Abstract
Introduction
The safety and efficacy of certolizumab pegol (CZP) 400 mg every 4 weeks (Q4W) monotherapy (FAST4WARD/NCT00548834) and in combination with methotrexate (MTX) (014/NCT00544154) in active rheumatoid arthritis (RA) has been published previously. This report outlines final long-term outcomes from the open-label extension (OLE) study (015/NCT00160693), which enrolled patients from these randomized controlled trials (RCTs).
Methods
Patients who withdrew from or completed the 24-week 014/FAST4WARD RCTs were enrolled and received CZP 400 mg Q4W with/without MTX. Exposure-adjusted event rates (ER) per 100 patient-years (PYs) of adverse events (AEs) and serious AEs (SAEs) were reported for all patients receiving ≥1 dose of CZP in RCTs or OLE (N = 427) between first CZP dose and up to 24 weeks after last CZP dose or study withdrawal. Efficacy assessments included clinical (ACR20/50/70 response rates, TJC, SJC) and patient-reported outcomes (HAQ-DI, PtGADA, pain, fatigue) to week 304 (5.8 years) in the CZP intent-to-treat population. SDAI and CDAI outcomes were analyzed post hoc. Outcomes for CZP monotherapy and CZP+MTX combination-therapy were compared.
Results
Globally, ERs of AEs and SAEs were 408.1 and 25.2 per 100 PY, respectively. Eleven patients had AEs leading to death (ER 0.6). Improvements in clinical and patient-reported outcomes during the 24-week RCTs were maintained to week 304, and were similar between all subpopulations.
Conclusions
The longest exposure duration to date with CZP 400 mg Q4W treatment confirmed the safety profile observed in previous studies. Initial improvements in signs and symptoms of RA, including PROs, were maintained in both CZP monotherapy and CZP + MTX combination-therapy patients.
Trial registration: ClinicalTrials.gov identifier, NCT00160693.
Funding: UCB Pharma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease associated with significant morbidity. RA treatment guidelines recommend use of methotrexate (MTX) or other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) prior to initiation of anti-tumor necrosis factor (TNF) therapy in combination with csDMARDs [1, 2]. However, the use of anti-TNFs as monotherapy is a potential treatment option for patients with RA who are intolerant to MTX or for whom MTX would be inappropriate. Around 30% of RA patients treated with a biologic receive it as monotherapy, primarily due to MTX intolerability [3–6].
Certolizumab pegol (CZP) is a PEGylated Fc-free anti-TNF approved in over 50 countries for the treatment of RA, ankylosing spondylitis, axial spondyloarthritis, psoriatic arthritis, and Crohn’s disease.
The safety and efficacy of CZP in RA, in combination with MTX and as a monotherapy, was initially investigated in two phase III randomized controlled trials (RCTs); the Study 014 RCT (NCT00544154) [7] and the FAST4WARD RCT (NCT00548834) [8]. Patients from these two 24-week, randomized, double-blind, placebo-controlled studies entered a single open-label extension (OLE) study (Study 015; NCT00160693). These early trials differed from later CZP clinical trials, such as RAPID 1 (NCT00152386) and RAPID 2 (NCT00175877) [9, 10], as patients received a CZP dose of 400 mg every 4 weeks (Q4W) compared to either 200 mg (with a loading dose of 400 mg every 2 weeks for the first 4 weeks) or 400 mg every 2 weeks in the RAPID trials, and patients in FAST4WARD did not receive concomitant MTX, unlike patients participating in the RAPID trials.
The primary objective of Study 015 was to assess the long-term (up to 7 years) safety and tolerability of CZP 400 mg subcutaneously Q4W. Secondary objectives were to assess the sustainability of CZP efficacy for treatment of the signs and symptoms of RA, including patient-reported outcomes (PROs), both in combination with MTX and as a monotherapy.
Methods
Study Design
Study 015 was a phase III, multi-center, open-label study of CZP 400 mg Q4W in patients who initially fulfilled the criteria for active RA [7, 8], which enrolled patients from two 24-week, randomized, double-blind, placebo-controlled studies of CZP: FAST4WARD and Study 014 (Fig. 1a). FAST4WARD and Study 014 had similar trial designs; however, FAST4WARD patients received CZP as a monotherapy while those in Study 014 received CZP in combination with MTX. Patients could enter the open-label extension (OLE; Study 015) after completing the RCT or withdrawing at or after 12 weeks of treatment, provided they had not withdrawn due to a possible drug-related adverse event (AE) or non-compliance.
Study design. *CZP monotherapy analysis excludes 1 FAST4WARD patient who was receiving MTX at baseline—patients were excluded from the CZP monotherapy analysis from the date of concomitant MTX administration. †CZP+MTX group includes all patients from Study 014 and one patient from FAST4WARD study who received MTX at baseline
During Study 015, all patients received CZP 400 mg subcutaneously Q4W and were permitted to take csDMARDs, either from study baseline or at any point, as needed. Visits were scheduled at OLE entry, week 1, week 4, and then every 4 weeks thereafter; treatment was continued until marketing approval in the respective countries was obtained (up to 7 years). Patients who attended a completion visit at site closure due to marketing approval were considered to have completed the study, irrespective of treatment duration at site closure.
This study was conducted in accordance with the current version of the applicable regulatory and International Conference on Harmonisation (ICH)-Good Clinical Practice (GCP) requirements, the ethical principles that have their origin in the principles of the Declaration of Helsinki of 1964, and the local laws of the countries involved. Informed consent was obtained from all patients for being included in the study.
Patients
Full inclusion criteria for the FAST4WARD and Study 014 RCTs have been reported elsewhere [7, 8]. Briefly, adults with adult-onset active RA of ≥6 months’ duration, defined as ≥9 tender joints (68 joint count), ≥9 swollen joints (66 joint count), with one of the following three criteria: ≥45-min duration of morning stiffness; erythrocyte sedimentation rate (ESR; Westergren) ≥28 mm/h; or C-reactive protein (CRP) >10 mg/l (>1.0 mg/dl), who had failed ≥1 prior csDMARD were eligible for study inclusion.
NSAIDs and oral corticosteroids ≤10 mg/day prednisone equivalent were allowed in both RCTs, if stable for ≥4 weeks prior to study entry and maintained throughout the RCT period. All csDMARDs in FAST4WARD, and csDMARDs apart from MTX (at least 6 months prior to study entry, with stable dose of 15–25 mg/week for at least 8 weeks prior to first study medication dose) in Study 014, were prohibited. Concomitant administration of any NSAID, oral corticosteroid, or csDMARD was permitted throughout Study 015, including addition of MTX for patients originally enrolled in FAST4WARD.
Safety
The primary objective of Study 015 was to investigate long-term safety of CZP treatment of active RA, as measured by adverse events (AEs) and serious AEs (SAEs).
The safety population in this report included all patients who received ≥1 dose of CZP in either the RCT or OLE. All events experienced between first CZP exposure and 24 weeks after the last visit/patient withdrawal were included. AEs and SAEs were assessed at every visit and classified by system organ class (SOC) and preferred term (PT) according to the MedDRA dictionary v9.0. AEs are reported as exposure-adjusted event rates (ER) per 100 patient-years (PYs), which include multiple occurrences of an AE in the same patient.
Efficacy Analyses
A secondary objective of the OLE was to investigate the sustainability of CZP efficacy and assess the long-term impact of CZP on physical function in patients who were able to continue CZP during the OLE.
Efficacy analyses are reported up to week 304 (5.8 years) from the RCT baseline; after this point, site closure due to marketing approval reduced overall patient numbers until they were no longer considered to allow meaningful analysis of efficacy outcomes. The efficacy outcomes are reported from the RCT baseline into the OLE for (1) all patients randomized to receive CZP at RCT baseline (CZP intent-to-treat [ITT] population), (2) all patients who received CZP as a monotherapy at RCT baseline (CZP monotherapy group: patients were only considered to be part of the analysis while receiving CZP monotherapy; once patients initiated concomitant MTX therapy, their data were excluded from this analysis) and (3) all patients who received CZP with MTX at RCT baseline (CZP+MTX group). Patients who were randomized to placebo at RCT baseline were not included in these analyses.
Pre-specified clinical efficacy assessments investigated in this study were ACR 20/50/70 responder rates [11], tender/painful joint count (TJC), and swollen joint count (SJC), and Disease Activity Score (DAS)28-3(CRP). PROs assessed included the HAQ functional disability index (HAQ-DI) [12], patient’s assessment of arthritis pain [PAAP 0–100 mm visual analogue scale (VAS)], patient’s global assessment of disease activity (PtGADA, 1–5 Likert scale), short-form 36 (SF-36) [13], and fatigue assessment (0–10 cm VAS) [14]. The DAS28-3(CRP) was assessed because PtGADA was not collected using a 0–100 scale as required for DAS28-4(CRP) [15]. Post hoc analysis included Clinical Disease Activity Index (CDAI) change from RCT baseline, CDAI remission (CDAI ≤2.8) [16], Simplified Disease Activity Index (SDAI) change from RCT baseline and SDAI remission (SDAI ≤3.3) [16], which were calculated using PtGADA and Physician’s Global Assessments of Disease Activity (PhGADA), (both collected using 1–5 Likert scales, and then converted to 10 cm VAS-like outputs as follows: 1 = 0 cm, 2 = 2.5 cm, 3 = 5 cm, 4 = 7.5 cm, 5 = 10 cm).
The proportion of patients achieving minimum clinically important difference (MCID) was also analyzed post hoc and was defined as HAQ-DI change >0.22 [17, 18], PAAP change >10 mm [19], PtGADA change >1 on 0–10 VAS (adjusted from 1 to 5 Likert scale), SF-36 physical component summary (PCS) and mental component summary (MCS) change >2.5 [20], and fatigue assessment change >1 cm.
Statistical Analysis
Kaplan–Meier analyses were used to estimate patient retention in the CZP ITT group and CZP monotherapy group. The analysis included a comparison of patients who withdrew for any reason with those who withdrew due to AE alone, or lack of efficacy (including addition of MTX for CZP monotherapy patients). Patients who withdraw due to another reason were censored at the time of withdrawal. Patients who did not withdraw were censored at their last scheduled visit.
Missing data due to withdrawal, missing assessment, or addition of MTX for monotherapy patients, were imputed using last observation carried forward (LOCF) for change from baseline in DAS28-3(CRP), fatigue assessment, HAQ-DI, PAAP, PtGADA, SJC, TJC, CDAI and SDAI. For ACR response rates, CDAI remission, SDAI remission and the proportion of patients achieving MCID, missing data were imputed using a combination of non-responder imputation (NRI) and LOCF, which was used to take into account the reason for withdrawal during the long follow-up of the OLE: in the RCT, all missing values were imputed by LOCF; for patients entering the OLE, LOCF was used for values missing due to study completion, missed assessment visit, or withdrawal not due to AEs, lack of efficacy, or use of MTX rescue medication in the monotherapy group during the OLE, with all other missing data imputed using NRI. Where the table or figure number is not indicated, data shown are present in the text only.
Results
Baseline Demographics and Patient Disposition
Overall, 427 patients received CZP in the RCT or OLE (safety population). Of these, 235 patients were originally randomized to CZP in combination with MTX or as a monotherapy in the RCTs (CZP ITT group). The other 192 patients included in the safety population had originally received placebo across both RCTs before entering the OLE and receiving CZP. Patients who received placebo in the RCTs but did not consent to the OLE were not included in the safety population. A total of 110 patients were randomized and received CZP monotherapy in the FAST4WARD RCT and were therefore initially included in the CZP monotherapy group. An additional 125 patients were randomized to receive CZP in combination with MTX (124 patients from Study 014 and one patient from FAST4WARD) (Fig. 1). The 192 patients who were randomized to placebo at RCT baseline were not included in the CZP monotherapy or combination groups. Overall, 402 patients entered the OLE. Patient demographics and disease characteristics were similar across all groups at baseline (Table 1).
Kaplan–Meier analysis of withdrawal due to lack of efficacy, adverse events, or not consenting to enter the OLE estimated a patient retention rate of 50.4% in CZP ITT patients up to week 364 (~7 years from RCT baseline; Figure S1). The Kaplan–Meier analysis of the CZP monotherapy group treated addition of MTX as a withdrawal due to lack of efficacy event for these patients, and estimated a patient retention rate of 44.8% at week 355 (week of last visit for monotherapy group). Accounting for patient withdrawal due to lack of efficacy alone and AEs alone provided estimated retention rates of 79.0% and 70.2% at week 364 for CZP ITT patients, and 65.4% and 76.1% at week 355 for patients receiving CZP monotherapy (Figure S1C and S1D).
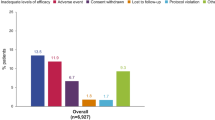
Patients were enrolled in the OLE between March 2003 and June 2004. As study sites were closed when CZP received marketing authorization (including FDA and EMA approval in 2009), a total of 67 patients remained in the study to week 364 of treatment (Fig. 1b). Of the patients who initiated CZP monotherapy, 18 subsequently received concomitant MTX in the RCT or OLE. The most common reason for withdrawal from the OLE was due to an AE [97 patients (24.1% of all patients entering the OLE)], followed by consent withdrawn and lack of efficacy [54 (13.4%) and 30 (7.5%), respectively] (Fig. 1b).
Safety
The longest CZP treatment duration in the complete safety population was 394 weeks (7.6 years), with a mean exposure of 213 weeks (4.1 years) and median exposure of 256 weeks (4.9 years). Of the patients that entered the OLE, 33 (8.3%) received concomitant corticosteroids and 328 (81.6%) received concomitant glucocorticoids during Study 015. Due to limitations in reporting, it was not possible to accurately track changes in the dosage of concomitant medication. The ER of SAEs was 25.2 per 100 PY, with the most frequent SAEs observed in the SOC categories infections/infestations, cardiac disorders and neoplasms benign, malignant and unspecified (incl. cysts and polyps) (ER = 4.5, 2.2, and 1.6 per 100 PY, respectively, Table 2).
The ERs per 100 patient-years in the safety population for serious infections (4.5) and AEs leading to withdrawal (7.8) were similar to previously reported rates for CZP therapy (Table 2). In the safety population, 11 AEs leading to death were reported (ER: 0.6 per 100 PY): seven cardiovascular events, two infections, one injury and one malignancy. The ER of AEs was highest during the first 3 months of treatment, which is a trend that has previously been reported. There was no increase in the rates of infections, cardiac disorders or malignancies over time (Table S1).
Clinical Efficacy
Rapid improvements in clinical disease activity reported in CZP ITT patients during the RCTs, including both CZP+MTX and CZP monotherapy group, were maintained over 304 weeks (Fig. 2). In the CZP ITT patient group (both combination and monotherapy patients) ACR20 and ACR50 responses were maintained between week 24 and week 304 (ACR20/50 52.3%/23.0% at week 24 and 42.6%/20.9% at week 304), whereas the ACR70 response rate continued to improve from 3.4% at week 24 to 6.4% at week 32 and was then maintained to week 304 (6.4%) (Fig. 2a). Similar results were reported in the CZP+MTX group (ACR20/50/70 51.2%/18.4%/0% at week 24 and 40.0%/16.0%/4.0% at week 304) and CZP monotherapy group (ACR20/50/70 52.7%/27.3%/7.3% at week 24 and 37.3%/22.7%/7.3% at week 304). As expected, observed case response rates for both combination and monotherapy patients in the CZP ITT group continued to improve throughout the OLE, with ACR20/50/70 rates at 49.5%/21.4%/2.9% (n = 210) at week 24, 72.3%/34.0%/11.3% (n = 141) at week 160 and 74.3%/37.1%/11.4% (n = 105) at week 304. Improvements were reported in TJC and SJC from baseline to week 24 OLE entry in CZP ITT and both treatment sub-groups. These decreases in TJC and SJC were maintained long-term to week 304 in all groups (Fig. 2d). The CZP ITT group observed case values were 15.7 and 10.7 at week 24 (n = 210), 7.4 and 4.6 at week 160 (n = 139), and 5.7 and 3.2 at week 304 (n = 105) for TJC and SJC scores, respectively.
Clinical efficacy outcomes for CZP ITT patients (N = 235), and the CZP monotherapy (n = 110) and CZP+MTX (n = 125) sub-populations: a ACR20/50/70 (NRI/LOCF); b mean SDAI (absolute values, LOCF); c Mean CDAI (absolute values, LOCF); d mean tender joint count (TJC; absolute values, LOCF) and swollen joint count (SJC; absolute values, LOCF)
In the CZP ITT, CZP+MTX and CZP monotherapy groups, the post hoc analysis of change from baseline in SDAI at week 24 (−23.6, −24.6 and −22.8, respectively) increased to week 304 (−26.9, −28.1 and −24.2). The post hoc analysis of change from baseline in CDAI was similar (week 24: −22.6, −23.8 and −21.5, week 304: −25.9, −27.8 and −22.8, in CZP ITT, CZP+MTX and CZP monotherapy groups, respectively). Absolute scores for CDAI and SDAI are presented in Fig. 2. At week 24, 3.4% of CZP ITT patients (3.3% of CZP+MTX and 3.6% of CZP monotherapy patients) were in SDAI remission, which rose to 7.7% (5.6 and 8.2%) by week 304. Similarly, CDAI remission was reported in 4.7, 4.0, and 5.5% of CZP ITT, CZP+MTX and CZP monotherapy patients at week 24, with remission rates increasing to week 304 (9.4, 8.0, and 9.1%).
Patient-Reported Outcomes
Rapid improvements in PROs observed during the RCT were maintained to week 304 in the CZP ITT group, including both patients receiving CZP + MTX and CZP monotherapy (Fig. 3). Proportions of patients reporting a MCID in HAQ-DI decreased from week 24 to week 304 (Fig. 4), although week 24 HAQ-DI mean scores were maintained to week 304 in the CZP ITT population (1.04–1.07) [including CZP+MTX (1.10–1.12) and CZP monotherapy (0.96–1.02) groups] (Fig. 3b). Observed case HAQ-DI values decreased between week 24 and week 304 in CZP ITT patients [week 24: 1.09 (n = 208), week 304: 0.83 (n = 105)].
Proportion of CZP ITT and CZP monotherapy patients achieving minimal clinically important difference (MCID) in HAQ-DI, pain (PAAP), fatigue, patient global assessment of disease activity (PtGADA), and SF-36 physical (PCS) and mental (MCS) components summary (% of patients, NRI/LOCF). Week 292 data presented where week 304 unavailable. Patients initially assigned to the CZP monotherapy group who achieved an MCID but also received MTX rescue medication prior to each visit were still included in the CZP ITT analysis, but excluded from the CZP monotherapy analysis
The proportion of patients reporting MCID in pain (PAAP), fatigue, PtGADA, and SF-36 PCS and MCS decreased between week 24 and week 304 (Fig. 4). Observed values for PAAP and PtGADA in CZP ITT patients were 39.0 and 2.7 (n = 210) at week 24, and 27.7 and 2.3 (n = 105) at week 304, respectively.
Discussion
This data represents the longest reported trial duration for CZP therapy to date, and demonstrates the long-term safety of CZP 400 mg Q4W maintenance dose over approximately 7.6 years and efficacy over just under 6 years, both in combination with MTX and as a monotherapy, which have not previously been published.
No new safety signals were observed over the course of the 7-year treatment period, with AE ERs comparable to other long-term anti-TNF studies [21–24]. AEs were consistent with other long-term evaluations of CZP in combination with MTX given as a 200 mg dose every 2 weeks (Q2W) [25–27]. Kaplan–Meier estimates of patient retention were high in both the CZP ITT and CZP monotherapy groups (up to 50% at 7 years), which is similar to retention rates described for longer-term trials of other anti-TNFs [22–24]. Although the Kaplan–Meier analyses of patient retention reported lower retention rates in the CZP monotherapy group compared to the ITT population, this was primarily driven by the use of MTX addition as a withdrawal due to lack of efficacy event in the CZP monotherapy group analyses. Lack of efficacy was given as the reason for withdrawal in 8.6% of ITT and 9.3% of CZP monotherapy patients.
Efficacy was sustained over the longer term (5.8 years) in these patients, both in terms of clinical measures and PROs. Improvements in clinical measures (ACR20/50/70, TJC, SJC, SDAI, and CDAI) achieved during the 24-week RCTs were maintained to week 304. Maintenance of response was similar between the CZP ITT, CZP+MTX, and CZP monotherapy groups in virtually all measures, especially depth of response as measured by ACR50 and ACR70 response rates.
PROs measure aspects of disease that cannot be easily assessed by physicians, as they quantify reports of patient health provided directly by the patient. Assessing PROs is therefore important for measuring the impact of the disease from the patient perspective [28–30]. Sustained efficacy in terms of PRO measures (HAQ-DI, PtGADA, pain, and fatigue) was observed to week 304 in CZP ITT and both CZP+MTX and CZP monotherapy groups, and was consistent with reports of sustained CZP+MTX efficacy in the RAPID 1 and 2 OLEs [25, 26].
The consistency between the long-term safety profile, patient retention and sustainability of response of CZP 400 mg Q4W reported in the present study and CZP 200 mg Q2W with MTX reported in the RAPID 1 and RAPID 2 OLEs [25, 26] support the use of the CZP 400 mg Q4W maintenance dose in clinical practice. This dosing schedule may be more convenient for some patients, and therefore has the potential to ease the burden of treatment for RA patients.
A number of limitations of the study design, previously described for the RCTs [7, 8], also apply to the present report of Study 015. The 1–5 Likert scale, which is much less sensitive to change than continuous VAS assessments as used in the RAPID trials, was used to collect the PtGADA and PhGADA in both the RCT and OLE studies. In addition, a combination of low initial CRP levels and the assay sensitivity resulted in a potential floor effect in terms of CRP level [7]. Therefore, three of the seven core ACR components had a limited sensitivity to change, and may have impacted the absolute response size observed. This limitation in data collection also impacted the post hoc analyses of CDAI and SDAI, as PtGADA and PhGADA needed to be converted to VAS-like outputs in order to calculate CDAI/SDAI change from baseline and remission rates. Therefore, TJC and SJC scores are reported in order to provide a more comparable measure of efficacy across studies. The safety population initially comprised 427 patients, of whom only 167 completed the OLE, which raises the possibility of selection bias in the safety analyses. Additionally, the OLE study design has limitations, such as the open-label nature of the study and the inherent survival bias, and the lack of a placebo control. However, given the ethical and regulatory restrictions for long-term placebo-controlled trials in rheumatology, OLE data are still valuable in informing long-term treatment choices. Comparisons between the CZP monotherapy and CZP ITT population were also limited in this study due to the lower treatment duration in the CZP monotherapy group (mean 3.4 vs. 4.1 years).
Conclusions
This study has demonstrated that CZP 400 mg Q4W had an acceptable safety profile over the long term (up to 7 years), and was also effective in maintaining initial improvements in signs and symptoms of RA, physical function, pain and fatigue over the longer term. These data support and build on previously published long-term data to 5 years for RAPID 1 and RAPID 2 OLEs [25, 26].
References
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(5):625–39.
Emery P, Sebba A, Huizinga TW. Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann Rheum Dis. 2013;72(12):1897–904.
Soliman MM, Ashcroft DM, Watson KD, Lunt M, Symmons DPM, Hyrich KL. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(4):583–9.
Mariette X, Gottenberg J-E, Ravaud P, Combe B. Registries in rheumatoid arthritis and autoimmune diseases: data from the French registries. Rheumatology (Oxford). 2011;50(1):222–9.
Lee SJ, Chang H, Yazici Y, Greenberg JD, Kremer JM, Kavanaugh A. Utilization trends of tumor necrosis factor inhibitors among patients with rheumatoid arthritis in a United States observational cohort study. J Rheumatol. 2009;36(8):1611–7.
Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, et al. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology (Oxford). 2012;51(7):1226–34.
Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68(6):805–11.
Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68(6):797–804.
Keystone E, Heijde D, Mason D Jr, Landewe R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58(11):3319–29.
Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):727–35.
Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20.
Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10(5):405–13 (discussion 15–20).
Khanna D, Pope JE, Khanna PP, Maloney M, Samedi N, Norrie D, et al. The minimally important difference for the fatigue visual analog scale in patients with rheumatoid arthritis followed in an academic clinical practice. J Rheumatol. 2008;35(12):2339–43.
Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8.
Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573–86.
Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol. 1993;20(3):557–60.
Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE Jr. Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000;43(7):1478–87.
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58.
Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2007;13(Suppl 9):S237–51.
Weinblatt ME, Bathon JM, Kremer JM, Fleischmann RM, Schiff MH, Martin RW, et al. Safety and efficacy of etanercept beyond 10 years of therapy in North American patients with early and longstanding rheumatoid arthritis. Arthritis Care Res (Hoboken). 2011;63(3):373–82.
Genovese MC, Schiff M, Luggen M, Le Bars M, Aranda R, Elegbe A, et al. Long-term safety and efficacy of abatacept through 5 years of treatment in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor inhibitor therapy. J Rheumatol. 2012;39(8):1546–54.
Burmester GR, Matucci-Cerinic M, Mariette X, Navarro-Blasco F, Kary S, Unnebrink K, et al. Safety and effectiveness of adalimumab in patients with rheumatoid arthritis over 5 years of therapy in a phase 3b and subsequent postmarketing observational study. Arthritis Res Ther. 2014;16(1):R24.
Smolen JS, Kay J, Doyle M, Landewé R, Matteson EL, Gaylis N, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17(1):14.
Keystone E, Landewe R, van Vollenhoven R, Combe B, Strand V, Mease P, et al. Long-term safety and efficacy of certolizumab pegol in combination with methotrexate in the treatment of rheumatoid arthritis: 5-year results from the RAPID 1 trial and open-label extension. Ann Rheum Dis. 2014;73(12):2094–100.
Smolen JS, van Vollenhoven R, Kavanaugh A, Strand V, Vencovsky J, Schiff M, et al. Certolizumab pegol plus methotrexate 5-year results from the rheumatoid arthritis prevention of structural damage (RAPID) 2 randomized controlled trial and long-term extension in rheumatoid arthritis patients. Arthritis Res Ther. 2015;17(1):245.
Bykerk VP, Cush J, Winthrop K, Calabrese L, Lortholary O, de Longueville M, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis. 2015;74(1):96–103.
van Gestel AM, Prevoo ML, van ‘t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39(1):34–40.
Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36(6):729–40.
Kirwan JR, Hewlett SE, Heiberg T, Hughes RA, Carr M, Hehir M, et al. Incorporating the patient perspective into outcome assessment in rheumatoid arthritis—progress at OMERACT 7. J Rheumatol. 2005;32(11):2250–6.
Acknowledgements
UCB Pharma sponsored the study, the development of the manuscript, and article processing charges. In addition to content approval by the authors, UCB signed off on the manuscript following a full review to ensure that the data presented in the publication are scientifically, technically, and medically supportable and did not contain any information which has the potential to damage the intellectual property of UCB. Additionally, UCB ensured that the publication complies with applicable laws, regulations, guidelines and good industry practice.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
The authors thank the patients, the investigators and their teams who took part in this study; we also acknowledge ‘Matladi N. Ndlovu, PhD, UCB Pharma, Brussels, Belgium for publication critical review coordination and Danielle Machin (Costello Medical Consulting, Cambridge, UK) for writing and editorial assistance, funded by UCB Pharma.
Disclosures
Roy Fleischmann: Research grants from Genetech Inc., Roche, Abbott, Amgen, UCB Pharma, Pfizer, BMS, Lilly, Sanofi-Aventis, MSD, Novartis, AstraZeneca and Janssen; Consultant for: Roche, Abbott, Amgen, UCB Pharma, Pfizer, BMS, Lilly, Sanofi-Aventis, Novartis, AstraZeneca and Janssen. Ronald F. van Vollenhoven: Research grants from AbbVie, BMS, GSK, Pfizer, Roche, UCB; Consultant for AbbVie, Biotest, BMS, GSK, Janssen, Lilly, Merck, Pfizer, Roche, UCB, Vertex. Jiri Vencovský: Consultant for: Abbott, Pfizer, MSD, UCB Pharma, Samsung Bioepics and Speakers Bureau: UCB Pharma, Pfizer, Abbott, MSD. Rieke Alten: Consultant for UCB Pharma. Owen Davies: Employee and shareholder of UCB Pharma. Irina Mountian: Employee of UCB Pharma. Marc de Longueville: Employee of UCB Pharma. David Carter: Former employee of UCB Pharma. Ernest Choy: Research grants from Abbott, Allergan, AstraZeneca, Boehringer Ingelheim, Chelsea Therapeutics, Chugai, Daiichi Sankyo, Eli Lilly, Ferring Pharmaceutical, GSK, Jazz Pharmaceuticals, MedImmune, Merrimack Pharmaceutical, MSD, Novartis, Pfizer, Pierre Fabre Medicament, Roche, Schering-Plough, Synovate, UCB Pharma; Consultant for Abbott, Allergan, AstraZeneca, Boehringer Ingelheim, Chelsea Therapeutics, Chugai, Daiichi Sankyo, Eli Lilly, Ferring Pharmaceutical, GSK, Jazz Pharmaceuticals, MedImmune, Merrimack Pharmaceutical, MSD, Novartis, Pfizer, Pierre Fabre Medicament, Roche, Schering-Plough, Synovate, UCB Pharma.
Compliance with Ethics Guidelines
This study was conducted in accordance with the current version of the applicable regulatory and International Conference on Harmonisation (ICH)-Good Clinical Practice (GCP) requirements, the ethical principles that have their origin in the principles of the Declaration of Helsinki of 1964, and the local laws of the countries involved. Informed consent was obtained from all patients for being included in the study.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/38F7F060237E7A26.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fleischmann, R., van Vollenhoven, R.F., Vencovský, J. et al. Long-Term Maintenance of Certolizumab Pegol Safety and Efficacy, in Combination with Methotrexate and as Monotherapy, in Rheumatoid Arthritis Patients. Rheumatol Ther 4, 57–69 (2017). https://doi.org/10.1007/s40744-017-0060-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-017-0060-8