decision-making [37]. Results from case-vignettes studies have been shown to be comparable to those obtained with practice audits or standardized patients while also measuring the quality of care [10]. Case-vignettes have the added advantage of avoiding differences in recruitment, the variability introduced by the disease severity, as well as other potential confounding factors that could interfere with the decision-making process.
The strengths of our study include the representativeness of participating rheumatologists with each region proportionally represented based on national demographics at the time of participation. We also note that only a small minority of the participants (15.6%) reported seeing less than one RA patient per week. An additional strength of the work was the high level of engagement obtained, as 94% of the participants with socio-demographic data completed all evaluations [38]. Finally, we believe that the design of our study, using case-vignettes with questionnaires following the DCE approach, enabled the assessment of preferences while limiting the risk of response bias.
A potential weakness of these findings relates to timeliness. As tsDMARDs had been available for less than 1 year for the treatment of RA in France, we focused this work on well-established csDMARD- and bDMARD-based strategies. Repeated or longitudinal studies will be needed to monitor the changing preferences of physicians as the field gains hands-on experience with the use of newer therapies. We also recognize that other factors, such as patient preference and continually evolving treatment recommendations, influence treatment decisions. We had to limit the number of variables of interest during the development of case-vignettes since the number of combinations increases quickly as more variables and categories are considered. The limited number of factors does not impact the validity of our results, but future studies should be carried out to understand the impact that patient preference and shared decision-making has on therapy selection [3, 39,40,41,42]. This is particularly important with the emergence of Janus kinase inhibitors, a new class of oral DMARDs, the first of which was approved by the European Medicines Agency and became available in France in 2017 [43, 44]. We note that the simulated population of patients may not be representative of the population seen by all rheumatologists, which may affect the generalizability of some of our results. However, this should be limited to the overall ranking of ABA and TCZ as results regarding the other options were aligned with findings from the literature.
Conclusions
This study provides information on the prescribing habits of French rheumatologists in RA after failure of a first-line strategy with MTX. We found a conservative trend with TNFi being the main therapeutic choice for most RA patients and ABA as the primary therapeutic choice for patients with pulmonary involvement or high risk of infection. We also found that an increase in the number of comorbidities resulted in a decreased preference for adding TNFi and TCZ, and in an increased preference of ABA and RTX, as well as of the other strategies. Understanding clinical decision-making will be particularly important as the therapeutic landscape for RA continues to evolve. The study should be repeated in the future to include new therapeutic options and explore patient preferences in the context of shared decision-making.
References
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1–25.
Nam JL, Takase-Minegishi K, Ramiro S, Chatzidionysiou K, Smolen JS, Van Der Heijde D, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1113–36.
Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–89.
Rein P, Mueller RB. Treatment with biologicals in rheumatoid arthritis: an overview. Rheumatol Ther. 2017;4(2):247–61.
Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ. 2016;353:i1777.
Favalli EG, Bugatti S, Biggioggero M, Caporali R. Treatment comparison in rheumatoid arthritis: head-to-head trials and innovative study designs. Biomed Res Int. 2014;2014:831603.
Reschovsky JD, Rich EC, Lake TK. Factors contributing to variations in physicians’ use of evidence at the point of care: a conceptual model. J Gen Intern Med. 2015;30(Suppl 3):S555–61.
Converse L, Barrett K, Rich E, Reschovsky J. Methods of observing variations in physicians’ decisions: the opportunities of clinical vignettes. J Gen Intern Med. 2015;30(Suppl 3):S586–94.
Fautrel B, Guillemin F, Meyer O, De Bandt M, Berthelot JM, Flipo RM, et al. Choice of second-line disease-modifying antirheumatic drugs after failure of methotrexate therapy for rheumatoid arthritis: a decision tree for clinical practice based on rheumatologists’ preferences. Arthritis Care Res. 2009;61(4):425–34.
Hifinger M, Hiligsmann M, Ramiro S, Watson V, Severens J, Fautrel B, et al. Economic considerations and patients’ preferences affect treatment selection for patients with rheumatoid arthritis: a discrete choice experiment among European rheumatologists. Ann Rheum Dis. 2017;76(1):126–32.
Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–15.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–13.
Coast J, Al-Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ. 2012;21(6):730–41.
Shekelle P. The appropriateness method. Med Decis Mak. 2004;24(2):228–31.
Prieto L, Alonso J. Exploring health preferences in sociodemographic and health related groups through the paired comparison of the items of the Nottingham health profile. J Epidemiol Community Health. 2000;54(7):537–43.
Richards JS, Dowell SM, Quinones ME, Kerr GS. How to use biologic agents in patients with rheumatoid arthritis who have comorbid disease. BMJ. 2015;351:h3658.
Brauer PM, Hanning RM, Arocha JF, Royall D, Goy R, Grant A, et al. Creating case scenarios or vignettes using factorial study design methods. J Adv Nurs. 2009;65(9):1937–45.
Flynn TN, Louviere JJ, Peters TJ, Coast J. Best–worst scaling: what it can do for health care research and how to do it. J Health Econ. 2007;26(1):171–89.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–77.
Kedra J, Granger B, Emilie S, Gaujoux-Viala C, Rat AC, Combe B, et al. Time to initiation of biologic disease-modifying antirheumatic drugs in the French cohort ESPOIR. Jt Bone Spine. 2020;88(1):105060
Sullivan E, Kershaw J, Blackburn S, Mahajan P, Boklage SH. Biologic disease-modifying antirheumatic drug prescription patterns among rheumatologists in Europe and Japan. Rheumatol Ther. 2020;7(3):517–35.
George MD, Sauer BC, Teng CC, Cannon GW, England BR, Kerr GS, et al. Biologic and glucocorticoid use after methotrexate initiation in patients with rheumatoid arthritis. J Rheumatol. 2019;46(4):343–50.
Frisell T, Baecklund E, Bengtsson K, Di Giuseppe D, Forsblad-d’Elia H, Askling J, et al. Patient characteristics influence the choice of biological drug in RA, and will make non-TNFi biologics appear more harmful than TNFi biologics. Ann Rheum Dis. 2018;77(5):650–7.
Esposito AJ, Chu SG, Madan R, Doyle TJ, Dellaripa PF. Thoracic manifestations of rheumatoid arthritis. Clin Chest Med. 2019;40(3):545–60.
Albrecht K, Zink A. Poor prognostic factors guiding treatment decisions in rheumatoid arthritis patients: a review of data from randomized clinical trials and cohort studies. Arthritis Res Ther. 2017;19(1):68.
Ton E, Bakker MF, Verstappen SM, Ter Borg EJ, van Albada-Kuipers IA, Schenk Y, et al. Look beyond the disease activity score of 28 joints (DAS28): tender points influence the DAS28 in patients with rheumatoid arthritis. J Rheumatol. 2012;39(1):22–7.
Ferreira RJO, Welsing PMJ, Jacobs JWG, Gossec L, Ndosi M, Machado PM, et al. Revisiting the use of remission criteria for rheumatoid arthritis by excluding patient global assessment: an individual meta-analysis of 5792 patients. Ann Rheum Dis. 2020;80:293–303.
Radner H, Yoshida K, Hmamouchi I, Dougados M, Smolen JS, Solomon DH. Treatment patterns of multimorbid patients with rheumatoid arthritis: results from an international cross-sectional study. J Rheumatol. 2015;42(7):1099–104.
Bengtsson K, Jacobsson LT, Rydberg B, Kvist G, Torstenson T, Dehlin M, et al. Comparisons between comorbid conditions and health care consumption in rheumatoid arthritis patients with or without biological disease-modifying anti-rheumatic drugs: a register-based study. BMC Musculoskelet Disord. 2016;17(1):499.
Png WY, Kwan YH, Lim KK, Chew EH, Lui NL, Tan CS, et al. A systematic review of the factors associated with the initiation of biologicals in patients with rheumatological conditions. Eur J Hosp Pharm. 2019;26(3):163–9.
Tatangelo M, Tomlinson G, Paterson JM, Ahluwalia V, Kopp A, Gomes T, et al. Association of patient, prescriber, and region with the initiation of first prescription of biologic disease-modifying antirheumatic drug among older patients with rheumatoid arthritis and identical health insurance coverage. JAMA Netw Open. 2019;2(12):e1917053.
Kalkan A, Husberg M, Hallert E, Roback K, Thyberg I, Skogh T, et al. Physician preferences and variations in prescription of biologic drugs for rheumatoid arthritis: a register-based study of 4,010 patients in Sweden. Arthritis Care Res (Hoboken). 2015;67(12):1679–85.
Curtis JR, Chen L, Harrold LR, Narongroeknawin P, Reed G, Solomon DH. Physician preference motivates the use of anti-tumor necrosis factor therapy independent of clinical disease activity. Arthritis Care Res (Hoboken). 2010;62(1):101–7.
Kalkan A, Roback K, Hallert E, Carlsson P. Factors influencing rheumatologists’ prescription of biological treatment in rheumatoid arthritis: an interview study. Implement Sci. 2014;9:153.
Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283(13):1715–22.
Martinez V, Attal N, Vanzo B, Vicaut E, Gautier JM, Bouhassira D, et al. Adherence of French GPs to chronic neuropathic pain clinical guidelines: results of a cross-sectional, randomized, “e” case-vignette survey. PLoS ONE. 2014;9(4):e93855.
Fraenkel L, Bogardus ST, Concato J, Felson DT, Wittink DR. Patient preferences for treatment of rheumatoid arthritis. Ann Rheum Dis. 2004;63(11):1372–8.
Alten R, Kruger K, Rellecke J, Schiffner-Rohe J, Behmer O, Schiffhorst G, et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adher. 2016;10:2217–28.
Taylor PC, Betteridge N, Brown TM, Woolcott J, Kivitz AJ, Zerbini C, et al. Treatment mode preferences in rheumatoid arthritis: moving toward shared decision-making. Patient Prefer Adher. 2020;14:119–31.
Harrison M, Spooner L, Bansback N, Milbers K, Koehn C, Shojania K, et al. Preventing rheumatoid arthritis: preferences for and predicted uptake of preventive treatments among high-risk individuals. PLoS ONE. 2019;14(4):e0216075.
Harrington R, Al Nokhatha SA, Conway R. JAK inhibitors in rheumatoid arthritis: an evidence-based review on the emerging clinical data. J Inflamm Res. 2020;13:519–31.
Angelini J, Talotta R, Roncato R, Fornasier G, Barbiero G, Dal Cin L, et al. JAK-inhibitors for the treatment of rheumatoid arthritis: a focus on the present and an outlook on the future. Biomolecules. 2020;10(7):1002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding and payment of the Rapid Service Fee was provided by Eli Lilly and Company, Indianapolis, IN, USA. All authors had complete access to the data used in this study and take full responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval this version for to be published.
Authors’ Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Baptiste Roux, Eric Senbel, Bruno Fautrel, and Frederick Durand. Bruno Fautrel, Frederick Durand, Eric Senbel, and Baptiste Roux planned and directed data interpretation. All authors contributed to manuscript drafting, provided critical revision throughout, and approved the final version for submission.
Medical Writing Assistance
Luke M. Healy, PhD, and Adam Clooney, PhD, (both employees of Eli Lilly and Company) provided writing and editorial support for this manuscript.
Disclosures
Baptiste Roux is an employee of FAST4. FAST4 was paid by Eli Lilly and Company to conduct the current study. Frederick Durand is an employee and shareholder of Eli Lilly and Company. Fatima-Zohra Badaoui is an employee of Eli Lilly and Company. Eric Senbel has received consultancy fees from Eli Lilly and Company. Bruno Fautrel has received research grant support and consultancy fees from Eli Lilly and Company.
Compliance with Ethics Guidelines
This study involved the elicitation of physician preference and was conducted in accordance with French regulatory requirements. The protocol and all administrative documents, including the financial agreement, were approved by the National Medical Council (Conseil National de l’Ordre des Médecins [CNOM]). The database was declared to the National Data Protection Authority (Commission Nationale de l’Informatique et des Libertés [CNIL]). Submission to an ethics committee was not required under French law.
Data Availability
All data generated or analyzed during this study are included in this published article as a supplementary information file.
Prior Presentation
Preliminary results from this analysis were presented in poster format at the European League Against Rheumatism (EULAR) 2020 Annual Meeting.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Senbel, E., Durand, F., Roux, B. et al. Elicitation of Rheumatologist Preferences for the Treatment of Patients with Rheumatoid Arthritis After the Failure of a First Conventional Synthetic Disease-Modifying Anti-Rheumatic Agent. Rheumatol Ther 8, 921–935 (2021). https://doi.org/10.1007/s40744-021-00311-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-021-00311-1