Abstract
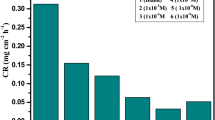
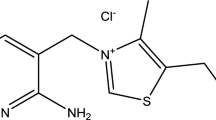
The corrosion inhibition of cupronickel alloy in 5% hydrochloric acid (HCl) was investigated in the absence and presence of diethylenetriamine (DETA) and ethylenediamine (EDA) as organic corrosion inhibitors. The effects of inhibitor concentration and temperature were investigated using mass loss method. The results obtained show that EDA act as an anticorrosion material for cupronickel alloy in hydrochloric acid with a maximum performance of 66.7%, while DETA was a poor inhibitor with maximum efficiency of 34.7%. The inhibition performance was found to increase with increase in inhibitor concentration and decrease with increase in temperature. The adsorption of both inhibitors on surface of metal was found to obey Freundlich isotherm. The values of the free energy of adsorption were below − 20 kJ/mol that is indicative of physisorption (physical adsorption). The proposed mechanism of the inhibition process suggests an adsorption of amine groups on metal surface. Mathematical models were also suggested to correlate the dissolution rate data as a function of temperature and inhibitor concentration. High correlation coefficients were obtained between experimental and predicted data. The mean value of residual between the experimental and predicted data was 0.457.






Similar content being viewed by others
References
Umoren SA, Obot IB, Igwe IO (2009) Synergistic inhibition between polyvinylpyrollidone and iodide ions on corrosion of aluminium in HCl. Open Corrosion J 2:1–7
Khadom AA (2010) Mathematical and quantum chemical studies for the corrosion inhibition of steel in HCl acid. Diyala J Eng Sci 03:106–121
Tahira MB, Abdullah MA, Nabi G, Rafique M, Sagir M (2019) Fabrication of heterogeneous photocatalysts for insight role of carbon nanofibre in hierarchical WO3/ MoSe2 composite for enhanced photocatalytic hydrogen generation. Ceram Int 45:5547–5552
Tahir MB, Sagir M, Shahzad K (2019) Removal of acetylsalicylate and methyl-theobromine from aqueous environment using nano-photocatalyst WO3-TiO2 @g-C3N4 composite. J Hazard Mater 363:205–213
Tahira MB, Abdullah MA, Nabi G, Rafique M, Sagir M (2018) Role of MoSe2 on nanostructures WO3-CNT performance for photocatalytic hydrogen evolution. Ceram Int 44:6686–6690
Sassi W, Dhouibi L, Bercot P, Rezrazi M, Triki E (2012) Effect of pyridine on the electrocrystallization and corrosion behavior of Ni–W alloy coated from citrate–ammonia media. Appl Surf Sci 263:373–381
Khadom AA (2011) Molecular Structure of Phenylthiourea as a Corrosion Inhibitor of Mild Steel in Hydrochloric Acid. Corros Sci Protect Technol 32(6):457–462
Khadom A, Musa AY, Kadhum AAH, Mohamad AB, Takriff MS (2010) Adsorption kinetics of 4-Amino-5-Phenyl-4H-1, 2, 4-Triazole-3-thiol on mild steel surface inhibitor. Port Electrochim Acta 28:221–230
Khadom A, Yaro AS, Kadhum AAH (2010) Adsorption mechanism of benzotriazole for corrosion inhibition of coppernickel alloy in hydrochloric acid. J Chil Chem Soc 55:150–152
Musa Y, Khadom AA, Kadhum AAH, Mohamad AB, Takriff MS (2012) The role of 4-amino-5-phenyl-4H-1, 2, 4- trizole-3-thiol on the inhibition of nickel-aluminum bronze alloy corrosion: electrochemical and quantum chemical studies. Res Chem Intermed 38:91–103
Yaro AS, Khadom AA, Ibraheem HF (2011) Peach juice as an anti-corrosion inhibitor of mild steel. Anti Corros Methods Mater 58(3):116–124
Poornima T, Nayak J, Nityananda SA (2011) Effect of 4-(N, N-diethylamino)benzaldehyde thiosemicarbazone on the corrosion of aged 18 Ni 250 grade maraging steel in phosphoric acid solution. Corros Sci 53:3688–3696
Khadom AA, Yaro AS, AlTaie AS, Kadum AAH (2009) Electrochemical, Activations and adsorption studies for the corrosion inhibition of low carbon steel in acidic media. Port Electrochim Acta 27(6):699–712
Khadom AA, Yaro AS, Musa AY, Mohamad AB, Kadhum AAH (2012) Corrosion inhibition of copper-nickel alloy: experimental and theoretical studies. J Korean Chem Soc 56(4):406–415
Obot IB, Umoren SA, Obi-Egbedi NO (2011) Corrosion inhibition and adsorption behaviour for aluminuim by extract of Aningeria robusta in HCl solution: Synergistic effect of iodide ions. J Mater Environ Sci 2:60–71
El-Sayed MS, Erasmus RM, Comins JD (2008) Inhibition of copper corrosion in acidic chloride pickling solutions by 5-(3-aminophenyl)-tetrazole as a corrosion inhibitor. Corros Sci 50(1):3439–3445
Larabi L, Benali O, Mekelleche SM, Harek Y (2006) 2-Mercapto-1-methylimidazole as corrosion inhibitor for copper in hydrochloric acid. Appl Surf Sci 253:1371–1378
Wang HL, Liu RB, Xin J (2004) Inhibiting effects of some mercapto triazole derivatives on the corrosion of mild steel in 10 M HCl medium. Corros Sci 46:2455–2466
Emregul KC, Kurtaran R, Atakol O (2003) An investigation of chloride-substituted Schiff bases as corrosion inhibitors for steel. Corros Sci 45:2803–2817
Lalitha A, Ramesh S, Rajeswari S (2005) Surface protection of copper in acid medium by azoles and surfactants. Electrochim Acta 51:47–55
Fouda AS, Abdallah M, El-Hoseiny M (2013) Acrylonitrile derivatives as corrosion inhibitors for Cu10Ni alloy in 0.5 M hydrochloric acid solution. Afr J Pure Appl Chem 7:252–263
Abd El Wanees S (1994) Amines as inhibitors for corrosion of copper in nitric acid. Anti-Corros Methods Mater 41(1):3–7
Stupnisek-Lisac E, Brnada A, Mance AD (2000) Secondary amines as copper corrosion inhibitors in acid media. Corros Sci 42:243–257
Khadom AA, Yaro AS, Kadum AAH (2010) Corrosion inhibition by naphthylamine and phenylenediamine for the corrosion of copper–nickel alloy in hydrochloric acid. J Taiwan Inst Chem Eng 41:122–125
Kuruvilla M, Prasad AR, John S et al (2017) Enhanced inhibition of the corrosion of metallic copper exposed in sulphuric acid through the synergistic interaction of cysteine and alanine: electrochemical and computational studies. J Bio Tribo Corros 3:5
Kuruvilla M, Prasad AR, Shainy KM et al (2019) Protection of metallic copper from the attack of sulphuric acid using HDMMA, a schiff base derived from L-cysteine and 2-hydroxy-1-naphthaldehyde. J Bio Tribo Corros 5:9
Khadom AA, Yaro AS (2011) Protection of low carbon steel in phosphoric acid by potassium iodide. Protect Metals Phys Chem Surf J 47:662–669
Yaro AS, Wael RK, Khadom AA (2010) Reaction kinetics of corrosion of mild steel in phosphoric acid. J Univ Chem Technol Metall 45:443–448
Orubite KO, Oforka NC (2004) Inhibition of the corrosion of mild steel in hydrochloric acid solutions by the extracts of leaves of Nypa fruticans Wurmb. Mater Lett 58:1772
Popova A, Sokolova E, Raicheva S, Christov M (2003) AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros Sci 45:33
Shereir LL (1977) Corrosion, 2nd edn. Newnes-Butterworths, London
Umoren SA, Ebenso EE (2007) The synergistic effect of polyacrylamide and iodide ions on the corrosion inhibition of mild steel in H2SO4. Mater Chem Phys 106:393
Sassi W, Zrelli R, Hihn J et al (2020) Silicate dip-coat mechanism as an inhibitor against copper dissolution into alkaline chloride media. J Bio Tribo Corros 6:50
Umoren SA, Obot IB, Ebenso EE (2008) Corrosion inhibition of aluminium using exudate gum from Pachylobus edulis in the presence of halide ions in HCl. E-journal Chemistry 5:355
Shams AM (2000) E1 Din, ME E1 Dahshan AM Taj El Din. Desalination 130:89
Kear G, Barker BD, Walsh FC (2004) Electrochemical corrosion of unalloyed copper in chloride media––a critical review. Corros Sci 46:109
Sherif EM, Park SM (2006) Effects of 2-amino-5-ethylthio-1, 3, 4-thiadiazole on copper corrosion as a corrosion inhibitor in aerated acidic pickling solutions. Electrochim Acta 51:6556
Ebenso EE (2003) Synergistic effect of halide ions on the corrosion inhibition of aluminum in H2SO4 using 2- acetyphenothioazine. Mater Chem Phys 79:58
Abiola OK (2006) Adsorption of 3-(4-Amino-2-Methyl-5-pyrimidyl methyl)-4-methyl thiazolium chloride on mild steel. Corros Sci 48:3078
Yaro AS, Al-Jendeel H, Khadom AA (2011) Cathodic protection system of copper–zinc–saline water in presence of bacteria. Desalination 270:193–198
Acknowledgement
Authors would like to thank College of Engineering in the University of Diyala for continues support and facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest arising from the involvement of other parties either internal or external to the University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmmod, A.A., Khadom, A.A. & Mahood, H.B. Experimental Modeling of Inhibition's Mechanism of Cupronickel Alloy by DETA and EDA into Acid Corrosive Media. J Bio Tribo Corros 6, 85 (2020). https://doi.org/10.1007/s40735-020-00381-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00381-8