Abstract
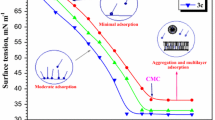
The electrochemical behavior of white cast iron in sulfamic acid was studied in the presence of hexadecyltrimethylammonium bromide as a corrosion inhibitor. The cooperative effect between counterions of sulfamic acid and CTAB was investigated as well as the impact of this phenomenon on the inhibition efficiency; for that purpose, gravimetric method, electrochemical impedance spectroscopy, potentiodynamic polarization curves and inductively coupled plasma spectrometry (ICP-OES) techniques were exploited. The CTAB molecule reveals remarkable qualities for the protection of white cast iron in sulfamic acid cleaning solutions. Sulfamate ions play a major role in this phenomenon by facilitating the attraction and adsorption of CTAB onto the surface of white cast iron. The inhibition efficiency remains almost stable (about 95%) for a concentration of 10−3 M in CTAB. The critical micelle concentration of CTAB in the H2O/NH2SO3H system was investigated by the conductimetry method. The CTAB adsorption onto the cast iron surface was studied by Langmuir adsorption isotherms. The surface characterization by RDX and SEM showed a good anticorrosion protection and a lower surface roughness in the presence of CTAB.






















Similar content being viewed by others
References
Singh B, Agarwal MK (2011) Research of cast iron in acidic medium in the industrial field as components in acid. Int J Res Sci Technol 1:2249
Arab N, Soltani MRN (2009) A study of coating process of cast iron blackening. J Appl Chem Res 9:13–23
Philippon J, Volfovsky C (2001) In: La conservation des métaux, 1th edn. CNRS Editions, Paris, pp 52–53
Motamedi M, Tehrani-Bagha AR, Mahdavian M (2011) A comparative study on the electrochemical behavior of mild steel in sulfamic acid solution in the presence of monomeric and gemini surfactants. J Electrochim Acta 58:488–496
El-Maksoud SAA (2008) The effect of organic compounds on the electrochemical behavior of steel in acidic media. Int J Electrochem Sci 3:528–555
Motamedi M, Tehrani-Bagha AR, Mahdavian M (2013) Effect of aging time on corrosion inhibition of cationic surfactant on mild steel in sulfamic acid cleaning solution. J Corros Sci 70:46–54
Morad M (2008) Corrosion inhibition of mild steel in sulfamic acid solution by Scontaining amino acids. J Appl Electrochem 38:1509–1518
Khamis A, Saleh MM, Awad MI (2013) Synergistic inhibitor effect of cetylpyridinium chloride and other halides on the corrosion of mild steel in 0.5 M H2SO4. J Corros Sci 66:343–349
Quraishi MA, Sardar R (2002) Aromatic triazoles as corrosion inhibitors for mild steel in acidic environments. J Corros Sci 58:748–755
Mahdavian M, Tehrani-Bagha AR, Holmberg K (2011) Comparison of a cationic gemini surfactant and the corresponding monomeric surfactant for corrosion protection of mild steel in hydrochloric acid. J Surfactants Deterg 14:605–613
Qiu LG, Xie AJ, Shen YH (2004) Understanding the adsorption of cationic gemini surfactants on steel surface in hydrochloric acid. J Mater Chem Phys 87:237–240
Migahed MA, Hegazy MA, Al-Sabagh AM (2012) Synergistic inhibition effect between Cu2+ and cationic gemini surfactant on the corrosion of downhole tubing steel during secondary oil recovery of old wells. J Corros Sci 61:10–18
Wang X, Yang H, Wang F (2012) Inhibition performance of a gemini surfactant and its co-adsorption effect with halides on mild steel in 0.25 M H2SO4 solution. Journal of Corrosion Science 55:145–152
Farag AA, El-Din MRN (2012) The adsorption and corrosion inhibition of some nonionic surfactants on API X65 steel surface in hydrochloric acid. J Corros Sci 64:174–183
Fuchs-Godec R, Pavlović MG (2012) Synergistic effect between non-ionic surfactant and halide ions in the forms of inorganic or organic salts for the corrosion inhibition of stainless-steel X4Cr13 in sulphuric acid. J Corros Sci 58:192–201
Free ML (2002) Understanding the effect of surfactant aggregation on corrosion inhibition of mild steel in acidic medium. J Corros Sci 44:2865–2870
Free ML, Wang W, Ryu DY (2004) Prediction of corrosion inhibition using surfactants. J Corros Sci 60:837–844
Singh P, Quraishi MA, Gupta SL, Dandia A (2016) Investigation of the corrosion inhibition effect of 3-methyl-6-oxo-4-(thiophen-2-yl)-4,5,6,7-tetrahydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile (TPP) on mild steel inhydrochloric acid. J Taibah Univ Sci 10:139–147
Sharma M, Chawla J, Singh G (2009) Cetyl trimethylammonium bromide as corrosion inhibi tor for mild steel in acidic medium. Indian J Chem Technol 16:339–343
Yang X, Li B, Wang H, Hou B (2011) Inhibition of CTAB on the corrosion of mild steel in hydrochloric acid. J Dispers Sci Technol 32:672–676
Hamid ZA, Atia AM, Helal AM, Megahed HE (2014) Clarification of the corrosion inhibition of mild steel in hydrochloric acid solutions via cetyltrimethyl ammonium bromide inhibitor. Egypt J Chem 57:353–371
Mukta S, Gurmeet S (2013) Adsorption and thermodynamic studies on acid corrosion of mild steel in the presence of additives. Int J Chem Appl 5:13–25
Fouda AS, Migahed MA, Atia AA, Mousa IM (2016) Corrosion inhibition and adsorption behavior of some cationic surfactants on carbon steel in hydrochloric acid solution. J Bio Tribo Corros 2:22
Li Xueming, Tang Libin, Liu Hongcheng, Guannan Mu, Liu Guangheng (2008) Influence of halide ions on inhibitive performance of cetyl trimethyl ammonium bromide in various concentrations of phosphoric acid for cold rolled steel. Mater Lett J 62:2321–2324
Mobin M, Parveen M, Rafiquee MZA (2017) Synergistic effect of sodium dodecyl sulfate and cetyltrimethyl ammonium bromide on the corrosion inhibition behavior of l-methionine on mild steel in acidic medium. Arab J Chem 10:1364–1372
Bakshi MS, Singh J, Singh K, Kaur G (2004) Mixed micelles of cationic 12-2-12 gemini with conventional surfactants: the head group and counterion effects. Colloids Surf A 237:61–71
Pérez-Rodríguez M, Prieto G, Rega C, Varela LM, Sarmiento F, Mosquera V (1998) A comparative study of the determination of the critical micelle concentration by conductivity and dielectric constant measurements. Langmuir 14:4422–4426
Sugihara G, Era Y, Funatsu M, Kunitake T, Lee S, Sasaki Y (1997) Micelle formation of dodecylammonium surfactant with mixed counterions: perfluorocarboxylate and alkanesulfonate ions. J Colloid Interface Sci 187:435
Castle JE, Qiu JH (1990) The application of ICP-MS and XPS to studies of ion selectivity during passivation of stainless steels. J Electrochem Soc 137:2031–2038
Kim DK, Muralidharan S, Ha TH, Bae JH, Ha YC, Lee HG, Scantlebury JD (2006) Electrochemical studies on the alternating current corrosion of mild steel under cathodic protection condition in marine environments. J Electrochim Acta 51:5259–5267
Ţoţea G, Ioniţă D, Demetrescu I (2014) ICP-MS determinations in sustaining corrosion data of 316 stainless steels in bioliquids. UPB Sci Bull B Chem Mater Sci 76:57–66
Nayak RS, Khanna B, Pasha A, Vinay K, Narayan A, Chaitra K (2015) Evaluation of nickel and chromium ion release during fixed orthodontic treatment using inductively coupled plasma-mass spectrometer: an in vivo study. J Int Oral Health 7:14–20
Behroozi Z, Danaei SM, Sardarian AR, Moshkelghosha V, Sardarian AR (2016) Evaluation of the corrosion of five different bracket-archwire combination: an in-vitro analysis using inductively coupled plasma mass spectrometry. J Dent Shiraz Univ Med Sci 17:262–267
Boukamp A (1993) Users manual equivalent circuit. 4.51 éd. Twente
Boukamp B (1989) Equivalent circuit user’s manual. 2° éd. Twente
Fiaud C (2004) Inhibiteurs de corrosion. Tech l’Ingénieur M160:14
Free M (2002) Understanding the effect of surfactant aggregation on corrosion inhibition of mild steel in acidic medium. J Corros Sci 44:2865–2870
Veleda APSR (1990) Proceedings of the seventh European symposium on corrosion inhibitors. J Mater Corros 42:149
El-Maksoud S (2004) The effect of hexadecyl pyridinium bromide and hexadecyl trimethyl ammonium bromide on the behaviour of iron and copper in acidic solutions. J Electroanal Chem 565:321–328
Özcan M, Solmaz R, Kardaş G, Dehri I (2008) Adsorption properties of barbiturates as green corrosion inhibitors on mild steel in phosphoric acid. Colloids Surf A 325:57–63
Gunasekaran G, Chauhan LR (2004) Eco friendly inhibitor for corrosion inhibition of mild steel in phosphoric acid medium. J Electrochim Acta 49:4387–4395
Bommersbach P (2005) Evolution of the properties of a corrosion-inhibiting film under the influence of temperature and hydrodynamic conditions. Thesis, LPCI
Kish JR, Stead NJ, Singbeil DL (2009) Corrosion control of type 316L stainless steel in sulfamic acid cleaning solutions. Corros Eng 65:491–500
Ashassi-Sorkhabi H, Seifzadeh D, Hosseini MG (2008) EN EIS and polarization studies to evaluate the inhibition effect of 3H-phenothiazin-3-one, 7-dimethylamin on mild steel corrosion in 1M HCl solution. J Corros Sci 50:3363–3370
Abdel-Fatah HT, Kamel MM, Hassan AA, Rashwan SA, El Wahaab SMA, El-Sehiety HE (2017) Adsorption and inhibitive properties of Tryptophan on low alloy steel corrosion in acidic media. Arab J Chem 10:S1164–S1171
Wahdan MH, Hermas AA, Morad MS (2002) Corrosion inhibition of carbon-steels by propargyltriphenylphosphonium bromide in H2SO4 solution. Mater Chem Phys 76(2):111–118
Larabi L, Harek Y, Traisnel M, Mansri A (2004) Synergistic Influence of Poly(4-Vinylpyridine) and Potassium Iodide on Inhibition of Corrosion of Mild Steel in 1M HCl. J Appl Electrochem 34:833–839
Gomma GK (1998) Mechanism of corrosion behaviour of carbon steel in tartaric and malic acid in the presence of Fe2+ ion. Mater Chem Phys 52:200–206
Chetouani A, Medjahed K, Sid-Lakhdar KE, Hammouti B, Benkaddour M, Mansri A (2004) Poly(4-vinylpyridine-poly(3-oxide-ethylene) tosyle) as an inhibitor for iron in sulphuric acid at 80 °C. J Corros Sci 46:2421–2430
Popova A, Sokolova E, Raicheva S, Christov M (2003) AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. J Corros Sci 45:33–58
Elayyachy M, Elkodadi M, Aouniti A, Ramdani A, Hammouti B, Malek F, Elidrissi A (2005) New bipyrazole derivatives as corrosion inhibitors for steel in hydrochloric acid solutions. J Mater Chem Phys 93:281–285
Mora-Mendoza JL, Turgoose S (2002) Fe3C influence on the corrosion rate of mild steel in aqueous CO2 systems under turbulent flow conditions. J Corros Sci 44:1223–1246
Bouklah M, Hammouti B, Lagrenee M, Bentiss F (2006) Thermodynamic properties of 2,5-bis(4-methoxyphenyl)-1,3,4-oxadiazole as a corrosion inhibitor for mild steel in normal sulfuric acid medium. J Corros Sci 48:2831–2842
Tariq Saeed M, Asrof Ali S (2003) Synthesis of some new bisquaternary ammonium salts as acid corrosion inhibitors for carbon steel. Anti-Corros Methods Mater 50:436–441
Emregül KC, Akay AA, Atakol O (2005) The corrosion inhibition of steel with Schiff base compounds in 2M HCl. J Mater Chem Phys 93:325–329
Chin RJ, Nobe K (1971) Electrochemical characteristics of iron in H2SO4 containing benzotriazole. J Electrochem Soc 118:545–548
Tang L, Li X, Li L, Mu G, Liu G (2006) The effect of 1-(2-pyridylazo)-2-naphthol on the corrosion of cold rolled steel in acid media: part 2: inhibitive action in 0.5 M sulfuric acid. J Mater Chem Phys 97:301–307
Tang L, Li X, Mu G, Li L, Liu G (2006) Synergistic effect between 4-(2-pyridylazo) resorcin and chloride ion on the corrosion of cold rolled steel in 0.5 M sulfuric acid. J Appl Surf Sci 252:6394–6401
Kliškic M, Radoševic J, Gudic S (1997) Pyridine and its derivatives as inhibitors of aluminium corrosion in chloride solution. J Appl Electrochem 27:947–952
Moretti G, Guidi F (2002) Tryptophan as copper corrosion inhibitor in 0.5 M aerated sulfuric acid. J Corros Sci 44:1995–2011
Elawady Y, Ahmed AI (1985) Effect of temperature and inhibitors on the corrosion of aluminum in 2n HCl solution-a kinetic-study. Indian J Chem 24:601–602
Lorenz W (1970) Zur Theorie partieller Ladungsübergangsreaktionen. Zeitschrift für Physikalische Chemie 65:244
Sherif ESM, Erasmus RM, Comins JD (2007) Effects of 3-amino-1,2,4-triazole on the inhibition of copper corrosion in acidic chloride solutions. J Colloid Interface Sci 311:144–151
Ahamad I, Gupta C, Prasad R, Quraishi MA (2010) An experimental and theoretical investigation of adsorption characteristics of a Schiff base compound as corrosion inhibitor at mild steel/hydrochloric acid interface. J Appl Electrochem 40:2171–2183
Singh AK, Quraishi MA (2010) Inhibiting effects of 5-substituted isatin-based Mannich bases on the corrosion of mild steel in hydrochloric acid solution. J Appl Electrochem 40:1293–1306
Benali O, Larabi L, Tabti B, Harek Y (2005) Influence of 1-methyl 2-mercapto imidazole on corrosion inhibition of carbon steel in 0.5 M H2SO4. Anti-Corros Methods Mater 52:280–285
Omar B, Mokhtar O (2011) Inhibition of cold rolled steel corrosion in sulphuric acid solution by 2-mercapto-1-methylimidazole: Time and temperature effects treatments. Arab J Chem 4:443–448
McCafferty E (2010) In introduction to corrosion science, 1st edn. Springer, New York, pp 359–360
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baymou, Y., Bidi, H., Ebn Touhami, M. et al. Corrosion Protection for Cast Iron in Sulfamic Acid Solutions and Studies of the Cooperative Effect Between Cationic Surfactant and Acid Counterions. J Bio Tribo Corros 4, 11 (2018). https://doi.org/10.1007/s40735-018-0127-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0127-2