Abstract
Introduction
Intra-articular (i.a.) hyaluronic acid is an accepted conservative therapy for knee osteoarthritis (OA). This study evaluated the safety and efficacy of a single i.a. injection of an innovative formulation of sodium hyaluronate 2.4% plus sodium chondroitin non-sulphated 1.6% of biotechnological origin (HA-SC) for the treatment of patients with radiographically confirmed symptomatic hip OA and moderate-to-severe pain.
Methods
In this prospective, multicenter, open-label, pilot study, HA-SC was administered using a standard ultrasound-guided procedure. Adverse events, global/local evaluation of tolerability, and use of rescue analgesics were recorded. Efficacy endpoints included visual analogue scale (VAS) measurement of hip pain, changes in Lequesne’s algofunctional Index, and assessment of global status.
Results
Treatment was well tolerated; adverse device events of moderate-to-severe intensity, most commonly, injection site pain/localized arthralgia occurred in 20.8% of subjects. Global evaluation of tolerability was rated as excellent or good (75.0%), fair (16.7%), and poor (8.3%) by subjects and 77.1, 14.6, and 8.3%, respectively, by investigators. There was a rapid and significant decrease in hip pain after a single injection; VAS pain score decreased from a mean of 67.5 mm at baseline to 29.3 mm by day 7, with the effects sustained during 6 months of follow-up (P < 0.0001). There were significant improvements in Lequesne’s Index for hip OA total scores at all time points during follow-up (P < 0.0001). The majority of subjects reported ‘Very much improved’ or ‘Slightly improved’ global improvement at any time point. Use of rescue paracetamol was generally low.
Conclusions
A single i.a. injection of an innovative HA-SC formulation was well tolerated, safe, and effective in the treatment of symptomatic hip OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Osteoarthritis of the hip is a chronic disease that imposes a high burden of global disability and impaired quality of life because of increasing pain, loss of mobility, and loss of independence with ageing or disease progression. |
Intra-articular (i.a.) hyaluronic acid is an accepted treatment in routine clinical practice to reduce pain, improve joint function, and limit joint damage in knee osteoarthritis. |
We examined in symptomatic hip osteoarthritis the efficacy and safety of an innovative formulation of hybrid cooperative complexes of sodium hyaluronate plus sodium chondroitin developed to address the anatomical and biomechanical differences between the knee and hip joints. |
What was learned from the study? |
A single i.a. injection of the hyaluronic acid/sodium chondroitin formulation was well tolerated, safe, and effective in the treatment of symptomatic hip osteoarthritis. |
The treatment provided a rapid and significant decrease in hip pain and improved joint functionality, starting immediately after the i.a. injection and maintained throughout 6 months of follow-up. |
Although conservative treatment of hip osteoarthritis remains challenging, this formulation could represent a promising, long-lasting, and effective treatment. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13200485.
Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease characterized by progressive damage of articular cartilage, joint space narrowing, subchondral bone remodeling, joint marginal osteophyte formation, and synovitis [1, 2]. OA, particularly of the hip and knee, imposes a high burden of global disability and impaired quality of life because of increasing pain, loss of mobility, and the consequent loss of independence as people get older or the disease progresses [1, 3, 4].
Although OA may affect any joint of the body, it most commonly affects the knee and the hip [1, 4]. Hip OA is the most common cause of difficulty in walking and is one of the most common causes of debilitating pain in the general population [4]. The burden of OA can only be expected to increase with the ageing and increasing rates of obesity of the world’s population [4, 5], and there will be an increasing demand for health services to treat hip and knee OA. The management of OA includes both pharmacological and non-pharmacological therapies; both approaches are required for optimal management [3, 6,7,8,9,10].
Analgesics, and in particular nonsteroidal anti-inflammatory drugs (NSAIDs), remain the mainstay of the pharmacological treatment of OA, although there are concerns over the potential of serious side effects with long-term use of these drugs, particularly in the elderly [3, 6]. Intra-articular (i.a.) corticosteroids may provide short-term pain relief in subjects who fail to respond to other conservative measures, but there are concerns about possible adverse events (AEs) with repeated use, including destructive joint damage in a small proportion of patients and an increased risk of infection [11,12,13,14]. None of these approaches has been shown to slow disease progression or reverse joint damage and, taken together, concerns related to the use of NSAIDs and i.a. corticosteroids have led to an increasing interest in types of drugs and modalities of administration that improve the clinical symptoms of OA with more favorable tolerability and safety profiles.
Accordingly, symptomatic slow-acting drugs for osteoarthritis (SYSADOA), including hyaluronic acid (hyaluronan) and chondroitin, have been the focus of extensive research and generated a growing awareness among clinicians for their potential beneficial therapeutic effect on the symptoms and outcomes of OA [15].
The use of i.a. injections of hyaluronic acid has gained acceptance among conservative therapies for OA, with demonstrated beneficial effects on pain and functional parameters and improved outcomes with an absence of clinically relevant AEs [16]. In routine clinical practice, i.a. hyaluronic acid is an accepted treatment for knee OA to reduce pain, improve joint function, and limit joint damage, supported by a number of evidence-based clinical practice guidelines, consensus statements, and decision algorithms [6, 7, 10, 17,18,19]. Although the onset of symptomatic benefit following i.a. hyaluronic acid may not be immediate, correspondingly, the therapeutic efficacy is prolonged (carry-over effect), with the effect size peaking by around 8 weeks and persisting for at least 6 months [20]. There is also some evidence that viscosupplementation with hyaluronic acid has potential disease-modifying effects in addition to symptomatic pain relief [16, 21]. However, there is considerable variability in treatment recommendations, in part due to a lack of methodological consistency in published studies and marked variations in effect size between different hyaluronic acid formulations, which add to the heterogeneity. Correspondingly, guideline support for viscosupplementation in hip OA is currently limited by a lack of strong clinical trial evidence due to substantial heterogeneity of available studies that restricts the pooled analysis of data. In addition, the deeper localization of the hip joint, and the proximity of femoral vessels and nerves, makes performing the injections under imaging control mandatory.
However, despite the discrepancies in the guidelines, there is overall clinical evidence that i.a. hyaluronic acid is a safe, well-tolerated, and beneficial therapy in hip OA [22, 23], significantly reducing pain and aiding functional recovery, although the level of evidence is lower than that for knee OA, in part because of the small sample size of some studies and the time-lapse before patients feel the benefits of viscosupplementation [24].
IBSA is a manufacturer of a portfolio of hyaluronic acid-based medical devices for i.a. viscosupplementation, formulated with a biocompatible pharmaceutical-grade, highly purified high molecular weight (around 1000 kDa) hyaluronic acid obtained with a multi-step biofermentation process. Although the current IBSA i.a. medical devices can be used in joints other than the knee, they are mainly made use of for knee viscosupplementation, and no medical device specifically designed for hip viscosupplementation has been available. As the largest ball-and-socket joint in the body, viscosupplementation of the hip necessitates the use of a large volume of hyaluronic acid solution, preferably with a high concentration of hyaluronic acid, which could be expected to unduly increase the viscosity of the solution, resulting in a gel-like solution requiring a high extrusion force and that is not easy to inject.
An innovative and patented formulation containing hybrid cooperative complexes of sodium hyaluronate 2.4% plus sodium chondroitin non-sulphated 1.6% of biotechnological origin has been developed,Footnote 1 providing a higher concentration of hyaluronic acid without a significant increase in viscosity, while maintaining the same high molecular weight of the other IBSA i.a. hyaluronic acid products.
Guided by the available experience in the safe and effective use of i.a. hyaluronic acid in the management of knee OA, this preliminary study was designed to evaluate the safety and efficacy of a single i.a. hip injection of the new formulation of sodium hyaluronate and sodium chondroitin (HA-SC) for the treatment of patients with symptomatic hip OA.
Methods
Ethical Considerations
Approval for the study was sought from and provided by the local Ethical Committee of each participating site (Comitato Etico dell’Università Campus bio-medico di Roma, Comitato Etico delle Province di Chieti e Pescara, Comitato Etico Aziende Sanitarie Umbria, Comitato Etico di Area Vasta Sud-Est (C.E.A.V.S.E.) c/o Azienda Ospedaliero Universitaria Senese, Comitato Etico dell'Università Federico II and Segreteria Comitato Etico Per Parma c/o Azienda Ospedaliero-Universitaria di Parma) and the National Regulatory Authorities of Italy, according to the specific national regulation, before starting the investigation. Written informed consent was obtained from each patient before starting the trial. The trial was conducted in accordance with the Declaration of Helsinki and its modifications, the rules of the International Conference on Harmonization (ICH) Good Clinical Practices (GCP), and ISO 14155, the European Union Council Directive 93/42/EEC amended by 2007/47/EC, the MEDDEV 2. 12–1 rev. 6 and amendments, and the local legislation in force on the conduct of clinical investigations with medical devices.
Design and Objectives
This was a prospective, multicenter, open-label, single-arm, pilot clinical investigation of patients with symptomatic hip OA treated with a single dose of i.a. sodium hyaluronate and sodium chondroitin (HA-SC) in the treatment of patients with symptomatic hip OA and suffering from moderate-to-severe pain.
Patient Population
Eligible subjects were male or female patients aged ≥ 40 years attending the outpatient clinics at the participating Italian public hospitals with symptomatic primary hip OA radiographically confirmed within the previous 6 months and continuous moderate-to-severe OA pain despite the failure of or non-response to regular use of analgesics and/or NSAIDs or other conservative treatments. Subjects were required to have documented Kellgren–Lawrence grade < 4 radiographic hip OA [25], and pain at the target hip ≥ 40 mm as measured on a visual analogue scale (VAS) after a wash-out period from analgesics and/or NSAIDs of > 5 times the drug half-life before the screening visit and confirmed at the baseline visit (visit 2). Exclusions from the study, which were related to circumstances considered likely to interfere with the study treatment or confound the evaluation of the affected joint, are summarized in Table 1. Participation in the study could be prematurely withdrawn at the initiative of the patient or investigator, for example, in the case of a significant AE or adverse device event (ADE).
Study Intervention
The investigational medical device (IMD) was a sterile 3-ml unit-dose syringe containing high molecular weight sodium hyaluronate 2.4% and sodium chondroitin non-sulphated 1.6% of biotechnical origin for i.a. administration. Each syringe provided 72 mg of sodium hyaluronate and 48 mg of sodium chondroitin. Biocompatible pharmaceutical-grade hyaluronic acid produced as a non-chemically modified, linear natural polymer free of cross-linking agents obtained from a safe and highly productive microbial strain is used in the patented formulation to closely mimic endogenous hyaluronic acid and ensure a good safety profile while providing satisfactory viscoelastic properties.
Non-sulphated chondroitin (sodium chondroitin) is used as a technological excipient with specific rheological properties that modulate the viscosity of high concentration, high molecular weight hyaluronic acid solutions, preserving the viscoelastic characteristics of hyaluronic acid that replicate the desirable properties of the synovial fluid of healthy subjects.
A standard ultrasound-guided procedure was used with the aim of ensuring the correct placement of the i.a. injection and minimizing the risk of adverse effects due to incorrect positioning of the needle [26]. In brief, subjects were examined supine with the hip in internal-rotation of 15 to 20°. A 7-MHz linear or 3.5-MHz convex transducer was used together with a sterile biopsy guide. The hip joint was scanned by means of an anterior parasagittal approach, lateral to the femoral vessels. The transducer was aligned with the long axis of the femoral neck to allow visualization of the acetabulum and the femoral head. Using an antero-superior approach, a 22-gauge spinal needle was advanced through the biopsy guide into the anterior capsular recess, at the level of the femoral head, using real-time imaging guidance software. Once the needle encountered the femoral head, it was retracted by 1 mm and the treatment was then injected into the hip joint. Intra-articular placement was verified with the real-time monitoring (direct visualization of viscous fluid or air bubbles) and utilizing power Doppler imaging (flow signals in i.a. recess). Color Doppler visualization prevented the inadvertent injection of blood vessels.
Data Collection
A total of seven visits were performed. A schematic representation of the study plan is shown in Fig. 1. Patients were followed up for 6 months to ensure long-term evaluation of the treatment.
Subjects underwent a screening visit (visit 1) between 1 and 10 days before treatment with the i.a. injection of HA-SC at the baseline/treatment visit (visit 2, day 0). Follow-up visits were performed after 7 days from treatment (visit 3, post-treatment follow-up), and after 30 (visit 4), 60 (visit 5), 90 (visit 6), and 180 days (visit 7, final visit).
Subjects who prematurely discontinued from the investigation between visit 2 and visit 3 attended an ‘early termination visit before visit 3′ where laboratory tests, assessment of local tolerability at the injection site, and investigator and subject assessment of global tolerability were scheduled. Subjects prematurely discontinued from the investigation between visit 3 and visit 7 attended an ‘early termination visit after visit 3′, in which procedures scheduled for visit 7 (day 180, final visit) were performed.
Hip pain was measured by VAS at any visit to the investigational site. Subjects also recorded, in a daily diary, the pain by VAS in the domiciliary setting in the first 7 days following treatment administration. Adverse events, any therapies taken for adverse events, and concomitant rescue paracetamol (not to exceed 3 g/day of paracetamol 500 mg tablets, to be interrupted at least 6 h before each control visit) were to be recorded in the subject’s diary at home during the entire investigational period.
Project management, monitoring of the investigation, data management, and reporting were carried out by the Contract Research Organisation (CRO), LB Research s.r.l., Via Lombardia 81, 22063 Cantù (CO), Italy, and statistical analysis was carried out by IBISMED s.r.l., Via Carlo D’Adda 8, 20143, Milan, Italy.
Outcome Measures
The primary objective was to evaluate the safety of i.a. HA-SC in patients with symptomatic hip OA. The secondary objective was to evaluate the performance (efficacy) of HA-SC in terms of pain and function of the affected hip joint. The primary safety endpoint was the number and type of ADE (i.e., AEs related to the medical device).
Secondary safety endpoints included local tolerability at the injection site, assessment of global tolerability by the patient and investigator, vital signs, and laboratory parameters.
The performance (efficacy) endpoints were changes in VAS pain from baseline to any post-baseline time point, changes in Lequesne’s algofunctional Index from baseline to any post-baseline time point, and subject and investigator assessment of change in global status (an overall evaluation of hip OA pain symptoms), according to a 5-point qualitative scale where 4 = very much improved, 3 = slightly improved, 2 = no change, 1 = slightly worsened, 0 = very much worsened. Consumption of concomitant analgesic treatments, defined as the number of subjects with the use of paracetamol and the number of used tablets, was recorded.
Statistical Methods
The safety (SAF) analysis set was defined as all enrolled subjects who signed the informed consent and received the medical device administration. The full analysis set (FAS) consisted of all subjects of the SAF who had a valid baseline VAS for pain as well as ≥ 1 post-baseline VAS pain assessment. The per-protocol analysis set (PPAS) was defined as all subjects of the FAS who also met all inclusion/exclusion criteria and who did not have any major deviation from the clinical investigational plan (i.e., inclusion criteria violation, use of forbidden concomitant medications).
The analysis of safety endpoints was performed in the SAF and performance endpoints on the FAS. Analysis of changes in VAS hip pain was repeated in the PPAS.
ADEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA®) version 22 and the number of subjects with ADEs, serious ADEs (SADEs), the severity of ADEs, and ADEs leading to withdrawal were recorded.
Statistical testing was conducted at the two-sided α = 0.05, and the 95% confidence interval (CI) was computed. As there was no control group, descriptive statistics were used according to the type of variable; for continuous variables, descriptive summary statistics included the number of non-missing values, number of missing values, mean, 95% two-sided mean CI, standard deviation (SD), median, lower, and upper quartile, minimum and maximum. Box plot graphs were also presented when appropriate. For categorical variables, descriptive summary statistics included counts and percentages per category.
The statistical analyses were performed using SAS® Statistical Analysis Software v 9.4 (SAS Institute, Cary, NC, USA), and the level of significance for all tests was 5%.
Results
The study was conducted at six sites in Italy; 48 subjects were enrolled and completed the study, with the first subject enrolled on the 1st of August 2017 and the last subject completing the study on the April 4, 2019. Therefore, all subjects were included in both the SAF and FAS populations. Five subjects had major protocol violations (non-allowed use of corticosteroids and NSAIDs) and were excluded from the PPAS, which therefore consisted of 43 subjects.
Patient demographics at baseline are presented in Table 2. The average age of the subjects was 61.2 years and more males (N = 29) than females (N = 19) were enrolled. All subjects were Caucasian, and the majority had bilateral hip OA, with the target hip balanced similarly between the left and right side. The diagnosis of hip OA was radiographically confirmed in all subjects, 94% of whom had Kellgren–Lawrence grade 2 or 3 radiographic hip OA. The mean time since diagnosis of hip OA was 44.9 (range, 0.7–220.6) months.
Prior and concomitant medications were reported in 83.3% (N = 40) of subjects overall. The most common medications by pharmacological subgroup were drugs for the cardiovascular system (23 subjects, 47.9%), drugs for the nervous system (21 subjects, 43.8%), and drugs for the alimentary tract and metabolism (12 subjects, 50.0%).
Safety and Tolerability
Treatment was generally well tolerated (Table 3). A total of 11 ADEs (primary safety endpoint) were reported in ten subjects (20.8%) overall (95% CI 10.0–35.0%). The intensity of ADEs was moderate (one event) in one subject (2.1%) and was severe (ten events) in nine subjects (18.8%). The most common ADEs by preferred term were injection site pain (five subjects, 10.4%) and arthralgia localized in the treated area (three subjects, 6.3%). One subject reported two ADEs of severe intensity (arthralgia and pain in the extremity). None of the other ADEs by preferred term were reported in more than one subject.
A total of 72 AEs was reported in 24 subjects (50.0%) overall. There was one serious AE (SAE) reported in one subject (2.1%), consisting of two events (atrial fibrillation and cardiac failure) not related to treatment. The intensity of AEs was mild (38 events) in 13 subjects (27.1%), moderate (19 events) in nine subjects (18.8%), and severe (12 events) in nine subjects (18.8%). None of the subjects discontinued the study due to AEs.
The global evaluation of tolerability was rated as excellent or good for 36 subjects (75.0%), fair by eight (16.7%), and poor by four (8.3%). Corresponding values reported by the investigators were excellent or good in 37 subjects (77.1%), fair in seven (14.6%), and poor in four (8.3%).
There were no substantial changes in mean values of heart rate, systolic blood pressure, diastolic blood pressure, body weight and BMI from baseline to any post-baseline time point; the only statistically significant change during the study was a mean decrease of 1.8 mmHg in diastolic blood pressure from baseline to visit 3 (day 7). Laboratory parameters were normal in the high majority of patients at baseline and/or day 7.
The results of local tolerability showed transient pain in the site of injection, with an increase in the mean VAS for pain from before injection until soon after injection, but the increase in pain intensity rapidly resolved, from a mean increase of 15.2 ± 39.8 mm soon after injection to 7.0 ± 38.6 mm 30 min after injection. Furthermore, at visit 3 (day 7), the mean VAS for pain in the site of injection was lower than that measured before injection, with a mean decrease from before injection of − 18.4 ± 29.4 mm.
Local erythema in the site of injection, absent in all subjects before injection, consisted of no more than faint redness in 20 subjects soon after injection. Thirty minutes after injection, one subject had moderate redness, and ten had faint redness, which persisted at day 7 in two subjects. Local erythema was absent soon after injection in 28 subjects, 30 min after injection in 37 subjects, and on day 7 in 46 subjects.
Efficacy
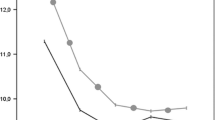
Changes in VAS pain score over the course of the study for the FAS are presented in Table 4 and Fig. 2. In summary, there was a rapid and significant decrease in hip pain after a single i.a. injection of HA-SC. VAS pain score decreased from a mean of 67.5 mm at baseline to 29.3 mm by visit 3 (day 7), with the effects sustained during 6 months of follow-up, at which time VAS was 22.8 mm (P < 0.0001 vs. baseline at any time point). Results in the PPAS were similar to those observed in the FAS (data not shown).
The mean Lequesne’s algofunctional Index for hip OA total score after the single injection of HA-SC decreased from a mean of 10.4 at baseline to 5.1 (P < 0.0001) at 6 months (Table 5; Fig. 3). The decrease was marked and remained significant at all evaluated time points (P < 0.0001).
The subjects’ assessment of global improvement showed an assessment of ‘Very much improved’ or ‘Slightly improved’ in the majority of subjects at any time point throughout the study. Consistent with the assessments of the subjects, the investigators reported ‘Very much improved’ or ‘Slightly improved’ for the majority of subjects at any time point.
Overall, 36 subjects (75.0%) used rescue paracetamol during the study. The mean number of tablets per day of rescue paracetamol (in subjects who took rescue paracetamol) was generally low and decreased from visit 3 (day 7) up to the end of observation at visit 7 (day 180). In the overall study period, subjects took a mean of 75.3 ± 105.8 tablets of rescue paracetamol, a mean daily number of 0.4 ± 0.6.
Discussion
The role of i.a. hyaluronic acid is well established in knee OA [20, 27,28,29,30,31], although its use in hip OA is less well documented. Anatomical and biomechanical differences between the knee and hip joints mean that the rheological properties of hyaluronic acid products formulated for use in knee OA are not ideal for administration in hip OA.
The primary objective of this study in subjects with radiographically documented hip OA, therefore, was to evaluate the safety of a single i.a. injection of an innovative medical device developed specifically for proper viscosupplementation of the hip joint. The formulation comprises a high molecular weight hyaluronic acid at a concentration appropriate for hip OA treatment in a hybrid cooperative complex with sodium chondroitin as an excipient to modulate the viscosity of the hyaluronic acid solution, mimicking the viscoelastic properties of healthy synovial fluid.
All 48 patients enrolled in the study received treatment, completed the investigation, and were therefore included in both the SAF set and the FAS. Overall, treatment with i.a. HA-SC was well tolerated, and tolerability was rated as excellent or good by the majority of subjects and investigators. Local tolerability was good-to-excellent, consisting of transient pain in the site of injection, an expected effect following i.a. injection and which tended to resolve rapidly in the few patients who experienced it. Local erythema, swelling, or hardening in the site of injection, occurred infrequently, was of mild intensity, and spontaneously resolved in a short time. Arthralgia localized to the treated area, for which the underlying disease might have had a contributory effect, in three subjects. There were no clinically significant changes in laboratory parameters or vital signs. None of the subjects had treatment-related SAEs and all subjects regularly completed the overall investigational period.
Treatment with i.a. HA-SC was associated with rapid and significant decreases in hip pain, which was statistically significant at 7 days and which were sustained up to the end of the observation at 6 months. The mean Lequesne’s algofunctional Index total score also markedly and significantly decreased from baseline to any post-baseline time point.
An assessment of ‘Very much improved’ or ‘Slightly improved’ health status was reported for the majority of subjects by both subjects and investigators at any time point. The use of rescue paracetamol was low and decreased over time.
Several other studies have shown that viscosupplementation with i.a. formulations of hyaluronic acid are a valid treatment option for hip OA [22, 32]. In one study, 114 patients with symptomatic hip OA received a single i.a. injection of hyaluronic acid 1.6% (two vials, 4 ml) and were followed up for 6 months [22]. There was a statistically significant reduction from baseline in Lequesne algofunctional index scores, VAS pain scores, and consumption of NSAIDs at all time points (P < 0.05), with no systemic and only mild side effects, consisting of local reaction at the injection site in seven subjects. More recently, in a study in 207 patients with Kellgren–Lawrence stage 2–3 hip OA treated with a single administration of i.a. hyaluronic acid, highly statistically significant improvements in pain, measured with the Brief Pain Inventory (BPI II), were recorded between baseline and the three following visits at 3, 6, and 12 months (P < 0.001) [23]. The evolution in the Harris Hip Score was also statistically significant between baseline and all following visits (P < 0.001), whereas VAS pain scores had improved at the month 3 visit (P < 0.001) and remained stable thereafter.
While, as noted, i.a. corticosteroids may be an appropriate intervention for relieving pain and providing functional recovery in hip OA in the short term, a recent meta-analysis has shown that i.a. hyaluronic acid is a valid and effective choice from the mid-term [33], although further trials with long-term follow-up are necessary.
Our study has some limitations; this was a small open-label, single-arm pilot study, and there was therefore no control group. However, extensive monitoring for AEs and ADEs was undertaken over the duration of the study up to the final visit at day 180, and the use of a number of validated clinical parameters and the kinetics of the performance effects observed on pain and function suggest that a tangible treatment effect was present.
Until i.a. viscosupplementation has been definitively shown to alter the natural history of hip OA and delay or prevent the need for hip arthroplasty, treatment with i.a. HA-SC should be considered a strategy for symptomatic relief. However, restoring the viscoelasticity of the synovial fluid to aid in the regeneration of damaged cartilage has the potential to become a promising therapeutic strategy for slowing the progression of hip OA. Our findings justify additional investigation in a larger patient population in the context of a controlled clinical study.
Conclusions
The results of this study show that a single i.a. injection of an innovative formulation containing hybrid cooperative complexes of sodium hyaluronate plus sodium chondroitin non-sulphated (HA-SC) was well tolerated and safe in the treatment of symptomatic hip OA. The treatment resulted in a rapid and significant decrease in OA hip pain (VAS) and functionality (Lequesne’s Index) that started immediately after the i.a. injection and was maintained throughout the 6-month follow-up period. Although conservative treatment of hip OA remains challenging, this formulation could represent a promising, long-lasting, and effective i.a. treatment.
Notes
Brand names: Sinogel®; Condrosulf® Intrarticolare; Condrosulf® Intraarticular; Artrocoat®
References
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–59.
Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59:333–9.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–89.
Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 Study. Ann Rheum Dis. 2014;73:1323–30.
Dagenais S, Garbedian S, Wai EK. Systematic review of the prevalence of radiographic primary hip osteoarthritis. Clin Orthop Relat Res. 2009;467:623–37.
Ariani A, Manara M, Fioravanti A, Iannone F, Salaffi F, Ughi N, et al. The Italian Society for Rheumatology clinical practice guidelines for the diagnosis and management of knee, hip and hand osteoarthritis. Reumatismo. 2019;71:5–21.
Bruyere O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. 2019;49:337–50.
Fernandes L, Hagen KB, Bijlsma JW, Andreassen O, Christensen P, Conaghan PG, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72:1125–35.
Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther KP, et al. EULAR evidence-based recommendations for the management of hip osteoarthritis: Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2005;64:669–81.
Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145–55.
Hunter JA, Blyth TH. A risk–benefit assessment of intra-articular corticosteroids in rheumatic disorders. Drug Saf. 1999;21:353–65.
Kompel AJ, Roemer FW, Murakami AM, Diaz LE, Crema MD, Guermazi A. Intra-articular corticosteroid injections in the hip and knee: Perhaps not as safe as we thought? Radiology. 2019;293:656–63.
Yamamoto T, Schneider R, Iwamoto Y, Bullough PG. Rapid destruction of the femoral head after a single intraarticular injection of corticosteroid into the hip joint. J Rheumatol. 2006;33:1701–4.
Hess SR, O’Connell RS, Bednarz CP, Waligora ACT, Golladay GJ, Jiranek WA. Association of rapidly destructive osteoarthritis of the hip with intra-articular steroid injections. Arthroplast Today. 2018;4:205–9.
Bruyere O, Burlet N, Delmas PD, Rizzoli R, Cooper C, Reginster JY. Evaluation of symptomatic slow-acting drugs in osteoarthritis using the GRADE system. BMC Musculoskelet Disord. 2008;9:165.
Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: results of an extensive critical literature review. Semin Arthritis Rheum. 2019;48:563–72.
Henrotin Y, Raman R, Richette P, Bard H, Jerosch J, Conrozier T, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45:140–9.
Raman R, Henrotin Y, Chevalier X, Migliore A, Jerosch J, Montfort J, et al. Decision algorithms for the retreatment with viscosupplementation in patients suffering from knee osteoarthritis: recommendations from the EUROpean VIScosupplementation COnsensus Group (EUROVISCO). Cartilage. 2018;9:263–75.
Rillo O, Riera H, Acosta C, Liendo V, Bolanos J, Monterola L, et al. PANLAR consensus recommendations for the management in osteoarthritis of hand, hip, and knee. J Clin Rheumatol. 2016;22:345–54.
Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis–meta-analysis. Osteoarthritis Cartilage. 2011;19:611–9.
Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13:216–24.
Migliore A, Massafra U, Bizzi E, Giovannangeli F, Tormenta S. Intra-articular ultrasound-guided injection of Sinovial® forte 1.6% in patients affected by symptomatic hip osteoarthritis: effectiveness and safety in a large cohort of patients. Eur J Inflamm. 2012;10:71–9.
Rivera F. Single intra-articular injection of high molecular weight hyaluronic acid for hip osteoarthritis. J Orthop Traumatol. 2016;17:21–6.
van den Bekerom MP, Lamme B, Sermon A, Mulier M. What is the evidence for viscosupplementation in the treatment of patients with hip osteoarthritis? Systematic review of the literature. Arch Orthop Trauma Surg. 2008;128:815–23.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502.
Migliore A, Martin LS, Alimonti A, Valente C, Tormenta S. Efficacy and safety of viscosupplementation by ultrasound-guided intra-articular injection in osteoarthritis of the hip. Osteoarthritis Cartilage. 2003;11:305–6.
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006. https://doi.org/10.1002/14651858.CD005321.pub2.
Miller LE, Block JE. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: Systematic review and meta-analysis of randomized, saline-controlled trials. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:57–63.
Wang F, He X. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: a meta-analysis. Exp Ther Med. 2015;9:493–500.
Xing D, Wang B, Liu Q, Ke Y, Xu Y, Li Z, et al. Intra-articular hyaluronic acid in treating knee osteoarthritis: a PRISMA-compliant systematic review of overlapping meta-analysis. Sci Rep. 2016;6:32790.
Vincent P. Intra-articular hyaluronic acid in the symptomatic treatment of knee osteoarthritis: a meta-analysis of single-injection products. Curr Ther Res Clin Exp. 2019;90:39–51.
Clementi D, D’Ambrosi R, Bertocco P, Bucci MS, Cardile C, Ragni P, et al. Efficacy of a single intra-articular injection of ultra-high molecular weight hyaluronic acid for hip osteoarthritis: a randomized controlled study. Eur J Orthop Surg Traumatol. 2018;28:915–22.
Ye Y, Zhou X, Mao S, Zhang J, Lin B. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279–87.
Acknowledgements
The authors would like to thank IBISMED (Milan, Italy) for the statistical analysis, funded by IBSA Institut Biochimique S.A., Lugano, Switzerland.
Funding
Funding for the study and the journal’s Rapid Service Fee was provided by IBSA Institut Biochimique S.A., Lugano, Switzerland.
Medical Writing Assistance
Medical writing support in the preparation of this article was provided by Ray Hill, an independent medical writer. Support for this assistance was funded by IBSA Institut Biochimique S.A., Lugano, Switzerland.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
A portion of this data was presented as part of the 2020 virtual congress of the European Congress of Rheumatology (EULAR) in June 2020.
Disclosures
Serafino Carta is a Consultant for Zimmer-Biomet. Rocco Papalia, Vincenzo Salini, Nicola Voglino, Mattia Fortina, Francesco Sadile, and Cosimo Costantino have nothing to disclose.
Compliance with Ethics Guidelines
Approval for the study was sought from and provided by the local Ethical Committee of each participating site (Comitato Etico dell'Università Campus bio-medico di Roma, Comitato Etico delle Province di Chieti e Pescara, Comitato Etico Aziende Sanitarie Umbria, Comitato Etico di Area Vasta Sud-Est (C.E.A.V.S.E.) c/o Azienda Ospedaliero Universitaria Senese, Comitato Etico dell'Università Federico II and Segreteria Comitato Etico Per Parma c/o Azienda Ospedaliero-Universitaria di Parma) and the National Regulatory Authorities of Italy, according to the specific national regulations, before starting the investigation. Written informed consent was obtained from each patient before starting the trial. The trial was conducted in accordance with the Declaration of Helsinki and its modifications, the rules of the International Conference on Harmonization (ICH) Good Clinical Practices (GCP), and ISO 14155, the European Union Council Directive 93/42/EEC amended by 2007/47/EC, the MEDDEV 2. 12-1 rev. 6 and amendments, and the local legislation in force on the conduct of clinical investigations with medical devices.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Details of the individual Ethical Committee and National Regulatory Authorities approvals are available on request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Papalia, R., Salini, V., Voglino, N. et al. Single-Dose Intra-Articular Administration of a Hybrid Cooperative Complex of Sodium Hyaluronate and Sodium Chondroitin in the Treatment of Symptomatic Hip Osteoarthritis: A Single-Arm, Open-Label, Pilot Study. Rheumatol Ther 8, 151–165 (2021). https://doi.org/10.1007/s40744-020-00255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-020-00255-y