Abstract
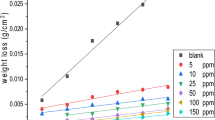
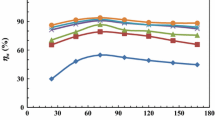
Inhibition performance of noncovalent functionalization of carbon nanotubes (CNTs) with biodegradable gemini surfactants on mild steel surface in 2 M hydrochloric acid solution was examined by potentiodynamic polarization, electrochemical impedance spectroscopy and quantum chemical calculations. Ultraviolet–visible (UV–vis) spectroscopy, thermogravimetric analysis, Raman analysis, and zeta-potential (Z-potential) measurements are also applied to discuss the stability of studied solutions. Ester-containing cationic surfactants; monomeric betainate, dodecyl esterquat gemini (ET), and dodecyl betainate gemini (BT) were used as potentially superior noncovalent functionalization agents for CNT-based formulations. For the first time, the anticorrosive efficiency of these surfactants on mild steel was investigated. The noncovalent functionalization of CNTs with ester-containing surfactants showed more appropriate inhibition properties at higher surfactant concentrations as a result of further dispersing ability. The best inhibition efficiency (IEE = 93%) is reported for BT (2.5 mM)-suspended nanotubes, while the effectiveness is decreased (IEE = 12%) dramatically at low concentration (0.1 mM). Surface observations are also employed to verify the corrosion protection of mild steel covered with noncovalent functionalization of CNTs. Density functional theory was employed for quantum chemical calculations, and a good correlation between experimental data and theoretical data has been obtained.












Similar content being viewed by others
References
Di Crescenzo A, Germani R, Del Canto E, Giordani S, Savelli G, Fontana A (2011) Effect of surfactant structure on carbon nanotube sidewall adsorption. Eur J Org Chem 2011:5641–5648
Javadian S, Yousefi A, Neshati J (2013) Synergistic effect of mixed cationic and anionic surfactants on the corrosion inhibitor behavior of mild steel in 3.5% NaCl. Appl Surf Sci 285:674–681
Yousefi A, Javadian S, Neshati J (2014) A new approach to study the synergistic inhibition effect of cationic and anionic surfactants on the corrosion of mild steel in HCl solution. Ind Eng Chem Res 53:5475–5489
Turhan MC, Li Q, Jha H, Singer RF, Virtanen S (2011) Corrosion behaviour of multiwall carbon nanotube/magnesium composites in 3.5% NaCl. Electrochim Acta 56:7141–7148
El-Hajjaji F, Messali M, Aljuhani A, Aouad MR, Hammouti B, Belghiti ME, Chauhan DS, Quraishi MA (2018) Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: electrochemical and molecular dynamics simulation studies. J Mol Liq 249:997–1008
Yousefi A, Javadian S, Dalir N, Kakemam J, Akbari J (2015) Imidazolium-based ionic liquids as modulators of corrosion inhibition of SDS on mild steel in hydrochloric acid solutions: experimental and theoretical studies. RSC Adv 5:11697–11713
Gerengi H, Mielniczek M, Gece G, Solomon MM (2016) Experimental and quantum chemical evaluation of 8-hydroxyquinoline as a corrosion inhibitor for copper in 0.1 M HCl. Ind Eng Chem Res 55:9614–9624
Roy P, Sukul D (2015) Protein–surfactant aggregate as a potential corrosion inhibitor for mild steel in sulphuric acid: zein–SDS system. RSC Adv 5:1359–1365
Shubha H, Venkatesha T, Vathsala K, Pavitra M, Punith Kumar M (2013) Preparation of self assembled sodium oleate monolayer on mild steel and its corrosion inhibition behavior in saline water. ACS Appl Mater Interfaces 5:10738–10744
Deyab M (2015) Application of nonionic surfactant as a corrosion inhibitor for zinc in alkaline battery solution. J Power Sources 292:66–71
Fouda AS, Zaki EG, Khalifa MMA (2019) Some new nonionic surfactants based on propane tricarboxylic acid as corrosion inhibitors for low carbon steel in hydrochloric acid solutions. J Bio Tribo Corros 5:31. https://doi.org/10.1007/s40735-019-0223-y
Baymou Y, Bidi H, Ebn Touhami M, Allam M, Rkayae M, Belakhmima RA (2018) Corrosion protection for cast iron in sulfamic acid solutions and studies of the cooperative effect between cationic surfactant and acid counterions. J Bio Tribo Corros 4:11. https://doi.org/10.1007/s40735-018-0127-2
Yousefi A, Aslanzadeh SA, Akbari J (2018) Experimental and DFT studies of 1-methylimidazolium trinitrophenoxide as modifier for corrosion inhibition of SDS for mild steel in hydrochloric acid. Anti-corros Methods Mater 65:107–122
Kumar AM, Gasem ZM (2015) In situ electrochemical synthesis of polyaniline/f-MWCNT nanocomposite coatings on mild steel for corrosion protection in 3.5% NaCl solution. Prog Org Coat 78:387–394
Chang K-C, Hsu M-H, Lu H-I, Lai M-C, Liu P-J, Hsu C-H, Ji W-F, Chuang T-L, Wei Y, Yeh J-M (2014) Room-temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitor for cold-rolled steel. Carbon 66:144–153
Chang C-H, Huang T-C, Peng C-W, Yeh T-C, Lu H-I, Hung W-I, Weng C-J, Yang T-I, Yeh J-M (2012) Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 50:5044–5051
Kirkland N, Schiller T, Medhekar N, Birbilis N (2012) Exploring graphene as a corrosion protection barrier. Corros Sci 56:1–4
Wang H (2009) Dispersing carbon nanotubes using surfactants. Curr Opin Colloid Interface Sci 14:364–371
Wang Q, Han Y, Wang Y, Qin Y, Guo Z-X (2008) Effect of surfactant structure on the stability of carbon nanotubes in aqueous solution. J Phys Chem B 112:7227–7233
Yousefi A, Aslanzadeh SA, Akbari J (2016) Effect of 1-ethyl-3-methylimidazolium bromide on interfacial and aggregation behavior of mixed cationic and anionic surfactants. J Mol Liq 219:637–642
Chami R, Bensajjay F, Alehyen S, El Achouri M, Bellaouchou A, Guenbour A (2015) Inhibitive effect of ester-quats surfactants in the series of (alcanoyloxy) propyl n-alkyl dimethyl ammonium bromide on the corrosion of iron in acid medium. Colloids Surf A 480:468–476
Jiang L, Gao L, Sun J (2003) Production of aqueous colloidal dispersions of carbon nanotubes. J Colloid Interface Sci 260:89–94
Tkalya EE, Ghislandi M, de With G, Koning CE (2012) The use of surfactants for dispersing carbon nanotubes and graphene to make conductive nanocomposites. Curr Opin Colloid Interface Sci 17:225–232
Duque JG, Densmore CG, Doorn SK (2010) Saturation of surfactant structure at the single-walled carbon nanotube surface. J Am Chem Soc 132:16165–16175
Aung NN, Zhou W, Goh CS, Nai SML, Wei J (2010) Effect of carbon nanotubes on corrosion of Mg–CNT composites. Corros Sci 52:1551–1553
Jeon H, Park J, Shon M (2013) Corrosion protection by epoxy coating containing multi-walled carbon nanotubes. J Ind Eng Chem 19:849–853
Tehrani-Bagha AR, Oskarsson H, Van Ginkel C, Holmberg K (2007) Cationic ester-containing gemini surfactants: chemical hydrolysis and biodegradation. J Colloid Interface Sci 312:444–452
Javadiana S, Motaee A, Sharifi M, Aghdastinat H, Taghavi F (2017) Dispersion stability of multi-walled carbon nanotubes in catanionic surfactant mixtures. Colloids Surf A 531:141–149
O’Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, Rialon KL, Boul PJ, Noon WH, Kittrell C (2002) Band gap fluorescence from individual single-walled carbon nanotubes. Science 297:593–596
Aghdastinat H, Javadian S, Tehrani-Bagha A, Gharibi H (2014) Spontaneous formation of nanocubic particles and spherical vesicles in catanionic mixtures of ester-containing gemini surfactants and sodium dodecyl sulfate in the presence of electrolyte. J Phys Chem B 118:3063–3073
Duan WH, Wang Q, Collins F (2011) Dispersion of carbon nanotubes with SDS surfactants: a study from a binding energy perspective. Chem Sci 2:1407–1413
Chen L, Xie H, Li Y, Yu W (2008) Applications of cationic gemini surfactant in preparing multi-walled carbon nanotube contained nanofluids. Colloids Surf A 330:176–179
White B, Banerjee S, O’Brien S, Turro NJ, Herman IP (2007) Zeta-potential measurements of surfactant-wrapped individual single-walled carbon nanotubes. J Phys Chem C 111:13684–13690
Hu H, Yu A, Kim E, Zhao B, Itkis ME, Bekyarova E, Haddon RC (2005) Influence of the zeta potential on the dispersability and purification of single-walled carbon nanotubes. J Phys Chem B 109:11520–11524
Sun G, Chen G, Liu J, Yang J, Xie J, Liu Z, Li R, Li X (2009) A facile gemini surfactant-improved dispersion of carbon nanotubes in polystyrene. Polymer 50:5787–5793
Menger FM, Keiper JS (2009) Gemini surfactants. Angew Chem Int Ed 39:1906–1920
Krishnegowda PM, Venkatesha VT, Krishnegowda PKM, Shivayogiraju SB (2013) Acalypha torta leaf extract as green corrosion inhibitor for mild steel in hydrochloric acid solution. Ind Eng Chem Res 52:722–728
Li X, Deng S, Fu H (2011) Inhibition by tetradecylpyridinium bromide of the corrosion of aluminium in hydrochloric acid solution. Corros Sci 53:1529–1536
Xu Z, Yang X, Yang Z (2010) A molecular simulation probing of structure and interaction for supramolecular sodium dodecyl sulfate/single-wall carbon nanotube assemblies. Nano Lett 10:985–991
Vigolo B, Penicaud A, Coulon C, Sauder C, Pailler R, Journet C, Bernier P, Poulin P (2000) Macroscopic fibers and ribbons of oriented carbon nanotubes. Science 290:1331–1334
Ouici H, Belkhouda M, Benali O, Salghi R, Bammou L, Zarrouk A, Hammouti B (2018) Adsorption and inhibition effect of 5-phenyl-1, 2, 4-triazole-3-thione on C38 steel corrosion in 1 M HCl. Res Chem Intermed. https://doi.org/10.1007/s11164-014-1556-2
Fattah-Alhosseini A, Joni MS (2015) Effect of immersion time on the electrochemical behaviour of AZ31B alloy. J Alloys Compd 646:685–691
Zeinabad HA, Zarrabian A, Saboury AA, Alizadeh AM, Falahati M (2016) Interaction of single and multi wall carbon nanotubes with the biological systems: tau protein and PC12 cells as targets. Sci Rep. https://doi.org/10.1038/srep26508
Harb SV, dos Santos FC, Pulcinelli SH, Santilli CV, Knowles KM, Hammer P (2016) Protective coatings based on PMMA–silica nanocomposites reinforced with carbon nanotubes, Chapter 7. https://doi.org/10.5772/62808
Antiohos D, Romano M, Chen J, Razal JM (2013) Carbon nanotubes for energy applications. In: Syntheses and applications of carbon nanotubes and their composites. InTech, London, pp 495–537
Daon J, Sun S, Jiang D, Cibien G, Leveugle E, Galindo C, Ziaei A, Ye L, Fu Y, Bai J, Liu J (2014) Electrically conductive thermal interface materials based on vertically aligned carbon nanotubes mats. In: 2014 20th international workshop on thermal investigations of ICs and systems (THERMINIC). IEEE, pp 1–4
Obot I, Macdonald D, Gasem Z (2015) Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: an overview. Corros Sci 99:1–30
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740
Javadian S, Darbasizadeh B, Yousefi A, Ektefa F, Dalir N, Kakemam J (2017) Dye-surfactant aggregates as corrosion inhibitor for mild steel in NaCl medium: experimental and theoretical studies. J Taiwan Inst Chem Eng 71:344–354
Javadian S, Ektefa F (2015) An efficient approach to explore the adsorption of benzene and phenol on nanostructured catalysts: a DFT analysis. RSC Adv 5:100799–100808
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yousefi, A., Javadian, S., Sharifi, M. et al. An Experimental and Theoretical Study of Biodegradable Gemini Surfactants and Surfactant/Carbon Nanotubes (CNTs) Mixtures as New Corrosion Inhibitor. J Bio Tribo Corros 5, 82 (2019). https://doi.org/10.1007/s40735-019-0274-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0274-0