Abstract
Introduction
Diagnosis of axial spondyloarthritis (SpA) can be delayed for several years mainly because of low awareness of axial SpA among non-rheumatologists who are the first interlocutors of potential SpA patients. One strategy to decrease the delay between appearance of first symptoms and diagnosis of axial SpA and to allow early management of the disease is to provide the non-rheumatologists with tools to identify patients requiring prompt referral to rheumatologists. This study was designed to evaluate in a real-world setting whether screening patients with chronic low back pain who consult physical medicine and rehabilitation (PMR) physicians, orthopedists, and ophthalmologists is useful in detecting axial SpA.
Methods
During this non-interventional cross-sectional study, data from 161 patients with chronic back pain, consulting an orthopedist, PMR physician, or ophthalmologist were collected during a single visit. Any patient who presented with at least four out of five symptoms of inflammatory back pain (IBP) and at least one additional SpA feature were to be referred to a rheumatologist. Analysis was purely descriptive.
Results
IBP was diagnosed in approximately half of the patients (89 patients) and 72 of them met the referral criteria. A total of 117 patients were finally referred to a rheumatologist and axial SpA was diagnosed for 37 of them.
Conclusions
The high prevalence of undiagnosed axial SpA in patients with chronic back pain visiting PMR physicians, orthopedists, and ophthalmologists suggests that these healthcare professionals may play a key role in the strategy developed to shorten the delay observed in the formal diagnosis of SpA.
Funding
Abbvie.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low back pain has been reported to affect 60–70% of adults during their lifetime and is a regular cause for seeking medical care [1, 2]. Low back pain is defined as chronic if symptoms persist for more than 3 months. Although chronic low back pain is most often degenerative, in about 5% of patients, the pain results from inflammation [3, 4] and is referred to as inflammatory back pain (IBP). One of the causes of IBP is axial spondyloarthritis (SpA), of which the prevalence is approximately 0.5–1% in the general population [5,6,7,8]. Chronic IBP has been identified as a major clinical feature of SpA and is experienced by most patients, whereas other peripheral or extra-articular manifestations (inflammation of peripheral joints with asymmetrical arthritis, predominantly of the lower limb; occurrence of enthesitis; uveitis) are present in approximately 40–60% of patients [6]. Axial SpA refers to patients with predominant axial involvement and axial complaints, and includes both ankylosing spondylitis (AS), for which evidence of sacroiliitis (another hallmark of SpA) is detected on X-rays, and non-radiographic axial SpA, where sacroiliitis is visible via magnetic resonance imaging (MRI) but not on X-rays [6]. Evidence has been gathered that non-radiographic axial SpA might be an early stage of AS, although not all cases of axial SpA will progress to AS [9, 10]. A strong association with Human Leucocyte Antigen-B27 (HLA-B27) has also been evidenced [11] and can be considered as the third main clinical feature of axial SpA.
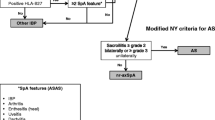
Based on these observations, over the past seven decades, several criteria and algorithms have been developed for the classification of AS and axial SpA. In 2009, the Assessment of SpondyloArthritis International Society (ASAS) released a new set of criteria for SpA in patients with chronic back pain [12,13,14]. The definition of IBP has been revised [15]. Sacroiliitis on imaging (X-rays or MRI) is the main criteria for the imaging arm, whilst the presence of HLA-B27 is the main criteria for the clinical arm. The ASAS criteria are met if at least one (in the imaging arm) or two (in the clinical arm) other SpA features are present [IBP, arthritis, enthesitis, uveitis, dactylitis, psoriasis, Crohn’s disease/ulcerative colitis, good response to nonsteroidal anti-inflammatory drugs (NSAIDs), family history of SpA, HLA-B27 and elevated C-reactive protein (CRP)] [12]. Although these criteria are intended for classification, they could also be a useful referral tool in the primary healthcare setting, allowing non-rheumatologists to determine whether referral to a rheumatologist is necessary.
Axial SpA has an early onset (generally in the second or third decade of life), which may hinder early diagnosis, as younger individuals may be less likely to promptly consult with a rheumatologist. These individuals may first turn to general practitioners or physical medicine and rehabilitation (PMR) physicians who are often the first interlocutors of potential patients with SpA. Non-rheumatologist specialists such as ophthalmologists and orthopedists are also consulted when extra-articular manifestations of the disease occur (for instance, uveitis or dactylitis) [16]. It is generally admitted that there is a delay of 5–10 years from the onset of symptoms (generally stiffness and axial pain) to a final diagnosis of AS [11, 14, 16]. On the other hand, efficient treatment strategies, such as NSAIDs, and more recently tumor necrosis factor (TNF) blockers, are available and seem to be most effective in the early stages of the disease [15]. Signs and symptoms associated with axial SpA (nocturnal pain, morning stiffness, fatigue, limitation of spinal mobility and ultimately ankylosis with disease progression) as well as symptoms associated with peripheral involvement, might seriously impact patient quality of life and have a non-negligible cost for society [17]. Facilitating an early diagnosis is therefore among the objectives clearly identified by ASAS to improve patient well-being [14, 18]. Some studies have highlighted the lack of awareness among general practitioners, especially regarding the disease spectrum and early detection [3, 19]. One strategy to shorten the delay between the occurrence of first symptoms and the final diagnosis of AS is to increase the awareness of SpA and AS among primary healthcare professionals and non-rheumatologist specialists, providing them with tools to identify these patients among the large population of patients with back pain. Recently, several referral strategies, mostly intended for general practitioners and primary care professionals, have been developed to allow earlier diagnosis [2, 10, 20, 21]. The SUSPECT study was designed to evaluate whether real-life screening of patients with chronic back pain who consult PMR physicians, orthopedists, and ophthalmologists is useful in detecting axial SpA.
Methods
Protocol Overview and Study Design
The SUSPECT study was approved by the ethics committee of Erasme Hospital (Brussels, Belgium) and conducted according to local regulations. It has therefore been performed in accordance with the ethical standard laid down in the 1964 Declaration of Helsinki and its later amendments. All patients gave written informed consent prior to their enrollment in the study.
SUSPECT was a non-interventional, cross-sectional study that took place between February 2011 and June 2013. No formal sample size calculation was performed. The planned number of patients (160) was defined on the basis of the recruiting capacity of the participating investigators. Data were collected during one routine visit to a non-rheumatologist investigator (orthopedists, PMR physicians, and ophthalmologists) practicing in university hospitals, regional hospitals, or in private practice. Patients aged 18–45 years at inclusion, with chronic back pain (>3 months) and back pain at night were eligible. Patients with diagnosed AS or SpA were excluded. The patients were evaluated for the presence of IBP based on the ASAS criteria [12] and SpA features according to the ASAS criteria for axial SpA [14]. Investigators were requested to refer any patient who presented with at least four of the five IBP symptoms and at least one additional SpA feature to a rheumatologist.
Demographic and baseline characteristics (duration of pain, presence of IBP symptoms, and SpA features) were collected during a single routine visit on a first case report form (CRF). For referred patients, investigators completed a second CRF based on the information provided by the rheumatologist (confirmation of diagnosis, diagnosis parameter, treatment initiated). Because this study was observational, HLA-B27 determination was not performed systematically (either by the investigator or the rheumatologist); however, the information was reported in the CRF when HLA-B27 results were available. The proportion of patients with HLA-B27-positive results was therefore not calculated. The number of patients with a positive HLA-B27 result is given for information purposes only; these data should be considered with caution.
Outcome Measures
The proportion of patients with confirmed diagnosis of axial SpA was evaluated and the characteristics of the patients referred to a rheumatologist were summarized.
Statistical Analysis
Descriptive analyses were performed using the SAS package for Windows, version 9.2 (SAS, Cary, NC, USA) on the full analysis set, which consisted of all enrolled patients with available information. Five subpopulations were defined: patients referred to a rheumatologist, patients agreeing to visit a rheumatologist, patients for whom feedback from the rheumatologist was available, patients with confirmed SpA diagnosis and patients with confirmed diagnosis not meeting the referral criteria (see flow chart in Fig. 1 for more details).
Results
Patient Demographics
A total of 27 investigators (three orthopedists, six ophthalmologists, and 18 PMR physicians) recruited 161 patients meeting the eligibility criteria for the study (patients aged between 18 and 45 years old, with chronic back pain and pain at night, having signed an informed consent and without known axial SpA). Patients ranged in age from 20 to 53 years, with an average age of 36 years [standard deviation (SD): 8 years]. Although the inclusion criteria stated an age limit of <45 years, 20 patients exceeded the age limit (eight patients had an age equal to 45 years). However, the decision was made to include these 20 patients in the full analysis set. Indeed, most of them (15/20) developed their back pain before 40 years of age and the others (5/20) had at least 2 SpA features. Of the 161 recruited patients, 46% were male.
Baseline Characteristics and Referral to a Rheumatologist
Of the 161 enrolled patients with back pain, 89 patients (55%) were diagnosed with IBP (at least four of five symptoms of IBP). The most frequently reported IBP symptoms (>85%) were age at onset younger than 40 years and pain at night (Table 1). The mean duration of back pain at the time of inclusion was 4.2 years (SD: 5.4 years; range, 2.5 months–25 years). A total of 130 patients (81%) presented at least one additional SpA feature. The most frequently reported additional SpA feature (collected before referral) was good response to NSAIDs (42%). A total of 72 patients met the referral criteria and 66 of them were referred to a rheumatologist. Although they did not meet referral criteria, 51 additional patients were referred to a rheumatologist. From these 51 patients, 46 fitted less of five IBP criteria and five had no SpA features according to the referring physician. Then, a total of 117 patients were advised to consult a rheumatologist, 104 agreed to do so and feedback was collected on 85 patients (Fig. 1). A diagnosis of SpA was confirmed for 37 patients (23.0% of the 161 enrolled patients, 31.6% of the referred patients, and 43.5% of the 85 patients with a rheumatologist feedback), 15 of which had not met the referral criteria.
Characteristics of Patients with Confirmed Axial SpA Diagnosis
In general, rheumatologists felt confident with their diagnosis (mean score >7 on a 0–10 scale). The main characteristics of patients with confirmed diagnosis of axial SpA are presented in Table 2. The mean age (34, SD: 8 years), sex ratio (41% male), and mean back pain duration (4.2, SD: 5.4 years) of patients with confirmed diagnosis of axial SpA are similar to those of the total study population. All patients meeting referral criteria had at least four IBP symptoms as specified in the protocol. Some discrepancies were observed between the SpA features recorded by the investigator and those recorded by the rheumatologist. For example, sacroiliitis on imaging (X-ray, MRI, or CT scan) was the most frequent SpA feature reported by the rheumatologist (57%, Table 2), whereas good response to NSAIDs for back pain was the most frequent SpA feature reported by investigators (42%, see previous paragraph).
Based on data from the rheumatologist evaluation, 33 of the 37 patients with confirmed diagnosis of SpA fulfilled the ASAS classification criteria for axial SpA (89%). The imaging arm criteria (sacroiliitis on imaging and at least one SpA feature) were met by 22 patients, whereas the clinical arm criteria (positive HLA-B27 result and at least two SpA features) were met by 11 patients.
Overall, a new diagnosis of axial SpA was confirmed by the rheumatologist for about one-third of the referred patients.
Discussion
In the SUSPECT study, 117 patients were referred to a rheumatologist (73% of the 161 enrolled patients) and diagnosis was confirmed for 37 patients, i.e., 23% of the enrolled patients and 32% of the referred patients. This proportion is similar to the prevalence observed in the literature [2, 8, 20, 22,23,24,25,26,27]. The primary healthcare professionals involved in the SUSPECT study were not general practitioners, but mainly PMR physicians and, to a lesser extent, orthopedists and ophthalmologists. Recently it was suggested that increasing awareness of SpA in primary healthcare professionals should not only focus on general practitioners but also target physicians who might encounter patients with potential extra-articular manifestations such as inflammatory bowel disease, psoriasis, or uveitis in their daily practices [28]. Our study shows that this is a valid point, underscoring the role of PMR physicians, orthopedists, and ophthalmologists in early diagnosis of SpA.
The fact that about 40% (15/37) of the patients with confirmed diagnosis did not meet the referral criteria proposed in the SUSPECT protocol suggests that these criteria (four of five IBP criteria and one additional SpA feature) may be too stringent. Several studies have questioned the use of IBP as the main clinical feature in the diagnosis of axial SpA [11, 24] due to the specificity of IBP with regards to its low prevalence among chronic back pain patients [6]. Shortly after the end of the study ASAS published recommendations for the early referral of patients with suspicion of axial SpA [29]: chronic back pain with onset before 45 years of age was retained as entry criterion; and IBP, along with other axial SpA characteristics (HLA-B27 positive, sacroiliitis on imaging, peripheral and/or extra-articular manifestation, positive family history for SpA, good response to NSAIDs and elevated acute phase reactants) was named as one of the additional parameters, which should lead to referral in those patients. This was defined in line with the ASAS classification criteria in which IBP was not proposed as a mandatory entry criterion but as a SpA feature. IBP is, and should remain, a key characteristic for screening patients in primary care settings; however, primary healthcare professionals should keep in mind that absence of IBP should not exclude a diagnosis of SpA [30]. This seems to be the case in the current study, where two-thirds of the patients with confirmed diagnosis that did not meet the referral criteria (10/15) did not meet the IBP criteria (presence of only three of the IBP symptoms). Overall, all the referred patients would have met the referral criteria as proposed by the recently published ASAS recommendation [29].
When the ASAS classification criteria for axial SpA were applied, 89% of the patients with confirmed diagnosis (33/37 patients) were classified as having axial SpA: one-third via the clinical arm of the ASAS criteria, which shows the importance of this arm for early diagnosis of axial SpA. These results are more or less in line with the results observed in 2 cohort studies, the DESIR and the SPACE cohorts [31, 32], which reported that 40% and 50% of patients, respectively, met the clinical arm criteria.
This study had several limitations; therefore, the results should be interpreted with caution. They are, however, mostly supportive of the current literature and informative for any healthcare professional dealing with early diagnosis of SpA. Because of the non-interventional nature of the study, it was not mandatory for the orthopedist, PMR physician or ophthalmologist to refer all patients included in the study to a rheumatologist. This might have led to a selection bias in the estimation of axial SpA in patients with chronic back pain. The fact that not all patients referred to a rheumatologist satisfied the referral criteria as stipulated in the protocol (44%) introduces another bias that may have complicated the interpretation of the sensitivity and specificity of the referral criteria used. This fact, combined with the limited number of patients included in the study, led to the decision to not compute the sensitivity and specificity of the referral criteria. Another limitation was that feedback from the rheumatologist was only received from 85 patients out of the 117 patients who were referred. It could be suggested that the rheumatologists provided feedback more readily in the case of a patient diagnosed with SpA, which may have led to another bias in the estimation of the prevalence of SpA.
Conclusions
The high percentage of axial SpA in patients with chronic back pain visiting PMR physicians, orthopedists, and ophthalmologists in this study suggests that these healthcare professionals may play a key role in shortening the delay between the first symptoms and the formal diagnosis of axial SpA. The fact that approximately 40% of the patients with a confirmed diagnosis did not meet the referral criteria of the study (four of five IBP criteria and one additional SpA feature) suggests that these criteria may be too stringent. When the ASAS classification criteria for axial SpA were applied, 89% of the diagnosed patients were classified as having axial SpA, with approximately one-third of patients diagnosed according to the clinical arm of the ASAS criteria.
Early diagnosis of SpA in patients with chronic back pain could be improved by appropriate education and information on axial SpA for non-rheumatologists. Overall, the recent developments that allow earlier diagnosis, namely, the detection of inflammation signs with MRI, the set-up of referral strategies (of which the SUSPECT study is a part), as well as the development of new drug therapies may make a positive difference in the diagnosis and early management of patients with axial SpA.
References
Kaplan W, Wirtz VJ, Mantel-Teuwisse A, Stolk P, Duthey B, Laing R. Priority medicines for Europe and the World 2013 update, WHO Library Cataloguing-in-publication data, ISBN 978 92 4 150575 8. 2013 [Internet. Accessed September 2015]. Available from http://www.who.int/medicines/areas/priority_medicines/MasterDocJune28_FINAL_Web.pdf?ua=1.
Van Hoeven L, Luime J, Han H, Vergouwe Y, Weel A. Identifying axial spondyloarthritis in Dutch primary care patients, ages 20–45 years, with chronic low back pain. Arthritis Care Res. 2014;66:446–53. doi:10.1002/acr.22180.
Jois RN, Macgregor AJ, Gaffney K. Recognition of inflammatory back pain and ankylosing spondylitis in primary care. Rheumatology. 2008;47:1364–6. doi:10.1093/rheumatology/ken224.
O’Shea FD, Boyle E, Salonen DC, et al. Inflammatory and degenerative sacroiliac joint disease in a primary back pain cohort. Arthritis Care Res. 2010;62:447–54. doi:10.1002/acr.20168.
Shaikh A. Ankylosing spondylitis: recent breakthroughs in diagnosis and treatment. J Can Chiropr Assoc. 2007;51:249–60.
Rudwaleit M, Taylor WJ. Classification criteria for psoriatic arthritis and ankylosing spondylitis/axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2010;5:589–604. doi:10.1016/j.berh.2010.05.007.
Costantino F, Talpin A, Said-Nahal R, et al. Prevalence of spondyloarthritis in reference to HLA-B27 in the French population: results of the GAZEL cohort. Ann Rheum Dis. 2013;74:689–93. doi:10.1136/annrheumdis-2013-204436.
Sieper J, Srinivasan S, Zamani O, et al. Comparison of two referral strategies for diagnosis of axial spondyloarthritis: the Recognising and Diagnosing Ankylosing Spondylitis Reliably (RADAR) study. Ann Rheum Dis. 2013;72:1621–7. doi:10.1136/annrheumdis-2012-201777.
Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 2005;4:1000–8.
Poddubnyy D, Sieper J. Similarities and differences between nonradiographic and radiographic axial spondyloarthritis: a clinical, epidemiological and therapeutic assessment. Curr Opin Rheumatol. 2014;26:377–83. doi:10.1097/BOR.0000000000000071.
Sieper J, Rudwaleit M, Khan MA, Braun J. Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol. 2006;20:401–17.
Rudwaleit M, Landewe R, van der Heijde D, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68:770–6. doi:10.1136/ard.2009.108217.
Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II). Ann Rheum Dis. 2009;68:777–83. doi:10.1136/ard.2009.108233.
Sieper J, Rudwaleit M, Baraliakos X, et al. The assessment of spondyloarthritis international society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68:ii1–44. doi:10.1136/ard.2008.104018.
Sieper J, Braun J. How important is early therapy in axial spondyloarthritis? Rheum Dis Clin North Am. 2012;38:635–42. doi:10.1016/j.rdc.2012.08.001.
van der Heijde D, Sieper J, Elewaut D, Deodhar A, Pangan AL, Dorr AP. Referral patterns, diagnosis, and disease management of patients with axial spondyloarthritis: results of an international survey. J Clin Rheumatol. 2014;20:411–7. doi:10.1097/RHU.0000000000000180.
Sieper J, van der Heijde D, Landewé R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the assessment of spondyloarthritis international society (ASAS). Ann Rheum Dis. 2009;68:784–8. doi:10.1136/ard.2008.101501.
Boonen A, Sieper J, van der Heijde D, et al. The burden of non-radiographic axial spondyloarthritis. Boonnen Semin Arthritis Rheum. 2015;44:556–62. doi:10.1016/j.semarthrit.2014.10.009.
Van Onna M, Gorter S, van Meerendonk A, van Tubergen A. General practitioners’ perceptions of their ability to identify and refer patients with suspected axial spondyloarthritis: a qualitative study. J Rheumatol. 2014;41:897–901. doi:10.3899/jrheum.131293.
Brandt HC, Spiller I, Song IH, Vahldiek JL, Rudwaleit M, Sieper J. Performance of referral recommendations in patients with chronic back pain and suspected axial spondyloarthritis. Ann Rheum Dis. 2007;66:1479–84. doi:10.1136/ard.2006.068734.
Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis. J Nat Rev Rheumatol. 2012;8:262–8. doi:10.1038/nrrheum.2012.39.
Sieper J, Rudwaleit M. Early referral recommendations for ankylosing spondylitis (including pre-radiographic and radiographic forms) in primary care. Ann Rheum Dis. 2005;64:659–63. doi:10.1136/ard.2004.028753.
Hermann J, Giessauf H, Schaffler G, Ofner P, Graninger W. Early spondyloarthritis: usefulness of clinical screening. Rheumatology. 2009;48:812–6. doi:10.1093/rheumatology/kep119.
Braun A, Saracbasi E, Grifka J, Schnitker J, Braun J. Identifying patients with axial spondyloarthritis in primary care: how useful are items indicative of inflammatory back pain? Ann Rheum Dis. 2011;70:1782–7. doi:10.1136/ard.2011.151167.
Poddubnyy D, Vahldiek J, Spiller I, et al. Evaluation of 2 screening strategies for early identification of patients with axial spondyloarthritis in primary care. J Rheumatol. 2011;38:2452–60. doi:10.3899/jrheum.110070.
Van Praet L, Bertin J, François D, De Brabanter G, Poriau S, Mielants H. Vroegtijdige verwijzing van patienten met een vermoeden van axiale spondylartropathie in eerste lijn: de Belgische resultaten van de RADAR-studie. Tijschr Voor Gneeskunde. 2012;. doi:10.2143/TVG.68.00.2000000.
Bentin J, Van Praet L, Malaise M, François D, Mielants H. Early referral of first line patients suspected of axial spondyloarthritis: the Belgian results of the RADAR study. Rev Med Liege. 2012;67:649–54.
Wallis D, Inman RD. Recognition of preclinical and early disease in axial spondyloarthritis. Rheum Dis Clin North Am. 2014;40:685–97. doi:10.1016/j.rdc.2014.07.011.
Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D, Assessment of SpondyloArthritis International Society (ASAS). Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483–7. doi:10.1136/annrheumdis-2014-207151.
Van Den Berg R, de Hooge M, Rudwaleit M, et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE)-cohort and from the Assessment of SpondyloArthritis international Society (ASAS)-cohort. Ann Rheum Dis. 2013;72:1646–53. doi:10.1136/annrheumdis-2012-201884.
Moltó A, Paternotte S, van der Heijde D, Claudepierre P, Rudwaleit M, Dougados M. Evaluation of the validity of the different arms of the ASAS set of criteria for axial spondyloarthritis and description of the different imaging abnormalities suggestive of spondyloarthritis: data from the DESIR cohort. Ann Rheum Dis. 2014;74:746–51. doi:10.1136/annrheumdis-2013-204262.
Van Den Berg R, de Hooge M, van Gaalen F, Reijnierse M, Huizinga T, van der Heijde D. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology. 2013;52:1492–9. doi:10.1093/rheumatology/ket164.
Acknowledgements
Sponsorship for this study and article processing charges was funded by AbbVie, Wavre, Belgium. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The authors would like to acknowledge Marie-Paule Derde of DICE nv. for the statistical analysis and Marie-Anne Thil and Kirsten Dumaz from Keyrus Biopharma (Belgium) for assistance in medical writing. Support for this assistance was funded by AbbVie, Wavre, Belgium. AbbVie was responsible for the study design, data collection, analysis, and interpretation of the data, and for the writing, review, and approval of the publication. All authors have access to the study data and contributed to the development of the publication; the authors maintained control over the final content. The data have been presented as oral presentation or poster on the following occasions: Oral presentation at the ‘Annual Belgian Congress of Rheumatology’, Brussels, 24–26 September 2014, Oral presentation at the ‘Annual Congress of the Belgian Society of Physical and Rehabilitation Medicine’, Leuven, 5–6 December 2014, Poster at the ‘9th International Congress on Spondyloarthropathies’, Gent, 23–25 October 2014.
Disclosures
Dr. Laure Tant has received research grants and consulting fees, and has given occasional conferences as a speaker for the following companies: AbbVie Inc., Pfizer, MSD, UCB, BMS, Roche, Celgene and Novartis. Prof. Dr. Herman Mielants has received research grants and consulting fees, and has served on speakers bureaus on behalf of AbbVie Inc., Pfizer and UCB. Prof. Valerie Gangji has received research grants and consulting fees, and has served on speakers bureaus on behalf of AbbVie Inc., Roche, MSD and Bone Therapeutics. Nadine Delmotte is an employee of AbbVie Inc. and may own stock or stock options. Dr. Maria Van den Enden is an employee of AbbVie Inc. and may own stock or stock options.
Compliance with Ethics Guidelines
The SUSPECT study was approved by the ethics committee of Erasme Hospital (Brussels, Belgium) and conducted according to local regulations. It has therefore been performed in accordance with the ethical standard laid down in the 1964 declaration of Helsinki and its later amendments. All patients gave written informed consent prior to their enrollment in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/5D47F06069E7B40D.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tant, L., Delmotte, N., Van den Enden, M. et al. High Prevalence of Undiagnosed Axial Spondyloarthritis in Patients with Chronic Low Back Pain Consulting Non-Rheumatologist Specialists in Belgium: SUSPECT Study. Rheumatol Ther 4, 121–132 (2017). https://doi.org/10.1007/s40744-016-0051-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-016-0051-1