Abstract
Introduction
The present study aimed to assess disease control, health resource utilization (HRU), and healthcare costs, and their predictors in gout patients across the USA, UK, Germany, and France.
Methods
Data were extracted from the PharMetrics Plus (USA), Clinical Practice Research Datalink–Hospital Episode Statistics (UK), and Disease Analyzer databases (Germany and France) for adult gout patients over a 3-year period: 2009–2011 (all dates +1 year for France). Patients had “prevalent established gout” (i.e., were treated with urate-lowering therapy [ULT] or eligible for ULT based on American College of Rheumatology guidelines) in the preindex panel-year, with January 1 of the second study year as the study index date. Assessments of disease control (uncontrolled gout definition: ≥1 serum urate (sUA) elevation or ≥2 flares; analysis limited to the subpopulation with sUA) data, HRU, and costs were in the second post-index panel-year, while potential predictors (demographics and gout treatment characteristics) were identified in the first post-index panel-year.
Results
Treatment rates were high (>70% with chronic urate-lowering treatment in all countries but France), while between 31.3% (France) and 62.9% (USA) of patients remained uncontrolled. Predictors of control included female gender and high adherence. In Germany, the UK, and France, lack of disease control predicted increased gout-attributed costs and increased HRU, both gout-attributed (also in the USA) and non-gout-attributed.
Conclusion
Gout management remains suboptimal, as many patients remain uncontrolled despite using urate-lowering treatment. Effective and convenient treatment options are needed to improve disease control and minimize additional HRU and costs.
Funding
AstraZeneca.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uncontrolled gout is a debilitating medical condition resulting from monosodium urate (MSU) crystal deposition throughout the body, manifesting as recurrent attacks of acute inflammatory arthritis of the peripheral joints. Gout affects about 1–4% of the population in Western developed countries, and is more prevalent in men [1–3]. The hallmark precursor to gout is hyperuricemia, defined as serum urate (sUA) levels >6.8 mg/dl (≈400 µmol/l); this predominantly results from inefficient renal uric acid excretion, rather than overproduction [4, 5]. Clinical diagnosis of gout is confirmed by the presence of characteristic MSU crystals in the joint fluid [2, 6]. While there is evidence of familial clustering in gout, risk factors include cardiovascular/metabolic diseases (e.g., obesity, arterial hypertension, diabetes, hypercholesterolemia, and renal failure) and menopause, as well as diets rich in purines, alcohol consumption, and thiazide diuretic use [4].
Management of gout encompasses both short-term control of acute attacks and long-term treatment to reduce sUA, thereby dissolving MSU crystals and preventing further acute manifestation of flares [4]. Treatment of acute attacks involves use of colchicine, nonsteroidal anti-inflammatory drugs (NSAID), or corticosteroids [7, 8]. At the first flare, dietary and lifestyle modifications are advised to prevent recurrence. For long-term management of gout, European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) treatment guidelines recommend urate-lowering therapies (ULTs) to decrease sUA to <6 mg/dl, while target levels <5 mg/dl are recommended for patients with recurrent acute attacks, tophi, or radiographic gout changes [7, 8]. British Society of Rheumatology guidelines recommend target sUA <5 mg/dl [9]. All guidelines recommend xanthine oxidase inhibitors (e.g., allopurinol, febuxostat), which inhibit uric acid production, as first-line therapy. When xanthine oxidase inhibitors are contraindicated or fail to achieve sUA targets, the addition or use of a uricosuric agent (e.g., probenecid, benzbromarone), which increases renal excretion of uric acid, is recommended [8]. Unfortunately, there is widespread evidence that patients with gout are not treated according to these guidelines and therefore their gout remains poorly controlled [10–13].
Gout progression can cause permanent joint destruction, bone erosion, and organ damage if hyperuricemia is left uncontrolled [14]. In addition to causing pain, disability, and diminished quality of life, poorly controlled gout is associated with significantly higher healthcare costs and loss in productivity [15–17]. In a US prospective study, patients with frequent gout attacks had a higher prevalence of comorbidities (chronic kidney disease, hypertension, dyslipidemia, ischemic heart disease, heart failure, and arthritis) than those with infrequent attacks [18]. They also had higher mean numbers of all-cause and gout-attributed outpatient and emergency department visits, as well as substantially greater healthcare costs than those with infrequent attacks [18]. Another study found higher outpatient, emergency, and inpatient services utilization among patients with gout than matched non-gout patients [19]; all-cause healthcare costs were also higher for gout patients, and increased with increasing sUA. Overall, however, data on the health and cost burden associated with gout are scarce. Moreover, significant proportions of patients continue to experience elevated sUA, recurrent flares, and tophi despite ULT [20, 21].
By analyzing data extracted from electronic medical record (EMR) and administrative claims databases, our study sought to investigate large populations of gout patients in the USA, UK, Germany, and France. Our objectives were: to assess the rate of uncontrolled gout and identify predictors of disease control (including ULT characteristics) in these populations; to estimate health resource utilization (HRU) and healthcare costs in that patient group; and to identify predictors of HRU and healthcare costs.
Methods
Data Sources
This study investigated gout patients in the USA, UK, Germany, and France using retrospective healthcare data extracted from EMR and administrative claims databases: US IMS PharMetrics Plus database [22–24]; UK Clinical Practice Research Database (CPRD) and Hospital Episode Statistics database [25, 26]; and IMS Disease Analyzers in Germany [27, 28] and France [29, 30], respectively. Details on these databases are in supplemental Table S1.
Study Design
This was an observational cohort study of established gout patients in four countries. For the USA, UK, and Germany, the study period ran from January 1, 2009 to December 31, 2011. All study objectives were assessed through a longitudinal panel design, where the study period was divided into fixed time periods of 1 calendar year (i.e., panel-years) (Fig. 1). This design was employed due to the dependence of analysis on various time-varying constructs (e.g., disease control, resource use, or cumulated costs) requiring measurement over defined time periods. The index-date was January 1, 2010. The 12-month period immediately preceding the index-date was defined as the preindex panel-year and was used to identify eligible patients and determine baseline characteristics. Treatment characteristics and predictors of the main outcomes (disease control status, HRU, costs) were assessed in the first post-index panel-year (full year 2010), while outcomes were assessed in the second post-index panel-year (full year 2011). For France, the same procedures were used, but the timeline was moved forward by 1 year to synchronize the study window with information collected through an observational study (only available for 2012), which was conducted in a subset of general practitioners/primary care physicians (GPs/PCPs) and included additional information on hospitalizations and laboratory results.
Patient Selection
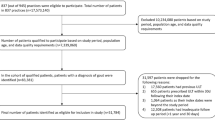
In all four countries, the study population consisted of adult patients (≥18 years at index-date) identified with established gout—i.e., receiving ULT or eligible for ULT according to ACR guidelines [8]—during the course of the preindex panel-year. ACR criteria were based on: a documented diagnosis code for gout or a prescription for colchicine or a colchicine combination; and a diagnostic code for moderate chronic kidney disease, urolithiasis, or tophus or the occurrence of two gout flares. Tophus coding was based on the International Classification of Diseases (ICD)-9 for US data; ICD-10 for German, French and UK hospital data; and Read codes for UK primary care data. Eligible patients were additionally required to be present in the database during the full 3-year period covered by the study. Patients with hematologic cancer, severe renal impairment (per diagnoses or laboratory values [estimated creatinine clearance <30 ml/min]), tumor lysis syndrome, or Lesch–Nyhan syndrome documented preindex were excluded.
For all analyses involving disease control status, the analysis population was limited to those with ≥1 sUA measurement during the period of assessment of control status.
Definition of Disease Control Status
Among those with ≥1 sUA measurement during the period of assessment of control status, a defined control status over the course of a panel-year was determined as follows: gout was considered controlled if no sUA elevation (>6 mg/dl), no diagnosis code for tophus, or no flare was documented, and as uncontrolled if ≥2 flares or a sUA elevation was reported. Control status was assessed in the second post-index panel-year and its predictors were identified in the first post-index panel-year; control status was also assessed in the first post-index panel-year as a potential predictor in different multivariate models. Remaining cases (e.g., one flare without sUA elevation) were labeled as “undefined control status”. Gout flare occurrence was defined by an office visit or hospitalization with a diagnosis of gout, followed by prescription of NSAID, colchicine, oral corticosteroid, or interleukin-1 antagonist within 3 days; or by an office visit or hospitalization with a diagnosis of joint pain, followed by prescription of colchicine within 3 days [31, 32].
Definition of Treatment Characteristics
Medications of interest in the context of this study were ULTs—xanthine oxidase inhibitors (allopurinol, febuxostat, or any combination including allopurinol or febuxostat), uric acid metabolism catalysts (pegloticase), and uricosuric agents (probenecid or sulfinpyrazone).
-
Patients were considered “chronic ULT-treated” if they had been continuously exposed to ULT for ≥60 consecutive days over the panel-year, regardless of the number of prescriptions or type of ULT. Discontinuation was defined as a gap of >50% of the days’ supply of the last prescription (starting from the end date of the supply in the last prescription).
-
Patients prescribed a ULT during the course of the panel-year but who did not qualify as chronic ULT-treated were categorized as patients “with less than 60 consecutive days’ supply of ULT” and reported as a distinct category.
-
Patients without a prescription for a ULT during the panel-year were categorized as “untreated patients”.
Persistence with ULT within each panel was defined as the number of consecutive days on any ULT, from treatment initiation until the first observed defined gap in days’ supply during the follow-up period (discontinuation) or the end of the panel, whichever occurred first. Adherence to ULT was calculated as persistence divided by the number of days in the panel (i.e., 365).
Identification of HRU
All healthcare resources utilized over the course of the second post-index panel-year were identified and split between gout-attributed and non-gout-attributed HRU. All visits or hospitalizations associated with a diagnosis of gout or joint pain were attributed to gout. Gout-attributed laboratory services included all sUA tests. Gout-attributed pharmacy services included all prescriptions for gout-related medications (i.e., ULT, anti-inflammatories, and colchicine). Non-gout-attributed utilization included all other outpatient, inpatient, and pharmacy services.
Country-specific limitations inherent to databases hindered collection of exactly the same information in all four countries; details are available in supplemental Table S1.
Valorization of HRU
For the USA, healthcare costs/charges associated with utilization were determined by the allowed amount (the amount the health plan allows for a particular service, including the paid amount plus any member liability), as documented in the PharMetrics data. For the remaining countries, costs were not available directly from databases, but were calculated by multiplying the number of units retrieved in the database by unit costs from published sources. Cost calculation for France was restricted to the subset of patients with complementary data on hospitalizations (n = 943). All costs were converted into 2011 United States dollars (USD) using historical “purchasing power parities for gross domestic product” rates as published by the Organisation for Economic Co-operation and Development (OECD) in 2011 (UK: 1 USD = 0.6997 GBP; Germany: 1 USD = 0.7842 €; France: 1 USD = 0.8443 €) [33].
Analytical Approach
Data were analyzed using SAS software version 9 (SAS Institute, Cary, NC, USA). Chi-square tests were conducted to compare the distribution of categorical variables, while the Wilcoxon rank sum test was used for continuous variables. Multivariate models were fit separately in each country and for each outcome to identify, in the first post-index panel-year: the drivers of disease control status, number of gout-attributed and non-gout-attributed GP/PCP visits, number of non-gout-attributed hospitalizations, and total gout-attributed costs in the second post-index panel-year. To determine predictors of disease control, a logistic regression model was constructed. To determine the drivers of resource utilization, Poisson regression models were fit as the dependent variables were discrete counts of events. For drivers of gout-attributed cost, a generalized linear model using gamma distribution with log-link function was fit to adjust for the skew typically found in cost data. In all multivariate models, the dependent variable was modeled as a function of the same set of demographic (age class, sex), treatment (chronic ULT-treated, treated for <60 days, untreated, and adherence to ULT in the first post-index panel), and clinical characteristics (Charlson Comorbidity Index [CCI] and control status in first preindex panel). Regression coefficients (or their transformation, e.g., odds ratios with 95% confidence intervals [CI]) and associated P values are reported. A P value <0.05 was considered statistically significant.
Compliance with Ethics Guidelines
This article is based primarily on previously and routinely collected data in the databases used for the study, in compliance with the rules for each database. The UK part of this study was approved by the Independent Scientific Advisory Committee for MHRA database research (ISAC) under protocol number 13_134, as required for use of CPRD data. Some complementary retrospective data were collected in France from a sample of GPs participating in the French Disease Analyzer database, with approval obtained from the “CNIL” (“Commission Nationale de l’Informatique et des Libertés”, ref: MMS/MKE/AR/144351). Beyond this, the current report does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Study Population
The total number of gout patients fulfilling the main eligibility criteria for HRU and cost-descriptive analysis was 105,112 in the USA, 29,758 in the UK, 49,722 in Germany, and 13,213 in France (Table 1; Table S2). The main reason for attrition across the four countries was non-continuous observation during follow-up (please see Table S2 in the supplemental material for details). Within the overall population, the number of evaluable patients with sUA laboratory values to assess control status in the second post-index panel-year was 2560 (2.4%) in the USA, 4385 (14.7%) in the UK, 20,397 (41.0%) in Germany, and 967 (7.3%) in France (Table 1). In this subpopulation, the average (standard deviation) age at index-date was similar in the UK (64.0 years [12.8]), Germany (68.9 [11.2]), and France (68.7 [11.2]) and substantially lower in the USA (53.6 [9.8]) (Table 1). The percentage of male patients ranged between 69.7% (Germany) and 88.6% (USA). The most frequent comorbidities recorded in the preindex panel were essential hypertension and hyperlipidemia. The total cohorts and the sUA cohorts were similar across characteristics (Table 1).
Description of Treatment Patterns in the First Post-index Panel-Year
A majority of patients in the total eligible population (i.e., not limited to patients with evaluable control status) were chronic ULT-treated during the first post-index panel-year (USA: 70.9%; UK: 86.3%; Germany: 81.6%), with the exception of France, where the percentage was 14.3% (Table 1), although the proportion of patients with some ULT, but not fulfilling defined criteria for chronic treatment due to gaps or short treatment, was highest in France: 32.7% (USA: 10.3%; Germany: 4.2%; UK: 3.1%). Conversely, the proportion of entirely untreated patients was 18.8% in the USA, 10.6% in UK, 13.9% in Germany, and 53.0% in France.
Among treated patients, most received only one ULT during the panel-year (USA: 83.3%; UK: 99.7%; Germany: 99.4%; France: 99.8%), with allopurinol the most commonly administered (USA: 89.5%; UK: 99.3%; Germany: 97.6%; France: 90.8%). The average daily allopurinol dose was 240.0 mg in the USA, 238.9 mg in UK, 253.8 in Germany, and 186.6 mg in France. A low percentage of patients received an average daily allopurinol dose >300 mg (USA: 1.4%; UK: 4.4%; Germany: 0.5%; France: 0.4%). Febuxostat was the second most commonly prescribed ULT in the USA (9.5%) and France (9.2%), but was seldom prescribed in the UK (0.2%) and Germany (0.4%). Other ULTs included sulfinpyrazone in the UK (0.7%) and probenecid or fixed allopurinol/benzbromarone combinations in Germany (both 1.3%). Treatment adherence to any ULT, measured in treated patients over the course of the whole panel, was 63.9% in the USA, 84.3% in UK, 69.0% in Germany, and 24.8% in France.
Rate of Uncontrolled Gout in the Second Post-index Panel-Year
Within the population with evaluable control status in the second post-index panel-year, the proportion of patients with uncontrolled gout was 62.9% in the USA (controlled: 32.7%; undefined: 4.4%), 55.8% in UK (41.7; 2.5%), 62.0% in Germany (36.8; 1.2%), and 31.3% in France (66.4; 2.3%). The proportion of patients with evidence of available sUA testing who had ≥1 elevated sUA (≥6 mg/dl) was 51.5% in the USA, 32.7% in UK, 42.3% in Germany, and 30.4% in France, the remaining uncontrolled patients being identified by occurrence of ≥2 flares. Tophi were documented in <0.1% of patients across the four countries. The rate of uncontrolled gout was consistently higher in patients untreated during the previous panel than in chronic ULT patients (USA: 77.5% vs. 55.4%; UK: 93.1% vs. 49.7%; Germany: 84.2% vs. 56.7%; France: 33.2% vs. 32.9%).
Multivariate logistic regression among the patients with defined control status in the second post-index panel-year showed that in the USA, UK, and Germany, the following characteristics in the first post-index year were associated with higher probability of being controlled in the second post-index year: female gender, chronic ULT-treated, and >80% adherent to ULT in the previous panel-year, as well as having fewer comorbidities reflected by CCI score (Table 2). In the USA and Germany, the probability of being controlled increased with age. The model could not be evaluated for France, due to the low number of patients with defined control status, resulting in non-convergence of the multivariate model (Table 2).
HRU in the Second Post-index Panel-Year
The proportion of patients in the total eligible population with ≥1 non-gout-attributed/gout-attributed GP/PCP visit, respectively, was 58.8/28.2% in the USA, 99.2/7.8% in UK, 97.9/0.5% in Germany, and 96.4/7.0% in France (Table 3). The proportion of patients with gout-attributed GP/PCP visits was consistently higher in uncontrolled than controlled patients (USA: 43.0% vs. 37.3%, P = 0.011; UK: 17.9% vs. 4.6%, P < 0.001; Germany: 0.7% vs. 0.2%, P < 0.001; France: 10.4% vs. 5.0%, P = 0.003).
The proportion of patients with ≥1 non-gout-attributed hospitalization was 10.0% in the USA, 27.2% in UK (calculated on the patient subset with linked hospitalization data, i.e., 60.4% of total eligible population), 12.6% in Germany, and 10.3% in France (calculated on the patient subset with additional hospitalization data; n = 943) (Table 3). About 31.2% of US patients and 4.6% of UK patients consulted a specialist for a gout-attributed reason (Table 3).
The multivariate analysis of HRU among the patients with defined control status in the first post-index panel-year showed that when adjusting simultaneously for demographics, treatment, and clinical characteristics, the significant predictors most frequently associated with higher numbers of non-gout-attributed GP/PCP consultations in the second post-index panel-year were older age (USA, UK, Germany), female gender (all countries), uncontrolled in the previous panel-year (UK, Germany, France), chronic ULT-treated in the previous panel-year (UK, Germany, France), and having higher CCI score (all countries) (Table 4). Similarly, a higher CCI score in the previous panel-year was associated with higher numbers of non-gout-attributed hospitalizations (USA, UK, Germany). Finally, more gout-attributed consultations with a GP/PCP were likely in patients with the following characteristics: being older (Germany, France), having a higher CCI score in the previous panel-year (USA, France), being uncontrolled in the previous panel-year (USA, UK, Germany, France), and not being ULT-treated in the previous panel-year (UK, Germany) (Table 4).
Healthcare Costs in the Second Post-index Panel-Year
The average all-cause healthcare cost per patient, expressed as 2011 USD and calculated over of the whole panel-year, was $13,514 in the USA, $2620 in UK, $1671 in Germany, and $1463 in France. Gout-attributed costs were lower than non-gout-attributed costs in all four countries (Table 3).
Based on multivariate analysis, patient characteristics resulting in higher gout-attributed costs were CCI score in the previous panel-year (USA, UK, Germany), being uncontrolled in the previous panel-year (UK, Germany, France), and being chronic ULT-treated in the previous panel-year (USA, Germany) (Table 4).
Discussion
Despite the high rate of ULT in the study population, >50% of patients with evaluable control status (i.e., with available sUA assessments) in all four countries remained uncontrolled, suggesting inadequacy of gout management in the real-world setting. The study also revealed poor compliance to treatment guidelines. Average allopurinol doses were below 300 mg in each country (most notably in France, at 186.6 mg), despite guideline recommendations that the dose can be advanced to 300 mg daily and above for those without renal impairment, in order to achieve target sUA in a substantial proportion of patients. Suboptimal dosing of allopurinol is recognized to be a common issue in the management of gout worldwide [13, 34, 35]. In addition, whereas EULAR and ACR guidelines recommend treating to a target sUA, including continuing measurements once the sUA target is achieved (every 6 months), the high percentage of patients with no sUA data—even in countries where all laboratory values were included in the data (Germany, UK)—clearly indicates that many patients are maintained on ULT without reassessment of sUA control.
In some cases (Germany, UK, France), lack of disease control resulted in increased utilization of healthcare resources (both gout-attributed and non-gout-attributed) and increased gout-attributed costs. In addition, it should be reiterated that the proportion of patients with gout-attributed GP/PCP visits was consistently and significantly higher in uncontrolled than controlled patients across all four countries. These findings suggest that in patients with established gout who received ULT treatment, longer persistence and higher adherence to ULT were associated with better control; however, this is only generalizable to the minority of patients with sUA testing. Overall, non-gout-attributed healthcare utilization and costs were higher than gout-attributed healthcare utilization and costs. This finding agrees with other studies assessing the economic burden of gout. Rai et al. [36] identified five studies reporting all-cause direct costs associated with gout patients; depending on the subpopulation studied, the all-cause annual direct costs ranged from $4733 (employed patients) to $18,362 (treatment-refractory patients), while gout-attributed costs ranged from $172 to $6179 across studies.
The assessment of disease control presented here must be viewed in light of the limitations in assessing clinical measures with retrospective data. First, the definition of controlled gout was met if there was no sUA elevation (>6 mg/dl), no diagnosis code for tophus, and no flare documented, while uncontrolled gout was defined by ≥2 flares or sUA elevation. As described below, in practice, the contribution of the “tophus” component of the definition of gout status was minimal, as tophi were under-documented. Incidences of flares can be reduced by prophylactic medications as well as ULT. However, guidelines recommend using prophylaxis for up to 6 months after initiation of ULT, while disease control in our study was assessed following the preindex 12-month period in established gout patients.
The reliability of gout diagnosis within databases in general represents a potential limitation seen for the majority of rheumatic diagnoses [37]. However, such differences are likely an artifact of comparing against disease definitions established to evaluate patients prospectively in a clinical setting or using epidemiologic surveys [38]. It is likely that the rate of uncontrolled disease in the overall population was underestimated, since uncontrolled gout was assessed through a composite endpoint including elevated sUA measurements, occurrence of flares, and tophi, each of which is subject to data-related limitations in estimations. Under-reporting of tophi, in particular, is relevant, as previous work has shown that patients use more resources when tophi are present [39, 40].
For the USA and France, sUA data were obtained from an external data source for only a subset of eligible patients, resulting in relatively low percentages of patients with evaluable control status in the second post-index panel-year (2.4% and 7.3%). In addition, the group in France on chronic ULT was very small (102 patients), and the uncertainty around sUA estimates was high, reflected in the very wide CI around the estimated effect of treatment on control status, odds ratio 1.02 (95% CI 0.06; 17.55) for treated versus not treated. Even in the UK and Germany—where all laboratory results were included in the main database—the low level of testing did not allow systematic evaluation of control status. Due to this limitation, all analyses involving gout control status were restricted to patients with available sUA data, and this additional eligibility criterion could have resulted in overestimation of the rate of uncontrolled gout in this subpopulation, since patients with suspected sUA elevation may have been more likely to be tested. Also, there is no specific diagnosis code for flares; consequently, identification of flares was based on an algorithm requiring a specific outpatient visit or hospitalization while, in the real world, many flares might be self-treated and therefore remain undetected in primary care databases.
The specificities of the various data sources used for this study should be taken into account when comparing results across the four countries. For instance, PharMetrics Plus is a claims database consisting of commercially insured working adults; this resulted in a US study population younger—and with potentially less severe gout—than in the other countries. The prevalence of chronic morbidities (especially hypertension and diabetes) was relatively low in the UK versus other published prevalence rates [41] or versus prevalence rates observed for instance in the German or US populations; one possible explanation resides in the specificity of the British National Health Service, where the GP/PCP plays a role of gatekeeper. Over the course of the patient’s affiliation to a practice, the data are centralized at the GP/PCP office; consequently, chronic diseases are coded when they first occur (or at the first visit if the patient is new to the practice) and are less likely to be systematically recoded at each new visit, and may consequently be missed when the look back period is limited to 1 year. A similar bias was observed in the French data, and to some extent probably affects also the German and US data due to the short look back period. The varying level of sUA data availability also resulted in cross-country variation in the assessment of disease control status.
Several data-related factors may also explain the high between-countries variability in estimates of resource utilization and related costs. In particular, the average number of GP/PCP consultations reported in the UK was much higher than in the USA, Germany, and France, because the CPRD data document all contacts between the patient and the practice (i.e., including phone calls or prescription renewals handled by a nurse); however, the valorization of consultations was made taking this into account and applying distinct unit costs to the different types of consultation.
The cost estimates, both gout- and non-gout-attributed, were in a higher range in the USA, which is because the billing information related to all healthcare services was a primary purpose of and directly available from the claims database, while costs had to be obtained from external sources in the other countries. Compared with the USA, the European databases also lacked some health-related data, likely contributing to underestimation of costs. In France and Germany, limited information (if any) was available on visits to specialists. Hospitalization data were only partially available in European countries, i.e., indirectly (from referrals, so only elective hospitalizations could be captured) in Germany and for a subset of patients in the UK and France; this resulted in low hospitalization rates, low counts of gout-attributed hospitalizations, and low associated cost estimates. More generally, the algorithms used to identify gout-attributed resources were very conservatively defined; also, they were very sensitive to attribution issues resulting from possible misclassification. Finally, even when data allowed for coding of diagnoses, the diagnosis of gout appeared to be underreported, as evidenced by the high number of patients receiving ULT with no diagnosis of gout documented in the same record. All this contributed to low counts of gout-attributed HRUs and possibly underestimated gout-attributed costs.
Conclusion
Despite the limitations, the study provides important new evidence on large patient populations in four countries indicating that current management of gout is consistently suboptimal in terms of sUA monitoring and treatment options. As a consequence, an important proportion of patients remain uncontrolled, even while treated with high-dose ULT, resulting not only in the symptomatic sequelae of continued flares and tophi, but also in continued subclinical urate crystal deposition. Additional effective and convenient treatment options are needed for these patients to improve disease control and minimize healthcare utilization and costs.
References
Soriano LC, Rothenbacher D, Choi HK, Rodriguez LAG. Contemporary epidemiology of gout in the UK general population. Arthritis Res Ther. 2011;13:R39–47.
Zhang W, Doherty M, Pascual E, et al. EULAR evidence based recommendations for gout. Part I: diagnosis. Report of a task force of the standing committee for international clinical studies including therapeutics (ESCISIT). Ann Rheum Dis. 2006;654:1301–11.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41.
Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223–33.
Neogi T. Gout. N Engl J Med. 2011;364:443–52.
Annemans L, Spaepen E, Gaskin M, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis. 2008;67:960–6.
Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312–24.
Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447–61.
Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatol (Oxf). 2007;46:1372–4.
Juraschek SP, Kovell LC, Miller ER 3rd, Gelber AC. Gout, urate-lowering therapy, and uric acid levels among adults in the United States. Arthritis Care Res (Hoboken). 2015;67:588–92.
Perez-Ruiz F, Carmona L, Yébenes MJ, et al. An audit of the variability of diagnosis and management of gout in the rheumatology setting: the gout evaluation and management study. J Clin Rheumatol. 2011;17:349–55.
Rashid N, Coburn BW, Wu YL, et al. Modifiable factors associated with allopurinol adherence and outcomes among patients with gout in an integrated healthcare system. J Rheumatol. 2015;42:504–12.
Sarawate CA, Brewer KK, Yang W, et al. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc. 2006;81:925–34.
Sanders S, Wortmann RL. Gout. In: Imbolden JB, et al., editors. Current rheumatology diagnosis and treatment, 2nd edn. New York: McGraw Hill; 2006.
Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res. 2013;75:1–4.
Khanna PP, Nuki G, Bardin T, et al. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: Results from a cross-sectional survey. Health Qual Life Outcomes. 2012;10:117.
Singh JA, Sarkin A, Shieh M, et al. Health care utilization in patients with gout. Semin Arthritis Rheum. 2011;40:501–11.
Saseen JJ, Agashivala N, Allen RR, Ghushchyan V, Yadao AM, Nair KV. Comparison of patient characteristics and gout-related health-care resource utilization and costs in patients with frequent versus infrequent gouty arthritis attacks. Rheumatology. 2012;51:2004–12.
Wu EQ, Patel PA, Yu AP, et al. Disease-related and all-cause health care costs of elderly patients with gout. J Manag Care Pharm. 2008;14:164–75.
Becker MA, Schumacher HR, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–61.
Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of hyperuricemia and gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63.
Curtis JR, Schabert VF, Yeaw J, et al. Use of a validated algorithm to estimate the annual cost of effective biologic treatment for rheumatoid arthritis. J Med Econ. 2014;17:555–66.
Bergvall N, Lahoz R, Reynolds T, Korn JR. Healthcare resource use and relapses with fingolimod versus natalizumab for treating multiple sclerosis: a retrospective US claims database analysis. Curr Med Res Opin. 2014;30:1461–71.
Bergvall N, Petrilla AA, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ. 2014;17:696–707.
Clarson LE, Hider SL, Belcher J, Heneghan C, Roddy E, Mallen CD. Increased risk of vascular disease associated with gout: a retrospective matched cohort study in the UK Clinical Practice Research Datalink. Ann Rheum Dis. 2015;74:642–7.
Gallagher AM, van Staa TP, Murray-Thomas T, et al. Population-based cohort study of warfarin-treated patients with atrial fibrillation: incidence of cardiovascular and bleeding outcomes. BMJ Open. 2014;4:e003839.
Kostev K, Dippel F-W, Bierwirth R. Resource consumption and costs of treatment in patients with Type 1 diabetes under intensified conventional therapy under German real-life conditions. J Diabetes Sci Technol. 2013;7:736–42.
Rathmann W, Kostev K, Gruenberger JB, Dworak M, Bader G. GIani G. Treatment persistence, hypoglycaemia and clinical outcomes in type 2 diabetes patients with dipeptidyl peptidase-4 inhibitors and sulphonylureas: a primary care database analysis. Diabetes Obes Metab. 2013;15:55–61.
Grandfils N, Detournay B, Attali C, et al. Glucose lowering therapeutic strategies for type 2 diabetic patients with chronic kidney disease in primary care setting in France: a cross-sectional study. Int J Endocrinol. 2013;2013:640632.
Misery L, Ansolabehere X, Grandfils N, Georgescu V, Taieb C. Nine-year follow-up of children with atopic dermatitis by general practitioners. Dermatology. 2014;228:344–9.
Halpern R, Fuldeore MJ, Mody RR, Patel PA, Mikuls TR. The effect of serum urate on gout flares and their associated costs: an administrative claims analysis. J Clin Rheumatol. 2009;15:3–7.
Wu EQ, Forsythe A, Guérin A, Yu AP, Latremouille-Viau D, Tsaneva M. Comorbidity burden, healthcare resource utilization and cost in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther. 2012;19:157–66.
Organisation for Economic Co-operation and Development. OECD StatExtracts. PPPs and exchange rates (historical rates 2011). Available at: http://stats.oecd.org/Index.aspx?DataSetCode=SNA_Table4. Accessed February 4, 2016.
Harrold LR, Mazor KM, Negron A, Ogarek J, Firneno C, Yood RA. Primary care providers’’knowledge, beliefs and treatment practices for gout: results of a physician questionnaire. Rheumatol (Oxf). 2013;52:1623–9.
Khanna P, Khanna D, Storgard C, Baumgartner S, Morlock R. A world of hurt: failure to achieve treatment goals in patients with gout requires a paradigm shift. Postgrad Med. 2016;128:34–40.
Rai SK, Burns L, De Vera MA, Haji A, Giustini D, Choi HK. The economic burden of gout: a systematic review. Semin Arthritis Rheum. 2015;45:75–80.
Harrold LR, Saag KG, Yood RA, et al. Validity of gout diagnoses in administrative data. Arthritis Rheum. 2007;57:103–8.
Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31:1582–7.
Morlock R, Flores NM, Annunziata K, Chapnick J, Nuevo J. Economic burden of controlled gout, uncontrolled gout, and gout exacerbated by common comorbidities: results from the 2012–2013 National Health and Wellness Survey. Value Health. 2015;18:A640–1.
Rudell K, Bobula J, Fu C, et al. Comparing burden of illness of tophaceous with non-tophaceous gout patients using a large US electronic health records database. Value Health. 2015;18:A158.
Zhu Y, Pandya B, Choi H. Comorbidities for gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125:679–87.
Acknowledgments
Sponsorship for this study and article processing charges were funded by AstraZeneca. Editorial assistance in the preparation of this manuscript was provided by Bill Wolvey of PAREXEL. Support for this assistance was funded by AstraZeneca. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Robert Morlock is an employee of Ardea Biosciences, a member of the AstraZeneca Group. Pierre Chevalier is an employee of IMS Health. Laura Horne is a former employee of AstraZeneca. Javier Nuevo is an employee of AstraZeneca. Chris Storgard is an employee of Ardea Biosciences, a member of the AstraZeneca Group. Lalitha Aiyer is a former employee of Ardea Biosciences. Dionne Hines is an employee of IMS Health. Xavier Ansolabehere is an employee of IMS Health. Fredrik Nyberg is an employee of AstraZeneca.
Compliance with Ethics Guidelines
This article is based primarily on previously and routinely collected data in the databases used for the study, in compliance with the rules for each database. The UK part of this study was approved by the Independent Scientific Advisory Committee for MHRA database research (ISAC) under protocol number 13_134, as required for use of CPRD data. Some complementary retrospective data were collected in France from a sample of GPs participating in the French Disease Analyzer database, with approval obtained from the “CNIL” (“Commission Nationale de l’Informatique et des Libertés”, ref: MMS/MKE/AR/144351). Beyond this, the current report does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/E5C4F0602C22FAF9.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Morlock, R., Chevalier, P., Horne, L. et al. Disease Control, Health Resource Use, Healthcare Costs, and Predictors in Gout Patients in the United States, the United Kingdom, Germany, and France: A Retrospective Analysis. Rheumatol Ther 3, 53–75 (2016). https://doi.org/10.1007/s40744-016-0033-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-016-0033-3