Abstract
Introduction
The objective of this study is to identify the potential risk factors for progression from subclinical to clinical psoriatic arthritis (PsA).
Methods
A retrospective, longitudinal, case–control study was conducted at a single hospital, including 25 patients with clinically confirmed PsA in the case group and 137 controls without confirmed PsA. All patients in both groups had a medical history of subclinical PsA. Various baseline covariates were collected from all patients when they had a status of subclinical PsA. Univariate, multivariate, stratified, and interaction analyses were employed to identify potential risk factors of transiting to clinical PsA from subclinical PsA.
Results
In multivariate logistic regression analysis, older age (OR 10.15, 95% CI 2.79–36.91, p = 0.00), alcohol drinking (OR 3.43, 95% CI 1.17–10.12, p = 0.03), elevated high-sensitivity C-reactive protein (hs-CRP) (OR 1.05, 95% CI 1.01–1.09, p = 0.03) were identified as risk factors for transition from subclinical to clinical PsA. Stratified and logistic regression analyses suggest a significant interaction between age and fatty liver. For patients aged less than 45 years old, the association between fatty liver and clinical PsA was statistically significant.
Conclusions
Older age, alcohol drinking, elevated hs-CRP, and the presence of fatty liver at less than 45 years old appear to increase the risk of transition from subclinical to clinical PsA. These findings call for a need to manage these risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
At present, early diagnosis and intervention are considered to be effective means for delaying the progression of PsA. |
Recently, the focus of research has shifted to identifying and even treating psoriasis patients at increased risk of transiting into PsA. |
The objective of this study is to identify the potential risk factors for progression from subclinical to clinical phase psoriatic arthritis (PsA). |
Our study showed drinking, older age, suffering from fatty liver with less than 45 years old, and elevated hs-CRP level might be correlated with an increased risk of developing clinical PsA among psoriasis patients complicated with subclinical PsA. |
Fatty liver screening could be performed after a patient is diagnosed with subclinical PsA, especially for young patients. Moreover, patients with subclinical PsA should drink appropriately. Inflammation should be controlled if the patient has elevated hs-CRP. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14055191.
Introduction
Psoriatic arthritis (PsA) is an immune-mediated chronic systemic arthritis with a prevalence of 30% among psoriasis patients [1]. The main clinical manifestations of PsA include peripheral arthritis, axial spondylarthritis, enthesitis, and dactylitis. These symptoms can occur alone or in combination with each other. Most patients with PsA have peripheral involvement, while less than 5% of them present solely axial involvement [2, 3]. Patients with PsA commonly have a serious decline in living quality and life expectancy due to arthralgia, fatigue, psychological stress, and various complications [4]. The presence of strong clinical heterogeneity and complicated pathogenesis has made the early diagnosis of PsA very challenging. There is still no universally accepted diagnostic criterion for PsA since it was categorized as an independent disease by the American College of Rheumatology in 1964 [5]. At present, the classification Criteria for Psoriatic Arthritis (CASPAR) [6] have been widely applied in clinical studies of PsA, owing largely to their high sensitivity and specificity [7]. However, it was originally put forward based on PsA patients with chronic conditions and requiring the presence of an inflammatory musculoskeletal precondition. Thus, the CASPAR criteria are not suitable for the diagnosis of early PsA.
In terms of treatment, a variety of biological agents and small molecular drugs have proven effects in curing skin lesions in patients with psoriasis. However, their curative effects on arthritis in patients with PsA are not satisfactory. In phase 3 clinical trials of many biological agents, more than half of the PsA patients failed to achieve the American College of Rheumatology 20 (ACR20) response rate [8,9,10,11]. Given that multiple inflammatory pathways have been activated before the onset of clinical symptoms, it is likely that once musculoskeletal symptoms occur, drug treatment may not improve the long-term clinical outcome [12]. Furthermore, the majority of PsA patients have skin lesions preceding arthritis by an average of 7 years [13]. This long progression course provides an opportunity to initiate early interventions to slow down the arthropathy process. Accordingly, over the past decade, increasing efforts have been devoted to identifying the risk factors of developing PsA.
Recent imaging studies have documented various preclinical changes in the musculoskeletal system in psoriasis patients with only skin lesions (PsO) in the absence of overt symptoms related to inflammatory arthropathy [12]. These findings have put forward the concept of subclinical PsA. Patients at a status of subclinical PsA have imaging abnormalities (such as synovitis, enthesitis, or early erosions detected by musculoskeletal ultrasound or magnetic resonance imaging) but do not have any apparent symptoms and signs of arthritis [12]. Although numerous studies have proposed several risk factors for transiting to PsA, they mostly focused on differentiating between PsO and PsA other than between subclinical PsA and clinical PsA.
The primary objective of this study is to identify the potential risk factors for progression from subclinical to clinical PsA.
Methods
Study Design and Patient Population
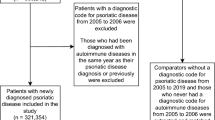
This is a retrospective case–control study with a longitudinal design, including a case group and a control group. All patients came from the Department of Dermatology, West China Hospital, Sichuan University between February 2016 and December 2019, and met the inclusion criteria described below. At initial enrollment, all patients in the case group had clinically confirmed PsA (defined below) while all control patients had subclinical PsA (defined below). All patients in both groups were followed backwards in their clinical record to the first diagnosis of subclinical PsA. The total follow-up intervals ranged from 1.5 years to 5.5 years, with a mean of 2.4 years.
All procedures performed in studies involving human participants were in compliance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of West China Hospital, Sichuan University (approval number: 2015(308)). Each participating patient signed an informed consent form.
Definition of PsA and Subclinical PsA
The classification of PsA was based on the CASPAR criteria [6], in which patients with inflammatory musculoskeletal disease must attain a score ≥ 3 among the following five categories: evidence of psoriasis (current psoriasis, a personal history of psoriasis, or a family history of psoriasis), presence of psoriatic nail dystrophy, a negative test for rheumatoid factor, dactylitis, and radiographic evidence of juxta-articular new bone formation. Psoriatic patients without positive symptoms and signs of arthritis but with abnormal musculoskeletal ultrasound changes (synovio-entheseal inflammation or early erosions) were defined as subclinical PsA [12]. According to the Outcome Measures in Rheumatology Clinical Trials [14], enthesitis was identified as abnormally hypoechoic (loss of normal fibrillar architecture) and/or thickened tendon or ligament at its bony attachment (may occasionally contain hyperechoic foci consistent with calcification), seen in two perpendicular planes with Doppler signals and/or bony changes including enthesophytes, erosions, or irregularity. Synovitis is characterized on grayscale ultrasound by intra-articular tissue that is abnormally thickened, hypoechoic or anechoic (relative to subdermal fat), non-displaceable, and poorly compressible. Bone erosion was identified with ultrasound based on the intra-articular discontinuity of the bone surface which is visible in two perpendicular planes.
Inclusion and Exclusion Criteria
Patients were enrolled when all the following inclusion criteria were met: (i) aged 18–65 years with no gender restriction; (ii) an established diagnosis of psoriasis confirmed by two dermatologists; (iii) a previous diagnosis of subclinical PsA as defined above. Patients were excluded if they met the following exclusion criteria: (i) a diagnosis of other arthritis including rheumatoid arthritis, osteoarthritis, gouty arthritis, ankylosing spondylitis, and similar conditions; (ii) presence of hematological system diseases and malignant tumors; (iii) presence of serious hepatic dysfunction, renal dysfunction, or other visceral organ dysfunction; (iv) pregnant or in lactation; (v) absence of clinical record required in this study.
Data Collection and Clinical Evaluation
In addition to the data collected at the initial enrollment as described above, baseline data were collected in the first diagnosis of subclinical PsA for all patients in the case group and control group through the Hospital Information System (HIS) in our hospital. Any required information missing in HIS was inquired through a telephone interview with patients by an experienced dermatologist. We excluded patients from whom any required information could not be traced. The details of demographic and clinical information for all patients are summarized in Table 1.
Regarding clinical characteristics, we focused on the skin, nail, scalp, disease course, systematic medicine, family history, and comorbidities. The Psoriasis Area and Severity Index (PASI) was used to assess psoriasis severity. At least two dermatologists evaluated the involvement of nails and scalp in each patient and classification of the psoriasis subtype based on medical records including original images on the affected areas taken at the first diagnosis of subclinical PsA. For the disease progression course, age at psoriasis onset, duration of psoriasis disease, and time of progression were recorded. Time of progression refers to the interval from the first diagnosis of subclinical PsA to the confirmation of clinical PsA for the case group and from the first diagnosis of subclinical PsA to the inclusion in this study for the control group. Systematic medication refers to oral administration of any drugs during the time of progression, including disease-modifying antirheumatic drugs, retinoic acid, biological agents, and non-steroidal anti-inflammatory drugs. All comorbidities were diagnosed according to the corresponding guidelines [15,16,17,18,19,20]. Fatty liver was detected by ultrasound according to the reported criteria [21]. Musculoskeletal ultrasound abnormalities were detected by two experienced sonographers based on images from grayscale and power Doppler ultrasound (Philips IU22).
All routine laboratory tests were conducted in the Department of Laboratory Medicine in our hospital. The normal reference ranges are as follows: high-sensitivity C-reactive protein (hs-CRP) < 3.5 mg/l, tumor necrosis factor-α (TNF-α) < 30 fmol/ml, erythrocyte sedimentation rate (ESR) < 21 mm/h, neutrophils 1.8–6.3 109/l, lymphocytes 1.1–3.2 109/l, uric acid 240–490 µmol/l, Ca + 2.11–2.52 mmol/l, triglyceride 0.29–1.83 mmol/l, cholesterol 2.80–5.70 mmol/l and blood glucose 3.90–5.90 mmol/l. We calculated the quartile of hs-CRP in the study patient population. The upper quartile was defined as the high hs-CRP, while the lower quartile was defined as the low hs-CRP.
In addition, we collected data concerning patients’ lifestyles, including alcohol drinking, smoking, sleeping habits, stress, and exercise activities. Alcohol consumption was categorized as following a previous report [22], including non-drinkers (< 12-g alcohol intake per month) and drinkers (≥ 12-g alcohol intake per month). Regular smokers were defined as smoking any tobacco product at least once a day according to the World Health Organization definition [23]. Sleep disorder was defined as any disorder that affects, disrupts, or involves sleep [24]. Stress was defined as any type of impact that causes physical, emotional, or psychological strain. Regular exercise was defined as any kind of recreational or sport physical activities for at least 7 h per week or at least 3 times per week.
Statistical Analysis
The continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR), while categorical variables were expressed as proportions (%). We used Student’s t test for normally distributed data with uniform variance, Welch’s t test for normally distributed data with nonuniform variance, and Mann–Whitney test for nonnormally distributed data. Pearson Chi-square test or Fisher’s exact test was used for comparing categorical outcomes. Mann–Whitney test was employed for ranked data. The alpha level was set to 0.05 with all tests two-sided. A p value < 0.05 was considered statistically significant. Univariate logistic regression was used to calculate the odds ratio (OR) between independent variables and outcome. Moreover, we compared patients having a high hs-CRP with those having a low hs-CRP and calculated the OR. After selecting clinically meaningful variables based on expert knowledge and previous research [12], we conducted a multivariate logistic regression model using the binary logistic regression method. The variable screening method was backward selection. In our previous report [25], and recent unpublished observations, there were substantial differences in clinical characteristics (e.g., musculoskeletal ultrasound abnormalities and the prevalence of comorbidities) between young and middle-aged patients with psoriasis. To control the heterogeneity caused by age, we stratified patients into youth (≤ 45 years) and middle-aged (> 45 years) layers. We further employed Breslow-Day and Tarone methods to check the consistency of OR values in each layer and verified interaction between fatty liver and age. Ultimately, five variables were selected based on suggestions from clinical experts to establish the final model. All analyses were performed using SPSS software (version 26.0). R software (version 4.0.2) was used for mapping a forest plot.
Results
Demographic and Clinical Characteristics of Patients
A total of 162 psoriasis patients who met study criteria were enrolled. Among them, 137 were classified as the control group, while 25 were classified as the case group. Their demographics and clinical characteristics are summarized in Table 1.
Results of Univariate Logistic Regression Analysis
Univariate logistic regression analysis was employed to explore which variables might be associated with the progression from subclinical to clinical PsA. This analysis demonstrated that odds for transition to clinical PsA per year of age, per unit of lymphocyte, per unit of PASI are 1.07 (95% CI 1.03, 1.12), 0.27 (95% CI 0.10, 0.75), 1.04 (95% CI 1.00, 1.08), respectively (Fig. 1). Compared to patients who have low hs-CRP, patients with high hs-CRP have a 2–37 higher odds (OR 8.77, 95% CI 2.06, 37.40) to transit from subclinical to clinical PsA.
Results of univariate logistic regression analysis. The 95% confidence intervals range from 0 to 8. BMI body mass index, PASI Psoriasis Area and Severity Index, hs-CRP high-sensitivity C-reactive protein, TNF-α tumor necrosis factor-α, ESR erythrocyte sedimentation rate, NLR neutrophil-to-lymphocyte ratio
Establishment of a Multivariable Logistic Regression Model
Based on expert knowledge and previous research [12], age, gender, alcohol drinking, PASI score, scalp involvement, nail involvement, and hs-CRP were selected. The backward selection was used for modeling, and the result is presented in Table 2. Alcohol drinking versus not leads to a 1.11 to ninefold increased risk to transit from subclinical to clinical PsA (OR 3.26, 95% CI 1.11, 9.54). Whereas, the odds ratios for transition to clinical PsA per year of age and per unit of hs-CRP are 1.08 (95% CI 1.03, 1.12) and 1.04 (95% CI 1.01, 1.08), respectively.
Stratified Analysis Based on Age
To control the heterogeneity caused by age, we stratified patients into youth (≤ 45 years) and middle-aged (> 45 years) layer. After univariate logistic regression analysis in each layer, we found that the odds ratio for clinically manifest PsA between young patients with and without fatty liver was 4.36 (95% CI 1.28, 14.92) (Table 3). Through the consistency test of OR, the difference of OR between layers was statistically significant. This implies that the difference between layers is not entirely caused by random errors, and that there might be an interaction between fatty liver and age.
Analysis of Interaction between Fatty Liver and Age
To further analyze the interaction between fatty liver and age, following the suggestions of clinical experts, we selected age, gender, hs-CRP, alcohol drinking, fatty liver, and the multiplicative interaction term (fatty liver*age) to construct the final multivariate logistic regression model. In this model, a binary qualitative variable age was used to replace the continuous variable age (Table 4). An age > 45 years, alcohol drinking, and increased hs-CRP were risk factors for transition to PsA with OR values of 10.15 (95% CI 2.79–36.91), 3.43 (95% CI 1.17–10.12), 1.05 (95% CI 1.01–1.09), respectively. Older age (> 45 years) has a 3 to 37-fold increased risk to transit from subclinical to clinical PsA, while alcohol drinking has a 1.2 to tenfold higher risk for transition than non-alcohol drinking. The odds ratio for transition to clinical PsA per unit of hs-CRP is around 1.01–1.09. Of special note, there was a negative multiplicative interaction between fatty liver and age. OR value of fatty liver was 5.41 (95% CI 1.41–20.82) in the model, while that of fatty liver*age was 0.10 (95% CI 0.01–0.76), which suggests that patients over 45 years old with fatty liver had 0.1 times higher risk of progression. Taken together with the results of the stratified analysis, patients with subclinical PsA at an age ≤ 45 years and in the presence of fatty liver constitute a high-risk group for transition to clinical PsA.
Discussion
In this study, we performed a retrospective, longitudinal, case–control study at a single medical center involving 25 patients with PsA and 137 controls. Various baseline demographic and clinical covariates were collected from all patients at a status of subclinical PsA, and analyzed by a series of statistical methods including univariate, multivariate, and stratified analyses. Our analysis has identified several risk factors that potentially increase the likelihood of progression from subclinical PsA to clinical PsA.
One of the most interesting findings in this study is that patients with subclinical PsA at an age ≤ 45 years and in the presence of fatty liver constitute a high-risk group for transiting to clinical PsA. All of these patients met the diagnostic criteria of nonalcoholic fatty liver disease (NAFLD), which has recently been renamed metabolic associated fatty liver disease (MAFLD) [15]. Our observation of the association between NAFLD and PsA progression is of considerable interest, given the previous reports that demonstrated that psoriasis patients have a notably higher risk of complications with NAFLD than healthy persons [26, 27], and that this risk appears to be higher in PsA patients than in patients with PsO [28]. Although these observations raise the speculation of a potential overlap in the pathogenesis between NAFLD and PsA, it waits further investigation whether the association of NAFLD with PsA is causal or due to unrecognized bias and limitations of these studies. A similar interesting finding is that the association between NAFLD and PsA transition is found in young patients (≤ 45 years old) rather than middle-aged patients (> 45 years old). The prevalence of NAFLD increases with age [29] and it is not susceptible for young people. The occurrence of fatty liver in young patients with subclinical PsA might be more indicative of their metabolic and immune disorders, which might be a reason for their rapid transition to PsA. This speculation warrants future investigation.
Other significant risk factors include an older age, alcohol drinking, and a high hs-CRP level. The finding of an older age as a risk factor to progress to PsA is not surprising, as has been seen in many other diseases. Mechanical stress and microdamage in bone and joints are known to increase with age and have important roles in the pathogenesis of spondyloarthropathies, which are usually difficult to be reversed or cured in older people [30]. Consistent with this conjecture, there are studies showing that psoriatic patients exceeding 45 years had more sites of enthesitis than those aged less than 45 years [25], and that mechanical stress and microdamage might be instrumental in inflammatory enthesitis as a primary driver [30].
The observation of alcohol drinking as a risk factor for PsA progression in this study is in contradiction with several previous studies demonstrating no association [31] or reverse association [32] between alcohol consumption and PsA. Clearly, further studies are required to determine whether alcohol consumption accelerates the transition from subclinical to clinical PsA and whether there is a dose–response relationship.
Our observation of hs-CRP as a risk factor for PsA progression is consistent with most of the previous studies [33, 34]. It would be worth exploring whether as pro-inflammatory CRP isoforms, pCRP*and monomeric CRP could actively participate in the pathogenesis of systemic inflammatory diseases (including rheumatoid arthritis), as proposed in previous studies [35].
Previous studies have shown several risk factors for PsA, including obesity and hyperlipidemia [12]. These factors were, however, not found to be associated with the risk of progression to PsA in our study. It is noteworthy that the previous study [12] is focused on patients with psoriasis while our study is focused on patients with subclinical PsA. The difference in the disease stages (psoriasis vs. subclinical PsA) as well as demographic factors (including racial and ethnic backgrounds) may have contributed to the different results between this and previous studies.
To the best of our knowledge, this is the first study focusing primarily on evaluating risk factors of progression from subclinical to clinical PsA. The main strength of this study lies in the inclusion of almost all commonly known variables that might be associated with PsA and the implementation of comprehensive multivariate and stratified analyses to eliminate the influence of confounding factors and ensure homogeneity. Certainly, this study has a few limitations. First, as a retrospective study, it has inherent defects including potential selection and information bias. In addition, our study is limited to a relatively small sample size at a single location, thus further research is needed with a larger sample size from multiple centers, preferably through a prospective longitudinal study design, to validate our findings. Moreover, our study focused on only the peripheral joints of patients; thus, the conclusions might be not applicable to subclinical PsA patients with exclusively axial involvement. Nevertheless, most patients with subclinical PsA present peripheral involvement; our conclusions are potentially valid for the majority of subclinical PsA patients. An additional limitation is that the patients included in this study were all moderate-to-severe psoriasis according to the PASI score; our conclusions are presumably valid for this patient population. Clearly, future studies are needed to include PsA patients with all degrees of severity and with both peripheral and axial involvement.
Conclusions
In conclusion, alcohol drinking, older age, the presence of fatty liver at less than 45 years old, and elevated hs-CRP level appear to be increased risk factors of transition from subclinical to clinical PsA. Our findings call for a need to effectively identify and manage these risk factors in routine clinical practice.
Change history
02 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40744-021-00364-2
References
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–70.
Feld J, Chandran V, Haroon N, Inman R, Gladman D. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol. 2018;14:363–71.
Gottlieb AB, Merola JF. Axial psoriatic arthritis: an update for dermatologists. J Am Acad Dermatol. 2021;84:92–101.
Augustin M, Vietri J, Tian H, Gilloteau I. Incremental burden of cardiovascular comorbidity and psoriatic arthritis among adults with moderate-to-severe psoriasis in five European countries. J Eur Acad Dermatol Venereol. 2017;31:1316–23.
Taylor WJ, Marchesoni A, Arreghini M, Sokoll K, Helliwell PS. A comparison of the performance characteristics of classification criteria for the diagnosis of psoriatic arthritis. Semin Arthritis Rheum. 2004;34:575–84.
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673.
McGonagle D, Tan AL. Are the classification criteria for psoriatic arthritis better than existing criteria for diagnosing psoriatic arthritis? Comment on the article by Taylor et al. Arthritis Rheum. 2007;56:699–700
Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–89.
Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76:79–87.
McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–46.
Ogdie A, Coates L. The changing face of clinical trials in psoriatic arthritis. Curr Rheumatol Rep. 2017;19:21.
Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019;15:153–66.
Tillett W, Charlton R, Nightingale A, et al. Interval between onset of psoriasis and psoriatic arthritis comparing the UK clinical practice research datalink with a hospital-based cohort. Rheumatology (Oxford). 2017;56:2109–13.
Wakefield RJ, Balint PV, Szkudlarek M, et al. OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487.
Eslam M, Sarin SK, Wong VW, et al. The Asian pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919.
Casey DE Jr, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American college of cardiology/American heart association task force on performance measures. J Am Coll Cardiol. 2019;74:2661–706.
Hata Y, Mabuchi H, Saito Y, et al. Report of the Japan atherosclerosis society (JAS) guideline for diagnosis and treatment of hyperlipidemia in Japanese adults. J Atheroscler Thromb. 2002;9:1–27.
Hamburger M, Baraf HS, Adamson TC 3rd, et al. 2011 Recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med. 2011;123:3–36.
Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–5.
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S13-S28.
Carol M. Rumack, Deborah Levine. Diagnostic ultrasound, 5th edition. 2018.
Shaper AG, Wannamethee SG. Alcohol intake and mortality in middle aged men with diagnosed coronary heart disease. Heart. 2000;83:394–9.
WHO policy on non-recruitment of smokers or other tobacco users: frequently questions. https://www.who.int/employment/FAQs_smoking_English.pdf, September 2008.
Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146:1387–94.
Tang Y, Yang Y, Xiang X, Wang L, Zhang L, Qiu L. Power doppler ultrasound evaluation of peripheral joint, entheses, tendon, and bursa abnormalities in psoriatic patients: a clinical study. J Rheumatol. 2018;45:811–7.
Gisondi P, Targher G, Zoppini G, Girolomoni G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol. 2009;51:758–64.
Ogdie A, Grewal SK, Noe MH, et al. Risk of incident liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis: a population-based study. J Invest Dermatol. 2018;138:760–7.
Candia R, Ruiz A, Torres-Robles R, Chávez-Tapia N, Méndez-Sánchez N, Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656–62.
van der Voort EA, Koehler EM, Dowlatshahi EA, et al. Psoriasis is independently associated with nonalcoholic fatty liver disease in patients 55 years old or older: results from a population-based study. J Am Acad Dermatol. 2014;70:517–24.
Jacques P, McGonagle D. The role of mechanical stress in the pathogenesis of spondyloarthritis and how to combat it. Best Pract Res Clin Rheumatol. 2014;28:703–10.
Eder L, Law T, Chandran V, et al. Association between environmental factors and onset of psoriatic arthritis in patients with psoriasis. Arthritis Care Res (Hoboken). 2011;63:1091–7.
Huidekoper AL, van der Woude D, Knevel R, et al. Patients with early arthritis consume less alcohol than controls, regardless of the type of arthritis. Rheumatology (Oxford). 2013;52:1701–7.
Gladman DD, Mease PJ, Choy EH, Ritchlin CT, Perdok RJ, Sasso EH. Risk factors for radiographic progression in psoriatic arthritis: sub analysis of the randomized controlled trial ADEPT. Arthritis Res Ther. 2010;12:R113.
van der Heijde D, Gladman DD, FitzGerald O, et al. Radiographic progression according to baseline C-reactive protein levels and other risk factors in psoriatic arthritis treated with tofacitinib or adalimumab. J Rheumatol. 2019;46:1089–96.
McFadyen JD, Zeller J, Potempa LA, Pietersz GA, Eisenhardt SU, Peter K. C-reactive protein and its structural isoforms: an evolutionary conserved marker and central player in inflammatory diseases and beyond. Sub cell Bio chem. 2020;94:499–520.
Acknowledgements
We thank the participants of the study.
Funding
This study including the Rapid Service Fee was funded by the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC18003), Chengdu, Sichuan, China and West China Precision Medicine Industrial Technology Institutes (2018-CY02-00058-GX).
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors made substantial contributions to all of these sections: conception and design of the study, data acquisition, analysis and interpretation, manuscript drafting and revising, and approval for submission and publication.
Disclosures
Yiyi Wang, Li Ding, Jihui Chen, Lingyan Zhang, Min Yang, Zhibin Liu, Liangliang Cheng, Tianjiao Lan, Gaojie Li, Yuanxia Gu, Yi Liu, and Wei Li have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of West China Hospital, Sichuan University (approval number:2015(308)), and each participating patient signed an informed consent form.
Data Availability
The datasets generated from the current study are available from the corresponding author upon request.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, Y., Ding, L., Chen, J. et al. Risk Factors for Progression from Subclinical to Clinical Phase of Psoriatic Arthritis: A Case–Control Study. Rheumatol Ther 8, 585–597 (2021). https://doi.org/10.1007/s40744-021-00295-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-021-00295-y