Abstract
Introduction
Implementation of a treat-to-target strategy is challenging when the patient and physician prioritize different goals. This study aimed to “translate” improvements in Clinical Disease Activity Index (CDAI) to concepts that resonate with patients (such as pain, fatigue, morning stiffness) by examining the association between changes in disease activity and patient-reported outcomes (PROs) in a national cohort of patients with rheumatoid arthritis (RA) initiating their first biologic treatment.
Methods
Patients in the Corrona registry with moderate or high disease activity (M/HDA) (defined by a CDAI score > 10), prior use of at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD), 12-month follow-up, and initiating their first tumor necrosis factor inhibitor (TNFi) between 1 January 2006 through 1 November 2015 were identified. Patients were stratified on the basis of CDAI during follow-up, and changes in PROs were compared with a test of trend using CDAI-defined groups.
Results
Of 1570 patients, 37% achieved sustained remission or low disease activity (remission/LDA), 15% had improving remission/LDA, 12% had worsening M/HDA, and 35% were in sustained M/HDA during 12-month follow-up. Those in sustained remission/LDA had greater magnitude of improvement in physical functioning, pain, fatigue, morning stiffness, patient’s global assessment, and quality of life compared with patients in sustained M/HDA (p < 0.001).
Conclusion
Reduction in disease activity was associated with improvements in PROs, with the greatest improvements seen in those who achieved sustained remission/LDA. These results reinforce the benefits of a treat-to-target approach to RA care and may improve dialogue between patients and providers, support shared decision-making, and reduce “clinical inertia.”
Funding
Corrona, LLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The burden of rheumatoid arthritis (RA) is well established in the literature. Patients with RA experience joint pain, fatigue, and prolonged morning stiffness. Furthermore, RA has detrimental effects on patients’ quality of life and physical functioning [1]. Despite the availability of biologic disease-modifying antirheumatic drugs (bDMARDs), the literature suggests that many patients with RA continue to experience moderate or even unacceptable levels of pain [2]. One potential contributing factor is that patients are often reluctant to discuss issues of personal importance, such as pain, fatigue, or their ability to function and participate in activities, with their physician [3].
The 2015 American College of Rheumatology (ACR) guideline for the treatment of RA advocates a treat-to-target strategy with remission as the optimum target and low disease activity (LDA) as an alternative target, especially for patients with longer-standing disease. In addition, the guideline advocates for an active patient–physician dialogue and collaborative decision-making [4]. Implementation of a treat-to-target strategy may be challenging when the patient and physician have different goals. Although rheumatologists may suggest adjustments in medications to achieve a composite index disease activity target, this may not have meaning for the patient; rather, patients often prioritize reduction of pain and improvement in function as their goals of therapy [1, 3].
The aim of this study was to investigate the association between changes in disease activity and patient-reported outcomes (PROs) among biologic-naïve patients with RA who were initiating treatment with tumor necrosis factor inhibitor (TNFi) therapy (adalimumab, etanercept, infliximab, certolizumab pegol, or golimumab) in the Corrona registry. This work is intended to give providers and patients information on how controlling disease activity will change the patient’s experience. “Translating” improvements in disease activity to concepts that resonate with patients (such as pain, fatigue, morning stiffness) may improve dialogue between patients and providers, support shared decision-making, and reduce “clinical inertia”, important components of an effective treat-to-target strategy [4,5,6].
Methods
Data Source
The Corrona registry is an independent, prospective, observational cohort of patients with RA (diagnosed by their rheumatologists) recruited at more than 169 private and academic practice sites across 40 states in the USA. As of 30 June 2016, Corrona had enrolled over 43,000 patients who met the ACR criteria for diagnosis of RA at enrollment. Approximately every 6 months, data are collected during routine clinical visits from both patients and their treating rheumatologists, who gather information on demographics, RA disease duration, prognosis, disease severity and activity, functional status, medical comorbidities, and RA medication use. Between-visit assessment of disease activity and PROs was not reported in this study.
This study was performed in accordance with the ethical principles of the Declaration of Helsinki. Registry participants were required to provide written informed consent and authorization prior to participating. For private practice sites participating within Corrona, approvals for data collection and analyses for this national study were obtained from the New England Institutional Review Board (IRB; approval #120160610), a central IRB. The local IRB of record was used for sites affiliated with an academic medical center. Further details on the Corrona registry have been published elsewhere [7].
Study Population
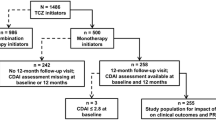
The study included biologic-naïve patients with RA in the Corrona registry who had prior use of at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD) and were initiating their first TNFi while in moderate or high disease activity (M/HDA), defined by the Clinical Disease Activity Index (CDAI) score greater than 10. The CDAI is a composite score (range 0–76) based on the summation of four RA disease indicators: swollen and tender joint counts (based on 28 joints), and Patient’s and Physician’s Global Assessment of Disease Activity [0–10 cm visual analog scale (VAS)] [8]. Patients with comorbid psoriasis, psoriatic arthritis, fibromyalgia, or age less than 18 years were excluded. The index date was the date of TNFi initiation on or after 1 January 2006 through 1 November 2015.
Disease Activity Group Definitions
To allow comparisons, the study cohort was stratified by disease activity categories. First, patients with CDAI no greater than 10 were classified as remission or low disease activity (remission/LDA) at each of their 6- and 12-month follow-up visits, whereas those with CDAI greater than 10 were identified as M/HDA [8]. Patients were then stratified into four mutually exclusive disease activity groups based on patterns of CDAI-defined disease activity observed over the 12-month follow-up period. Sustained remission/LDA was defined as remission/LDA at both the 6- and 12-month visit. Patients who were in M/HDA at both the 6- and 12-month visit were classified as sustained M/HDA. Those who changed CDAI categories between the 6- and 12-month visits were classified as either improving remission/LDA or worsening M/HDA, based on the direction of change (Supplemental Table 1).
Data Collection
Baseline demographic, clinical, and disease characteristics were reported at the index date. A CDAI score was required at baseline and at 6- and 12-month (window ± 3 months) post-index visits in the Corrona registry. If more than one visit occurred within a given time window, the visit closest to 6 or 12 months was used. Patient-reported measures available at baseline, 6 and 12 months included the modified Health Assessment Questionnaire (mHAQ; developed as a short form of the HAQ with eight questions, one from each of the eight categories of the HAQ, range 0–3), patient assessments of pain and fatigue (0–100 mm VAS), duration (hours) of morning stiffness, Patient’s Global Assessment of Disease Activity (PtGA; 0–100 mm VAS), and the EuroQol-5 dimensions-3 level (EQ-5D-3L) US Index score. The eight-item mHAQ asks patients to rate their difficulty performing the physical function activities of dressing, getting out of bed, lifting a cup, walking, bathing, bending, turning faucets, and getting into and out of a car [9]. The EQ-5D is a standardized measure of health-related quality of life comprised of five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, with each rated on a three-level scale: no problems, some problems, and extreme problems. The EQ-5D US Index is reported as a single value, ranging from − 0.102 (where zero is a health state equivalent to death; negative values are valued as worse than death) to 1 (perfect health), with higher scores indicating better health utility [10,11,12,13].
Statistical Methods
A retrospective cohort design was used. Patient characteristics and outcomes were compared across the CDAI-defined disease activity groups with a test of trend using CDAI groups as the ordinal predictor in linear regression for continuous measures and logistic regression for dichotomous or categorical measures. Wald F test was used for testing statistical significance of the CDAI group as an ordinal predictor of the continuous outcomes in linear regression, and Wald Chi-square test was used for testing such statistical significance of the dichotomous or categorical outcomes in logistic regression. For the outcomes of interest, i.e., the change in CDAI score and PROs at 12 months, both unadjusted and adjusted test of trend (Wald F test) analyses using linear or logistic regression were conducted. No imputations were made for missing data. Index patient characteristics, including baseline CDAI and baseline values of the corresponding outcome, were included in the adjusted model (Supplemental Tables 2 and 3).
As part of the study, the proportions of patients who met or exceeded a minimum clinically important difference (MCID) in physical functioning (mHAQ, decrease of 0.25 units) [14], patient pain (decrease of 10 mm on a 100-mm scale) [15], and patient fatigue (decrease of 10 mm) [16] at the 12-month follow-up visit were reported.
Biologic DMARD treatment patterns were reported during the 12 months following TNFi initiation. Patients were stratified by switching status into three mutually exclusive groups: patients who remained on the index TNFi, patients who switched to an alternative bDMARD, and patients who discontinued bDMARD use and had not switched to another bDMARD by 12 months. Patients who switched to an alternative bDMARD were further subdivided by switch to a subsequent TNFi or a non-TNFi. Among patients who switched, time (months) to switch was reported, as well as reason for switching when available. For those who switched more than once during the observation period, only the first switch was reported.
Results
Baseline Patient Characteristics
There were 1570 patients who fulfilled the study inclusion and exclusion criteria; 585 (37%) achieved sustained remission/LDA, 239 (15%) were identified as improving remission/LDA, 190 (12%) were categorized as worsening M/HDA, and 556 (35%) were in sustained M/HDA through 12 months from the index date of initiation of TNFi therapy (Supplemental Table 1).
Table 1 summarizes the baseline characteristics in the overall study cohort and by disease activity group. Race/ethnicity, age, duration of RA, marital status, smoking status, number of previous csDMARDs, and RA-related concomitant medications were comparable between the groups. The proportions of sero-positive patients [rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP)], patients with private insurance, those with a college education, and those with full-time work were highest in the sustained remission/LDA group. Higher proportions of female patients and patients with greater mean body mass index (BMI) were observed in the M/HDA groups than in the sustained remission/LDA group.
Association of Disease Activity and Patient-Reported Outcomes
In the overall study population, patients experienced improvements in mean CDAI and PRO measures of physical function (mHAQ), pain, fatigue, duration of morning stiffness, PtGA, and quality of life (EQ-5D) at 6 and 12 months following initiation of TNFi (Table 2). There was a significant trend toward improved 6- and 12-month mean PROs with better disease control on the basis of CDAI. Those in sustained remission/LDA had greater magnitude of improvement through 12 months in physical functioning, pain, fatigue, morning stiffness, patient’s global assessment, and quality of life compared with patients in sustained M/HDA (p < 0.01) (Table 2). When adjusted for the impact of baseline CDAI and PRO scores, results were consistent; both unadjusted and adjusted regression analyses suggested significantly greater change at 12 months in important PROs (mHAQ, pain, fatigue) for patients achieving better disease control (Supplemental Table 2). In addition, a larger magnitude of change in median CDAI and PRO values was observed in patients who achieved sustained remission/LDA over the 12-month follow-up period, compared with patients in sustained M/HDA. Similar absolute changes were seen in those in sustained remission/LDA and improving remission/LDA (Fig. 1 and Supplemental Table 4).
Change from baseline to 12-month median CDAI and PRO measures. CDAI Clinical Disease Activity Index, mHAQ modified Health Assessment Questionnaire, M/HDA moderate or high disease activity, PRO patient-reported outcome, PtGA Patient’s Global Assessment of Disease Activity, Rem/LDA remission or low disease activity, VAS visual analog scale
Minimum Clinically Important Difference
The proportions of patients who met or exceeded the MCID for mHAQ, pain, and fatigue were higher in patients achieving sustained remission/LDA than in the sustained M/HDA group (p < 0.001). Nearly 70% of patients in sustained remission/LDA achieved the MCID for pain; however, the proportions achieving MCID for mHAQ and fatigue were lower (Table 3).
12-Month bDMARD Treatment Patterns
The majority of patients (63% overall) remained on their index TNFi through 12 months regardless of disease activity during follow-up. A small number (14%) of patients discontinued their index TNFi and were not receiving a bDMARD, whereas 22% of patients switched to another bDMARD by 12 months (Fig. 2). Patients in sustained remission/LDA had the highest proportion of patients remaining on their index TNFi. Patients in worsening or sustained M/HDA had higher rates of discontinuation or switching than patients in sustained remission/LDA (Fig. 2). Among patients remaining on their index TNFi, the largest proportion (46%) achieved sustained remission/LDA; however, 27% of patients remained on the index TNFi despite being in sustained M/HDA over the 12-month follow-up period (Supplemental Table 5).
Switching to an alternative TNFi occurred twice as often as did switching to a non-TNFi biologic, and occurred at a median of 6 and 6.5 months, respectively. Among the approximately 75% of patients with a reported reason for their switch from the index TNFi to another bDMARD, lack of efficacy was the most common reason (61% of switches). Safety issues accounted for 15% of switches to another biologic. Median time to switch did not differ substantially by reason for the switch or by the biologic class switch (subsequent TNFi or non-TNFi) (Table 4 and Supplemental Table 5).
Discussion
This study examined the association between changes in disease activity and PROs among biologic-naïve patients with RA who were initiating treatment with TNFi therapy while in M/HDA. The study found that 37% of patients achieved sustained remission/LDA, 15% had improving remission/LDA, 12% had worsening M/HDA, and 35% were in sustained M/HDA during the year following TNFi initiation. Reduction in the assessed level of disease activity aligned with improvements in the PROs examined. Those who achieved sustained remission/LDA had a greater magnitude of change in physical functioning (mHAQ), pain, fatigue, morning stiffness, PtGA, and quality of life (EQ-5D) over the 12-month follow-up period than did patients who remained in M/HDA at 12 months. These findings suggest that achieving and maintaining remission or LDA for the longest time possible is associated with the best outcomes, consistent with the philosophy of a treat-to-target strategy of care [4, 17]. Of note, in this cohort of patients, patients at the lower end of the spectrum of M/HDA at TNFi initiation experienced a greater magnitude of change in PROs at 12 months, compared with patients who had higher baseline levels of disease activity.
These findings from the Corrona registry, a real-world cohort of patients with RA, are similar to those reported in several recent phase III randomized clinical trials (RCTs), including RA-BEACON (baricitinib vs. placebo), ORAL STEP (tofacitinib vs. placebo), and RADIATE (tocilizumab vs. placebo). These trials enrolled patients with a history of inadequate response to at least one TNFi. Patients were maintained on stable csDMARD background therapy in both the active and control treatment arms. Efficacy data aligned with respective changes in PROs in these studies; patients randomized to the active treatment arms demonstrated greater clinical efficacy, as well as greater magnitude of improvement in most PROs, compared with placebo-treated patients [18,19,20,21].
Patients who remained in M/HDA through 12 months in the current study experienced improvements, albeit modest, in physical functioning, pain, fatigue, morning stiffness, PtGA, and quality of life. Up to 40% of these patients experienced a clinically meaningful reduction in pain. These patients may be satisfied with these minor improvements and unaware of the degree of improvement possible with further reduction in disease activity, contributing to patients’ hesitancy or refusal to change therapy regimens [22, 23].
Switching biologics occurred in 22% of the population, occurring more frequently in those with higher levels of disease activity. That being said, among patients with sustained M/HDA, only one-third switched biologics leaving the remaining two-thirds to have either continued on the index TNFi or discontinued the TNFi despite being in moderate or high disease activity at both time points over the year.
There are several potential reasons for this lack of treatment acceleration, including clinical inertia [5, 6] and system-level barriers such as high copayments for medications, formulary limitations, and the prior authorization process which delays access to needed medication [24]. In addition, it is not clear whether the patients were aware of the disease activity or engaged in shared decision-making regarding treatment acceleration [25]. Lastly, patients may be hesitant to make medication changes even when indicated because of concerns about medication side effects or other concerns [26,27,28].
The study provides additional evidence of the benefits for the treat-to-target paradigm. Although measuring disease activity using validated measures and titrating medications until achievement of the disease activity target is advocated, often this is not done in clinical practice [29]. The results of our study may persuade patients and providers who are hesitant to alter therapy in the setting of active disease to rethink their position given the greater relief of symptoms and improvement in physical functioning and quality of life with each further reduction in disease activity. Our findings may also help guide patient and provider conversations and we suggest that translating improvements in disease activity to outcomes that are important to patients (such as pain, fatigue, and physical function) could improve the dialogue and support shared decision-making between the rheumatologist and their patient, reducing clinical inertia. Additional research is warranted to determine whether this approach of focusing on symptoms, functional status, and quality of life when setting treatment goals achieves better outcomes for patients with RA.
Strengths and Limitations
There are several strengths of this study, including longitudinal disease activity information on a national cohort of patients with RA followed by a broad distribution of US rheumatologists. However, the study has limitations. We can identify only treatments that were agreed to by patients and not those that were offered and declined. Although we examined a national cohort of patients, they may not be representative of the total RA patient population and rheumatologists across the USA in terms of clinical characteristics and RA treatment practices. However, prior work using national Medicare data showed that patients with RA enrolled in the Corrona registry are similar in terms of age and gender but more likely to receive biologics as compared to Medicare RA patients not cared for by a Corrona provider [30].
This study reported PROs at baseline, 6 and 12 months but does not provide information between these time points. Counts for some variables were reduced because of missing data, and no adjustments for missing data were made. Treatment adjustments made after the 12-month follow-up visit were not captured, nor were subsequent switches in patients who had more than one bDMARD switch during the observation period. The study did not report dose adjustments, and it is not known if patients who continued on the index TNFi had upward dose titration. Among patients who switched to an alternative bDMARD during follow-up, the remaining observation period to assess the effect of switching on disease activity and PROs was limited to a few months in some cases.
Conclusions
In the 12 months following TNFi initiation by biologic-naïve patients with RA, reduction in the assessed level of disease activity was associated with improvements in PROs, with the greatest improvements seen in those who achieved sustained remission/LDA. These results reinforce the need for a treat-to-target approach to RA care with treatment acceleration until the target level of disease activity is achieved. In addition, our findings may be useful in discussions between patients and providers because they quantify improvements that are important to patients including pain, fatigue, morning stiffness, physical function, and quality of life. Unfortunately, substantial numbers of patients did not achieve sustained remission/LDA in the 1 year following initiation and many of those patients remained on the initial TNFi. Given the improvements associated with lower levels of disease activity, patients and providers may wish to consider alternative therapies sooner in those with persistent active disease.
References
Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. 2016;36:685–95.
Lee YC, Cui J, Lu B, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther. 2011;13:R83.
Strand V, Wright GC, Bergman MJ, Tambiah J, Taylor PC. Patient expectations and perceptions of goal-setting strategies for disease management in rheumatoid arthritis. J Rheumatol. 2015;42:2046–54.
Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1–25.
Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825–34.
Phillips LS, Twombly JG. It’s time to overcome clinical inertia. Ann Intern Med. 2008;148:783–5.
Kremer JM. The CORRONA database. Clin Exp Rheumatol. 2005;23:S172–7.
Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res. 2012;64:640–7.
Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53.
EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72.
Szende A, Oppe M, Devlin N, editors. EQ-5D value sets: inventory, comparative review and user guide, vol 2. Dordrecht: Springer; 2007. pp 1–102.
Ramos-Goñi JM, Rivero-Arias O. Eq5d: a command to calculate index values for the EQ-5D quality-of-life instrument. Stata J. 2011;11:120–5.
Wolfe F, Pincus T. Listening to the patient: a practical guide to self-report questionnaires in clinical care. Arthritis Rheum. 1999;42:1797–808.
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21.
Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. J Rheumatol. 2007;34:280–9.
Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75:3–15.
Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243–52.
Smolen JS, Kremer JM, Gaich CL, et al. Patient-reported outcomes from a randomized phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON). Ann Rheum Dis. 2017;76:694–700.
Strand V, Burmester GR, Ogale S, et al. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study. Rheumatology. 2012;51:1860–9.
Strand V, Burmester GR, Zerbini CA, et al. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: patient-reported outcomes from a phase III trial. Arthritis Care Res. 2015;67:475–83.
Wolfe F, Michaud K. Resistance of rheumatoid arthritis patients to changing therapy. Arthritis Rheum. 2007;56:2135–42.
Curtis JR, Shan Y, Harrold L, Zhang J, Greenberg JD, Reed GW. Patient perspectives on achieving treat-to-target goals: a critical examination of patient-reported outcomes. Arthritis Care Res. 2013;65:1707–12.
Happe LE, Clark D, Holiday E, Young T. A systematic literature review assessing the directional impact of managed care formulary restrictions on medication adherence, clinical outcomes, economic outcomes, and health care resource utilization. J Manag Care Spec Pharm. 2014;20:677–84.
Corrigan J. Crossing the quality chasm: a new health system for the 21st century. Building a better delivery system: a new engineering/health care partnership. Washington, DC: The National Academies Press; 2001. p. 95–7.
van Hulst LT, Kievit W, van Bommel R, van Riel PL, Fraenkel L. Rheumatoid arthritis patients and rheumatologists approach the decision to escalate care differently: results of a maximum difference scaling experiment. Arthritis Care Res. 2011;63:1407–14.
Fraenkel L, Cunningham M. High disease activity may not be sufficient to escalate care. Arthritis Care Res. 2014;66:197–203.
Harrold L, Reed GW, Harrington JT, et al. A cluster-randomized trial of a behavioral intervention to incorporate a treat-to-target approach in the clinical care of rheumatoid arthritis patients in the United States [abstract]. Arthritis Rheumatol. 2015;67:3821–2.
Harrold LR, Reed GW, Kremer JM, et al. Identifying factors associated with concordance with the American College of Rheumatology rheumatoid arthritis treatment recommendations. Arthritis Res Ther. 2016;18:94.
Curtis JR, Chen L, Bharat A, et al. Linkage of a de-identified United States rheumatoid arthritis registry with administrative data to facilitate comparative effectiveness research. Arthritis Care Res. 2014;66:1790–8.
Acknowledgements
Funding
This study was funded by Corrona, LLC. Corrona, LLC has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, BMS, Crescendo, Eli Lilly and Company, Genentech, GSK, Horizon Pharma USA, Janssen, Momenta Pharmaceuticals, Novartis, Pfizer, Roche, and UCB. Article processing charges were funded by Eli Lilly and Company. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Cynthia J. Larmore, MSN, RN is an employee and shareholder of Eli Lilly and Company; Natalie N. Boytsov, PhD is an employee and shareholder of Eli Lilly and Company; Carol L. Gaich, PharmD is an employee and shareholder of Eli Lilly and Company; Xiang Zhang, PhD is an employee and shareholder of Eli Lilly and Company, Indianapolis; Andre B. Araujo, PhD is an employee and shareholder of Eli Lilly and Company, Indianapolis; Sabrina Rebello, MPH is an employee of Corrona, LLC; Bob A. Salim, PhD is an employee of Axio Research, LLC; George W. Reed, PhD is an employee and shareholder of Corrona; Leslie R. Harrold, MD, MPH is an employee and shareholder of Corrona; Leslie R. Harrold is also an employee of the University of Massachusetts Medical School, has a research grant to the University of Massachusetts Medical School from Pfizer, and has been a consultant to Roche and Bristol-Myers Squibb.
Compliance with Ethics Guidelines
This study was performed in accordance with the ethical principles of the Declaration of Helsinki. Registry participants were required to provide written informed consent and authorization prior to participating. For private practice sites participating within Corrona, approvals for data collection and analyses for this national study were obtained from the New England Institutional Review Board (IRB; approval #120160610), a central IRB. The local IRB of record was used for sites affiliated with an academic medical center.
Data Availability
The datasets generated and analyzed during the current study are not publicly available because of proprietary rights but are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/2D1DF06022604BA0.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Larmore, C.J., Boytsov, N.N., Gaich, C.L. et al. Examination of Patient-Reported Outcomes in Association with TNF-Inhibitor Treatment Response: Results from a US Observational Cohort Study. Rheumatol Ther 5, 215–229 (2018). https://doi.org/10.1007/s40744-017-0092-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-017-0092-0