Abstract
Introduction
Enthesitis is a core outcome domain assessed in psoriatic arthritis (PsA) clinical trials. Limited evidence describes the impact of enthesitis on patient-reported outcomes (PROs) and physician satisfaction with current treatment options. The objective of this analysis is to characterize the impact of enthesitis on PROs and physician satisfaction with currently available treatment in clinical practice settings.
Methods
Cross-sectional survey of rheumatologists, dermatologists, and their consulting patients with PsA in Australia, Canada, European Union (EU5), and the USA conducted in 2018. Physicians assessed current presence and severity of enthesitis, overall disease severity, other symptoms experienced, and their satisfaction with the current treatment. PsA participant self-reported data included current pain level, EQ5D, Psoriatic Arthritis Impact of Disease (PsAID12), Health Assessment Questionnaire Disability Index (HAQ-DI), and Work Productivity and Activity Impairment Index (WPAI-SHP). Bivariate descriptive analyses were conducted to describe features and outcomes in participants with and without enthesitis.
Results
Rheumatologists (454) and dermatologists (238) provided information for 3157 participants with PsA. Mean participant age was 49.2 years, and 45.9% were female. Enthesitis was present currently in 6.5% (205) of participants with PsA. Those with enthesitis had worse overall disease severity compared to those without enthesitis (12.2% vs 2.2% severe) and had more extraarticular manifestations, including nail psoriasis, dactylitis, and sacroiliitis. Enthesitis was associated with more pain, worse quality of life (QoL), increased disability, and a negative impact on work. Participants with enthesitis had higher NSAIDs and opioid pain medication use but similar biologic use. Physicians were significantly less satisfied with current PsA treatment in participants with enthesitis versus without enthesitis.
Conclusions
Participants with psoriatic arthritis with enthesitis experienced significantly higher disease burden than those without enthesitis but were not more likely to receive advanced therapies. Physicians were significantly more dissatisfied with treatment in patients with enthesitis than in those without it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Enthesitis is a core outcome domain assessed in psoriatic arthritis (PsA) clinical trials. |
Limited evidence describes the impact of enthesitis on patient-reported outcomes (PROs) and physician satisfaction with current treatment options. |
The objective of this real-world cross-sectional study was to characterize the impact PsA enthesitis had on PROs and to explore the contribution of enthesitis to physician-reported satisfaction with their patients’ treatment. |
Patients with enthesitis had worse overall disease severity, more extraarticular manifestations, more pain and opioid use, worse quality of life (QoL), and physicians were significantly less satisfied with current PsA treatment compared to patients without enthesitis. |
Our findings underscore the importance of assessing patients with PsA for enthesitis and ensuring its optimal management. Data on PsA treatment efficacy specifically for enthesitis are needed to expand treatment options for patients and physicians. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13019816.
Introduction
Enthesitis, inflammation of the site where the tendon, ligament, or joint capsule inserts into the bone, is recognized as a core feature of psoriatic arthritis (PsA) and is recommended by the Group for the Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) as one of the main musculoskeletal outcome domains to assess in PsA clinical trials [1, 2]. The presence of enthesitis can differentiate PsA from other types of arthritis, such as rheumatoid arthritis or osteoarthritis [3].
The prevalence of enthesitis in patients with PsA has been described in several observational studies. Prevalence estimates reported from cross-sectional studies range from 11% to 49% [4,5,6,7,8].
Enthesitis has been suggested as an important indicator of PsA disease severity [3] and radiographic damage in both peripheral and axial joints [9]. Several studies have described a higher disease activity in patients with PsA with enthesitis compared to those without enthesitis. Data from the CORRONA Psoriatic Arthritis/Spondyloarthritis registry, as well as a national registry from Turkey, found that patients with enthesitis had a greater number of tender or swollen joints, more extra-articular manifestations, and higher disease activity scores when compared to patients without enthesitis [7, 10]. Similar results were seen in the University of Toronto PsA cohort where enthesitis was found to be independently associated with the presence of extra-articular symptoms (e.g., tenosynovitis and dactylitis), as well as a higher inflamed joint count [11].
Real-world data also demonstrates worse patient-reported outcomes (PROs) in patients with PsA with enthesitis versus those without enthesitis, including greater pain and fatigue, worse functional status and quality of life, and detrimental impacts on work productivity [7, 10, 12, 13].
The impact of enthesitis on physician satisfaction with PsA treatments has not been well characterized. Enthesitis is considered a feature resistant to disease-modifying anti-rheumatic drug (DMARD) treatment, and more intense treatment is usually needed [2, 14]. Limited research exists on the importance of different PsA clinical manifestations, as weighed by physicians or patients, on overall disease activity. Dandorfer and colleagues compared the patients’ and physicians’ perspectives on PsA manifestation contributions to their assessment of global PsA burden. The study found arthritis symptoms had the highest contribution to global PsA burden for patients. When axial symptoms, enthesitis, and dactylitis occurred concurrently, enthesitis accounted for approximately one-third of the global PsA burden for patients. From the physician’s perspective, the physicians’ global assessment had a good correlation with the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) [15]. This study suggests that enthesitis is an important manifestation to both patients and physicians that will impact treatment success and satisfaction.
The objective of this real-world cross-sectional study was to characterize the impact PsA enthesitis had on PROs and to explore the contribution of enthesitis to physician-reported satisfaction with their patients’ treatment.
Methods
Data were drawn from a large-scale cross-sectional survey of dermatologists, rheumatologists, and their consulting patients with PsA across Australia, Canada, Japan, the European Union 5 (EU5), and the USA. In Japan, physicians also included orthopaedic surgeons and internal medicine. The data were collected from April to November 2018 using established methods [16].
Study Design and Population
A non-interventional, cross-sectional survey captured current and historic PsA data from physicians on their consulting patients with PsA and concomitant PRO. Physicians were eligible to complete the survey if they had more than 3 years’ experience and were treating patients with PsA.
Physicians were identified via publicly available lists and invited to participate in the study. Participating physicians were asked to complete a patient record form (PRF) for a minimum of three consecutive adult patients they were actively treating who had a rheumatologist-confirmed PsA diagnosis, were at least 18 years of age, and who were not currently enrolled in a clinical trial. There was no selection by treatment received. The same patients were invited, by their physicians, to complete a voluntary questionnaire. Clinical diagnosis of PsA was based upon the physicians’ individual judgment with no restrictions on how the diagnosis was determined for inclusion in the study.
Data Collection
Data were collected from physician-reported PRFs and voluntary patient-completed forms (PSCs). Physician-reported data included patient demographics, clinical characteristics (including the current presence and severity [mild, moderate, or severe] of enthesitis), overall (PsA) disease severity (mild, moderate, or severe), other PsA symptoms experienced, and their own satisfaction with disease control under their current treatment plan (5-point Likert scale ranging from very satisfied to very dissatisfied). Physicians also provided information on the number of active PsA joints and current psoriasis severity by body surface area (BSA, range 0–100%) affected.
Patient-reported data included information on general health, disease history and symptoms, medications, and PROs including the current level of pain (1–10 numeric rating scale [NRS] with 10 = worst), Psoriatic Arthritis Impact of Disease (PsAID12) questionnaire [17], Health Assessment Questionnaire-Disability Index (HAQ-DI) [18, 19], EuroQoL 5D (EQ5D) [20, 21], and the Work Productivity and Activity Impairment scale (WPAI) [22, 23]. The PsAID is a PsA-specific health status instrument developed by the European League Against Rheumatism (EULAR). The instrument, designed for use in observational studies, includes 12 items covering both physical and psychological aspects of the disease, each item scored with a 0–10 numeric rating scale. The questionnaire uses a weighted scoring system based on the relative importance of symptoms to patients with pain, fatigue, and skin problems having the highest relative weights. The total score ranges from 0 to 10 (where 10 represents the worst health score). A PsAID12 score of 4 is considered a patient acceptable symptom state [17]. The HAQ-DI is an index measuring physical function originally developed for assessment of patients with rheumatoid arthritis but commonly used in PsA clinical trials. The score ranges from 0 to 3 with higher scores indicating higher disability. The EQ5D utility score includes five domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression and is converted to a score that is anchored at 0 for death and 1 for perfect health. The WPAI assesses the individual’s ability to work and perform regular activities. It includes four scores: absenteeism, presenteeism, overall work impairment, and activity impairment outside of work. Each score ranges from 0% to 100% with higher percentages reflecting greater impairment.
Endpoints, Data Analysis, and Statistical Methods
Patients were grouped for the analysis according to whether the physician indicated enthesitis was or was not currently present based on their clinical judgement. Patient demographics, clinical characteristics, and PROs were described within each group (patients currently with enthesitis versus currently without enthesitis) and overall, using n and mean and standard deviation (for numeric variables) or n and percentage (for categorical variables). Differences in outcomes between patients with enthesitis or without enthesitis were assessed using bivariate analysis. Student’s t test or Mann–Whitney tests were used for continuous data and Fisher’s exact tests were used for categorical data; 95% significance level was used. Statistical analysis was performed using Stata (version 15.1).
Ethical Considerations
The research was conducted in accordance with national market research and privacy regulations (European Pharmaceutical Market Research Association, US Department of Health and Human Services National Institutes of Health, and the Health Insurance Portability and Accountability Act). Ethical approvals were sought and granted by the Western Institutional Review Board (Study ID number 1183030) for the USA and Canada and by the Freiburg Ethics Commission (FEKI) for all other countries. Participants provided informed consent to participate in the study and did not provide any identifiable information. All responses were de-identified before the dataset was received for analysis to preserve respondent (physician and patient) confidentiality, and all patients and physicians were assigned a study number in order to allow linkage of data during collection and data processing.
Results
Data were collected from 454 rheumatologist and 238 dermatologist participants for 3200 participants with PsA (1514 from rheumatologists and 1686 from dermatologists) from nine countries representing Asia Pacific (n = 443; 13.8%), Europe (n = 1966; 61.4%), and North America (n = 791; 24.7%). Information on the presence of current enthesitis (referred to simply as enthesitis throughout the rest of the document) was available for 3157 participants with PsA (n = 2952 without enthesitis; n = 205 with enthesitis). Physician-reported enthesitis severity was 54.0% mild, 41.3% moderate, and 4.6% severe for the entire sample. Enthesitis severity reported by physician speciality was 58.3% mild, 35.9% moderate, and 5.8% severe by rheumatologists, and 47.8% mild, 49.3% moderate, and 2.9% severe by dermatologists. Self-reported data was completed by up to 1416 participants. The remaining results focus on the entire sample, as the information from rheumatologists’ and dermatologists’ patients were similar, but any notable differences are highlighted.
Patient Demographic and Clinical Characteristics
Across the 3157 participants with PsA, 45.9% were female, with a mean (standard deviation [SD]) age of 49.2 (13.3) years, a mean (SD) body mass index 26.9 (6.5) kg/m2, mean (SD) time since diagnosis of PsA 4.9 (6.0) years, and 57.4% were in full-time employment. Enthesitis was present at the time of the survey in 6.5% (n = 205) of participants with PsA; rheumatologist patients: 8.8% (132/1498); dermatologist patients: 4.4% (73/1659). Participants with and without enthesitis were similar demographically, although those with enthesitis were less likely to be working full time (47.2%) compared to those without enthesitis (58.1%) and had a larger percentage on long-term sick leave (7.5% vs 2.1% in those without enthesitis) (Table 1).
Participants with enthesitis had worse overall physician-rated disease severity compared to those without enthesitis (52.2% as moderate–severe, compared to 23.2%, p < 0.001). Participants with enthesitis had a greater mean number of joints affected by PsA (6.1 vs 3.0, p < 0.0001). Few physician (rheumatologist/dermatologist) participants in this real-world setting utilized standardized assessment tools that are commonly used in clinical trials. Those that were more frequently used included the 68 tender joint count (TJC) (n = 697), 66 swollen joint count (SJC) (n = 742), and disease activity score in 28 joints (DAS28) (n = 326). Across these three assessments, participants with enthesitis had higher joint counts (TJC 7.8 vs 3.5, p < 0.0001; SJC 6.3 vs 3.0, p = 0.0004) and higher disease activity score (DAS28 3.7 vs 2.2, p < 0.0001) (Table 2).
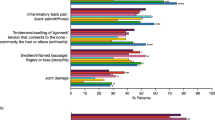
Participants with enthesitis also exhibited a greater number of other PsA manifestations compared to the group without enthesitis, including nail psoriasis (33.2% vs 14.8%, p < 0.0001), dactylitis (31.7% vs 5.9%, p < 0.0001), and sacroiliitis (10.7% vs 3.4%, p < 0.0001). A higher percentage of dermatologists’ patients overall were identified as having nail psoriasis compared to rheumatologists’ patients, while a higher percentage of rheumatologists’ patients overall were identified as having dactylitis compared to dermatologists’ patients (Tables S1, S2). Inflammatory back pain was also statistically significantly higher in the group with enthesitis vs without from dermatologists (24.7% vs 8.3%, p < 0.0001) (Table S2), while it was numerically higher in the rheumatologist’s patients (Tables S1, S2). The mean BSA affected by psoriasis was not statistically significant between the two groups (BSA with enthesitis = 7.3%; BSA without enthesitis = 5.9%, p = 0.0764) (Table 2). The same trend was seen comparing rheumatologists’ and dermatologists’ patients, although a higher BSA overall was reported by dermatologists (Table S2).
Patient-Reported Outcomes
The number of participants with results varies for each of the PROs related to some missing data from the self-completed questionnaires. Participants with enthesitis experienced a higher mean (SD) level of pain (4.27 [2.29]) compared to those without enthesitis (2.92 [1.84]); p < 0.0001) and a worse level of disability as assessed by the HAQ-DI (with enthesitis 0.89 [0.71], without enthesitis 0.47 [0.55]; p < 0.0001). Participants with enthesitis reported worse QoL compared to those without enthesitis, as measured by both the PsAID12 and EQ5D (Figs. 1, 2). In participants with enthesitis, the mean PsAID12 score was 4.29 (2.70) compared to the group without enthesitis with a mean score of 2.36 (1.98) (p < 0.0001). For the EQ5D, mean scores were 0.67 (0.22) in participants with enthesitis and 0.81 (0.17) in those without enthesitis (p < 0.0001).
Participants with enthesitis also reported a greater impact on work compared to those without enthesitis including statistically higher (worse) presenteeism, overall work impairment, and activity impairment outside of work (Fig. 3). Similar results were seen comparing patients from rheumatologists and dermatologists (data not shown).
Medication Use
Participants with enthesitis were more likely to be taking medication for their pain compared to those without enthesitis, including non-steroidal anti-inflammatory drugs (NSAIDs) (44.4% vs 22.9%, p < 0.001), non-opioid analgesics (10.7% vs 5.4%, p = 0.0045), and opioid analgesics (8.3% vs 1.9%, p < 0.0001). A higher percentage of participants with enthesitis were also taking csDMARDs (44.9% vs 34.2%, p = 0.0024). However, both groups of participants received targeted synthetic (12.2% with enthesitis, 9.3% without; p = 0.1754) or biologic DMARDS (58.5% with enthesitis, 58.4% without; p = 1.000) in approximately comparable percentages (Table 3). Similar results were also seen in participants with PsA with severe enthesitis (subjective assessment by physician participants) where 50% (n = 4) were receiving a biologic therapy. Comparing patients from rheumatologists and dermatologists revealed similar trends across medication use; however, notable differences included a statistically significant higher use of non-opioid analgesics and targeted synthetic DMARDs in dermatologists’ patients with enthesitis (Tables S3, S4).
Physician and Patient Satisfaction with Treatment
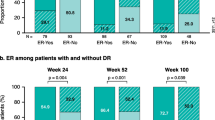
Overall physicians were more likely to be satisfied than dissatisfied with treatment in their participants with PsA. However, physician participants were significantly less likely to be satisfied with disease control with the current treatment provided in participants with enthesitis (57.6% satisfied) versus without enthesitis (84.4% satisfied, p < 0.0001) (Table 4). The three most common reasons physicians selected for not being satisfied were lack of efficacy overall (52.3%), lack of pain control (36.9%), and not effective in addressing the joint symptoms (32.9%).
From the patient perspective, 52.2% of patients with enthesitis were satisfied with their treatment versus 79.7% of patients without enthesitis, p < 0.0001. The four most common statements patients selected for lack of satisfaction were overall I don’t feel it works very well (51.7%), I still get temporary worsening/flares (42.5%), my pain isn’t sufficiently controlled (42.5%), and I still feel tired/fatigued (40.2%). The reported satisfaction rates and reasons for dissatisfaction between rheumatologists and dermatologists, as well as between the participants with PsA were similar, with a slightly higher trend in reported rates of dissatisfaction in patients with enthesitis from dermatologists compared to rheumatologists (Tables S5, S6).
Discussion
In this cross-sectional survey of participants with PsA from nine countries, clinician- and patient-reported outcomes were compared in participants who had current enthesitis to those who did not have current enthesitis. Enthesitis was clinically identified by physicians in 6.5% (n = 205) of their patients. The overall percentage was higher when reported by rheumatologists than dermatologists (9% vs 4%). This prevalence of enthesitis is lower than observed in other studies, possibly because of underreporting/underassessment of enthesitis in clinical practice. Enthesitis outcome measures were used infrequently in routine clinical practice in this sample, with physicians identifying specific instrument use in only 4% of patients (MASES 1.87%, Leeds Enthesitis Index (LEI) 1.77%, and Spondyloarthritis Research Consortium of Canada (SPARCC) 0.48%). Another factor that could contribute to lower prevalence of enthesitis compared to some other published literature is that this was a largely treated population.
In this study, participants with PsA with enthesitis experienced more severe disease than those without enthesitis. This finding supports assertions that enthesitis is an indicator of disease severity and complements other studies showing similar results. In the cross-sectional retrospective study from the Corrona PsA/SpA registry, Mease and colleagues also found higher TJC and SJC in 420 patients with enthesitis (identified by SPARCC) compared with 1147 without (TJC 6 vs 0, p < 0.0001; SJC 1 vs 0, p < 0.0001) and higher DAS28 C-reactive protein (CRP) (3.2 vs 2.6, p < 0.0001) [7]. Similarly, in a national registry from Turkey, Sunar et al. found higher TJC and DAS28 erythrocyte sedimentation rate (ESR) in 251 patients with PsA with enthesitis (subjective assessment) compared to 879 without (TJC 6 vs 4, p = 0.001; DAS28 ESR 3.6 vs 3.3, p = 0.001) [10]. Our study found a higher prevalence of other PsA disease manifestations in patients with enthesitis, including dactylitis, nail symptoms, and inflammatory back pain, which is similar to the studies by Mease and Sunar [7, 10]. The Corrona PsA/SpA registry found dactylitis in 27% of patients with enthesitis (versus 10% without) [7]. The registry study from Turkey found dactylitis in 13.1% of patients with enthesitis (versus 6.5% without), as well as a higher prevalence of nail symptoms (28.7% with fingernail pitting versus 21.3% without) [10]. Association of enthesitis with inflammatory back pain is also corroborated by the registry from Turkey where 59.4% of patients with enthesitis reported chronic inflammatory back pain (versus 39% without enthesitis) [10].
We identified worse patient-reported outcomes in participants with enthesitis compared to those without, including higher levels of pain, worse physical function, and lower quality of life as measured by both the PsAID12 and EQ5D. Our study corroborates results from the Corrona PsA/SpA, Turkey registries, and Toronto cohort, which reported higher (worse) physical function (HAQ) [7, 10] and pain scores [7, 11] in patients with enthesitis. Lower quality of life associated with enthesitis has been demonstrated with both disease-specific (PsAQoL) and generic (SF-36) [10, 24] instruments. Lastly, we found participants with enthesitis had a statistically significant impact on work productivity, a similar trend seen in the Corrona registry [7].
While current guidelines recommend the use of biologic therapies in patients with severe enthesitis who have failed NSAIDs or local steroid injections [14], in this study, patients with enthesitis were not more likely to be receiving biologics, with almost 60% of patients (58.5% [n = 120] with enthesitis overall and of those 50% [n = 4] with severe enthesitis) receiving biologics and 58.4% [n = 1724] without enthesitis receiving such treatments. Similar treatment patterns were seen in the Corrona PsA/SpA registry where 59% of patients with PsA with enthesitis and 60% without enthesitis were receiving biologics [7]. There was a higher use of csDMARDs in patients with enthesitis compared to those without even though strong evidence to support their effectiveness in enthesitis is lacking [25]. Use of pain medications, including NSAIDs, non-opioid and opioid analgesics, was also significantly higher in patients with enthesitis versus those without. Overall, physicians were significantly less satisfied with current treatment for patients with enthesitis than those without enthesitis.
Limitations
Data analyzed for this study were collected by physicians from a non-randomized sample of patients. Physicians were asked to complete data capture forms on a consecutive series of patients to reduce selection bias. No formal source data verification procedures were implemented. The sample is representative of the consulting patient population, so there is a potential over-representation of well-motivated patients or patients with less severe PsA. The severity assessment of enthesitis was subjective (mild–severe), as there currently is not a validated instrument for evaluating enthesitis severity. Individuals with fibromyalgia were not excluded, which could impact the identification and assessment of enthesitis in patients with both conditions. Recall bias is a common limitation of surveys; however, data in the study were collected at the time of consultation and using patient medical records to limit recall bias. The cross-sectional design cannot be used to demonstrate cause and effect, and all data were purely descriptive with no covariate adjustment. Participants voluntarily completed self-completion forms, and therefore PRO measures were based only on those who agreed to participate; thus, base sizes fluctuated where information was not completed by the participants.
Conclusions
Participants with PsA with enthesitis experienced more severe disease and worse outcomes than those without enthesitis. Physicians are less satisfied with their ability to treat patients with enthesitis versus without. In our survey only 58% of participants with enthesitis, and 50% of those with severe enthesitis, received biological therapies. Our findings underscore the importance of assessing patients with PsA for enthesitis and ensuring its optimal management. Data on PsA treatment efficacy specifically for enthesitis are needed to expand treatment options for patients and physicians.
References
Apostolakos J, Durant TJ, Dwyer CR, et al. The enthesis: a view of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4:333–42.
Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–71.
Kaeley GS, Eder L, Aydin SZ, Gutirrez M, Bakewell C. Enthesitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum. 2018;48(1):35–433.
Kalyoncu U, Bayindir Ö, Ferhat Öksüz M, et al. The Psoriatic Arthritis Registry of Turkey: results of a multicentre registry on 1081 patients. Rheumatology. 2016;56(2):279–86.
Love TJ, Gudbjornsson B, Gudjonsson JE, Valdimarsson H. Psoriatic arthritis in Reykjavik, Iceland: prevalence, demographics, and disease course. J Rheumatol. 2007;34(10):2082–8.
Gladman DD, Chandran V. Observational cohort studies: lessons learnt from the University of Toronto Psoriatic Arthritis Program. Rheumatology. 2011;50(1):25–31.
Mease PJ, Karki C, Palmer JB, et al. Clinical characteristics, disease activity, and patient-reported outcomes in psoriatic arthritis patients with dactylitis or enthesitis: results from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. Arthritis Care Res. 2017;69(11):1692–9.
Zisman D, Gladman DD, Stoll ML, et al. The juvenile psoriatic arthritis cohort in the CARRA Registry: clinical characteristics, classification, and outcomes. J Rheumatol. 2017;44(3):342–51.
Polachek A, Cook R, Chandran V, Gladman DD, Eder L. The association between sonographic enthesitis and radiographic damage in psoriatic arthritis. Arthits Res Ther. 2017;19(1):189.
Sunar I, Ataman S, Nas K, et al. Enthesitis and its relationship with disease activity, functional status, and quality of life in psoriatic arthritis: a multi-center study. Rheumatol Int. 2020;40(2):283–94.
Polachek A, Li S, Chandran V, Gladman DD. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res. 2017;69(11):1685–91.
Gezer O, Batmaz I, Sariyildiz MA, et al. Sleep quality in patients with psoriatic arthritis. Int J Rheum Dis. 2017;20(9):1212–8.
Baskan B, Oten E, Sivas F, et al. The relationship between vitamin D, vertebral deformity and quality of life in psoriatic arthritis. Acta Reumatol Port. 2016;41(4):350–8.
Gossec L, Smolen JS, Gaujoux-Viala C, et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis. 2012;71(1):4–12.
Dandorfer SWH, Rech J, Manger B, Schett G, Englbrecht M. Differences in the patient’s and physician’s perspective of disease in psoriatic arthritis. Semin Arthritis Rheum. 2012;42(1):32–41.
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes - a means to understand. Curr Med Res Opin. 2008;24(11):3063–72.
Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73(6):1012–9.
Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–45.
Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9(5):789–93.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108.
EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65.
Tillett W, Lin CY, Zbrozek A, Birt J. A threshold of meaning for work disability improvement in psoriatic arthritis measured by the work productivity and activity impairment questionnaire. Rheumatol Ther. 2019;6(3):379–91.
Wervers K, Luime JJ, Tchetverikov I, et al. Influence of disease manifestations on health-related quality of life in early psoriatic arthritis. J Rheumatol. 2018;45(11):1526–31.
Orbai AM, Weitz J, Siegel EL, et al. Systematic review of treatment effectiveness and outcome measures for enthesitis in psoriatic arthritis. J Rheumatol. 2014;41(11):2290–4.
Acknowledgements
Funding
The study and the Rapid Service Fee were funded by Eli Lilly and Company (Indianapolis, Indiana, USA). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Editorial Assistance
Antonia Baldo and Barbara Jackson from Syneos Health assisted with editing, which was funded by Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the whole work, and have given their approval for this version to be published.
Prior Presentation
Data included in this manuscript was previously presented as a poster at the American College of Rheumatology 2019 Annual Meeting, November 8–13, 2019, Atlanta, Georgia.
Disclosures
Ana-Maria Orbai: AbbVie, Amgen, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, Sun Pharma, UCB. Julie A. Birt, William N. Malatestinic and Aubrey T. Sprabery: Employees of Eli Lilly and Company. Anthony M. Reginato: Eli Lilly and Company, Novartis, Horizon. Elizabeth A. Holdsworth and Nicola Booth have nothing to disclose.
Compliance with Ethics Guidelines
The research was conducted in accordance with national market research and privacy regulations (European Pharmaceutical Market Research Association, US Department of Health and Human Services National Institutes of Health, and the Health Insurance Portability and Accountability Act). Ethical approvals were sought and granted by the Western Institutional Review Board (Study ID number 1183030) for the USA and Canada and by the Freiburg Ethics Commission (FEKI) for all other countries. Participants provided informed consent to participate in the study and did not provide any identifiable information. All responses were de-identified before the dataset was received for analysis to preserve respondent (physician and patient) confidentiality, and all patients and physicians were assigned a study number in order to allow linkage of data during collection and data processing.
Data Availability
All data generated or analyzed during this study are included in the published article or as supplementary information files or can be made available on request.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Orbai, AM., Birt, J.A., Holdsworth, E.A. et al. Impact of Enthesitis on Psoriatic Arthritis Patient-Reported Outcomes and Physician Satisfaction with Treatment: Data from a Multinational Patient and Physician Survey. Rheumatol Ther 7, 937–948 (2020). https://doi.org/10.1007/s40744-020-00242-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-020-00242-3