Abstract
Air pollution and the various chemicals that are a part of this complex mixture have been associated with several adverse infant health outcomes. One major area of research is describing the underlying biological mechanism between air pollution and adverse infant health outcomes. Metabolomics, a new omics field, studies small molecules present in a biological matrix and may provide insight into underlying biological mechanism. We conducted a narrative review of the literature to identify studies utilizing metabolomics with air pollution, or some potential component of it, and adverse infant health. We identified seven studies that met our inclusion criteria. These studies described a range of potential air pollutants including tobacco smoke, PAH, NO2, PM2.5, O3, BC, heavy metals, and PFAS. The studies mainly focused on gestational age and weight outcomes. Metabolic analysis revealed many altered metabolomic pathways including those related to amino acid metabolism, glycan metabolism, lipid metabolism, and cofactor and vitamin metabolism. These studies provide valuable insight into the potential biological mechanisms that underpin the association between air pollution and adverse gestational outcomes. Future studies should utilize longitudinal study design and use complex mixture analysis for air pollution exposure assessment, as well as focus on the use of more toxicologically relevant target tissue for infant health outcomes.

Similar content being viewed by others
Data Availability
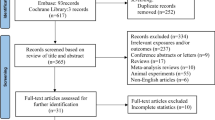
Data were abstracted from seven published articles. Abstracted data are available upon request or can be abstracted directly from these seven published studies (see Table 1).
References
Bourdrel T, Bind MA, Béjot Y, Morel O, Argacha JF. Cardiovascular effects of air pollution. Arch Cardiovasc Dis. 2017;110(11):634–42. https://doi.org/10.1016/j.acvd.2017.05.003.
CDC. Air pollutants. Centers for Disease Control and Prevention. https://www.cdc.gov/air/pollutants.htm
Polivka BJ. The Great London Smog of 1952. Am J Nurs. 2018;118(4):57–61. https://doi.org/10.1097/01.NAJ.0000532078.72372.c3.
Checa Vizcaíno MA, González-Comadran M, Jacquemin B. Outdoor air pollution and human infertility: a systematic review. Fertil Steril. 2016;106(4):897-904.e1. https://doi.org/10.1016/j.fertnstert.2016.07.1110.
Gaskins AJ, Mínguez-Alarcón L, Fong KC, et al. Exposure to fine particulate matter and ovarian reserve among women from a fertility clinic. Epidemiology. 2019;30(4):486–91. https://doi.org/10.1097/ede.0000000000001029.
Liang W, Zhu H, Xu J, et al. Ambient air pollution and gestational diabetes mellitus: an updated systematic review and meta-analysis. Ecotoxicol Environ Saf. 2023;255.
Guo C, Qian Y, Xu R, et al. Exposure to ambient air pollution from the preconceptional period and risk of gestational hypertension. Sci Total Environ. 2023;885.
Zhu W, Zheng H, Liu J, et al. The correlation between chronic exposure to particulate matter and spontaneous abortion: a meta-analysis. Chemosphere. 2022;286(Pt 2): 131802. https://doi.org/10.1016/j.chemosphere.2021.131802.
Liu J, Chen Y, Liu D, et al. Prenatal exposure to particulate matter and term low birth weight: systematic review and meta-analysis. Environ Sci Pollut Res Int. 2023;30(23):63335–46. https://doi.org/10.1007/s11356-023-26831-7.
Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–11. https://doi.org/10.1016/j.envres.2012.05.007.
Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60(8):612–6. https://doi.org/10.1136/oem.60.8.612.
Gaskins AJ, Tang Z, Hood RB, et al. Periconception air pollution, metabolomic biomarkers, and fertility among women undergoing assisted reproduction. Environ Int. 2021;155.
Boamah-Kaali E, Jack DW, Ae-Ngibise KA, et al. Prenatal and postnatal household air pollution exposure and infant growth trajectories: evidence from a rural Ghanaian pregnancy cohort. Environ Health Perspect. 2021;129(11): 117009. https://doi.org/10.1289/ehp8109.
Clasen TF, Chang HH, Thompson LM, et al. Liquefied petroleum gas or biomass for cooking and effects on birth weight. N Engl J Med. 2022;387(19):1735–46. https://doi.org/10.1056/NEJMoa2206734.
Barn P, Gombojav E, Ochir C, et al. The effect of portable HEPA filter air cleaner use during pregnancy on fetal growth: the UGAAR randomized controlled trial. Environ Int. 2018;121(Pt 1):981–9. https://doi.org/10.1016/j.envint.2018.08.036.
Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–9. https://doi.org/10.1080/004982599238047.
Liang D, Li Z, Vlaanderen J, et al. A state-of-the-science review on high-resolution metabolomics application in air pollution health research: current progress, analytical challenges, and recommendations for future direction. Environ Health Perspect. 2023;131(5):56002. https://doi.org/10.1289/ehp11851.
Sumner LW, Amberg A, Barrett D, et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics. 2007;3(3):211–21. https://doi.org/10.1007/s11306-007-0082-2.
Li Y, Xiao N, Liu M, et al. Dysregulation of steroid metabolome in follicular fluid links phthalate exposure to diminished ovarian reserve of childbearing-age women. Environ Pollut. 2023;330. https://doi.org/10.1016/j.envpol.2023.121730.
Prince N, Begum S, Mínguez-Alarcón L, et al. Plasma concentrations of per- and polyfluoroalkyl substances are associated with perturbations in lipid and amino acid metabolism. Chemosphere. 2023;324: 138228. https://doi.org/10.1016/j.chemosphere.2023.138228.
Walker DI, Marder ME, Yano Y, et al. Multigenerational metabolic profiling in the Michigan PBB registry. Environ Res. 2019;172:182–93. https://doi.org/10.1016/j.envres.2019.02.018.
Hood RB, Liang D, Chiu YH, et al. Pesticide residue intake from fruits and vegetables and alterations in the serum metabolome of women undergoing infertility treatment. Environ Int. 2022;160: 107061. https://doi.org/10.1016/j.envint.2021.107061.
Chen M, Guan Y, Huang R, et al. Associations between the maternal exposome and metabolome during pregnancy. Environ Health Perspect. 2022;130(3):37003. https://doi.org/10.1289/ehp9745.
Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83(11):713–20. https://doi.org/10.1016/j.earlhumdev.2007.08.002.
Jovandaric MZ, Babic S, Raus M, Medjo B. The importance of metabolic and environmental factors in the occurrence of oxidative stress during pregnancy. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms241511964.
Pereira B, Figueiredo B, Pinto TM, Míguez MC. Effects of tobacco consumption and anxiety or depression during pregnancy on maternal and neonatal health. Int J Environ Res Public Health. 2020. https://doi.org/10.3390/ijerph17218138.
Tan Y, Barr DB, Ryan PB, et al. High-resolution metabolomics of exposure to tobacco smoke during pregnancy and adverse birth outcomes in the Atlanta African American maternal-child cohort. Environ Pollut. 2022;292.
Chadeau-Hyam M, Athersuch TJ, Keun HC, et al. Meeting-in-the-middle using metabolic profiling - a strategy for the identification of intermediate biomarkers in cohort studies. Biomarkers. 2011;16(1):83–8. https://doi.org/10.3109/1354750x.2010.533285.
Vineis P, Demetriou CA, Probst-Hensch N. Long-term effects of air pollution: an exposome meet-in-the-middle approach. Int J Public Health. 2020;65(2):125–7. https://doi.org/10.1007/s00038-019-01329-7.
Agarwal P, Singh L, Anand M, Taneja A. Association between placental polycyclic aromatic hydrocarbons (PAHS), oxidative stress, and preterm delivery: a case-control study. Arch Environ Contam Toxicol. 2018;74(2):218–27. https://doi.org/10.1007/s00244-017-0455-0.
Duarte-Salles T, Mendez MA, Meltzer HM, Alexander J, Haugen M. Dietary benzo(a)pyrene intake during pregnancy and birth weight: associations modified by vitamin C intakes in the Norwegian mother and child cohort study (MoBa). Environ Int. 2013;60:217–23. https://doi.org/10.1016/j.envint.2013.08.016.
Langlois PH, Hoyt AT, Desrosiers TA, et al. Maternal occupational exposure to polycyclic aromatic hydrocarbons and small for gestational age offspring. Occup Environ Med. 2014;71(8):529–35. https://doi.org/10.1136/oemed-2013-101833.
Padula AM, Noth EM, Hammond SK, et al. Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth. Environ Res. 2014;135:221–6. https://doi.org/10.1016/j.envres.2014.09.014.
Porpora MG, Piacenti I, Scaramuzzino S, Masciullo L, Rech F, Benedetti Panici P. Environmental contaminants exposure and preterm birth: a systematic review. Toxics. 2019. https://doi.org/10.3390/toxics7010011.
Rennie MY, Detmar J, Whiteley KJ, et al. Vessel tortuousity and reduced vascularization in the fetoplacental arterial tree after maternal exposure to polycyclic aromatic hydrocarbons. Am J Physiol Heart Circ Physiol. 2011;300(2):H675–84. https://doi.org/10.1152/ajpheart.00510.2010.
Choi H, Rauh V, Garfinkel R, Tu Y, Perera FP. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008;116(5):658–65. https://doi.org/10.1289/ehp.10958.
Suter MA, Aagaard KM, Coarfa C, et al. Association between elevated placental polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts from Superfund sites in Harris County, and increased risk of preterm birth (PTB). Biochem Biophys Res Commun. 2019;516(2):344–9. https://doi.org/10.1016/j.bbrc.2019.06.049.
Wang L, Guo P, Tong H, et al. Traffic-related metrics and adverse birth outcomes: a systematic review and meta-analysis. Environ Res. 2020;188: 109752. https://doi.org/10.1016/j.envres.2020.109752.
Kloog I, Chudnovsky AA, Just AC, et al. A new hybrid spatio-temporal model for estimating daily multi-year PM(2.5) concentrations across Northeastern USA using high resolution aerosol optical depth data. Atmos Environ (1994). 2014;95:581–90. https://doi.org/10.1016/j.atmosenv.2014.07.014.
Lee HJ, Koutrakis P. Daily ambient NO2 concentration predictions using satellite ozone monitoring instrument NO2 data and land use regression. Environ Sci Technol. 2014;48(4):2305–11. https://doi.org/10.1021/es404845f.
Di Q, Rowland S, Koutrakis P, Schwartz J. A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. J Air Waste Manag Assoc. 2017;67(1):39–52. https://doi.org/10.1080/10962247.2016.1200159.
Abu Awad Y, Koutrakis P, Coull BA, Schwartz J. A spatio-temporal prediction model based on support vector machine regression: ambient black carbon in three New England States. Environ Res. 2017;159:427–34. https://doi.org/10.1016/j.envres.2017.08.039.
Dettwiler M, Flynn AC, Rigutto-Farebrother J. Effects of non-essential “toxic” trace elements on pregnancy outcomes: a narrative overview of recent literature syntheses. Int J Environ Res Public Health. 2023. https://doi.org/10.3390/ijerph20085536.
Heng YY, Asad I, Coleman B, et al. Heavy metals and neurodevelopment of children in low and middle-income countries: a systematic review. PLoS ONE. 2022;17(3): e0265536. https://doi.org/10.1371/journal.pone.0265536.
Zhao S, Yang X, Xu Q, et al. Association of maternal metals exposure, metabolites and birth outcomes in newborns: a prospective cohort study. Environ Int. 2023;179: 108183. https://doi.org/10.1016/j.envint.2023.108183.
Mitrovic-Jovanovic A, Dragojevic-Dikic S, Zamurovic M, et al. Comparison of electrolytic status (Na+, K+, Ca2+, Mg2+) in preterm and term deliveries. Clin Exp Obstet Gynecol. 2012;39(4):479–82.
Tarannum F, Faizuddin M, Madaiah H. Gingival crevicular fluid prostaglandin E2 level as a predictor of preterm low birth weight: a pilot investigation. J Oral Sci. 2011;53(3):293–300. https://doi.org/10.2334/josnusd.53.293.
Laine JE, Bailey KA, Rubio-Andrade M, et al. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers Of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect. 2015;123(2):186–92. https://doi.org/10.1289/ehp.1307476.
Maitre L, Fthenou E, Athersuch T, et al. Urinary metabolic profiles in early pregnancy are associated with preterm birth and fetal growth restriction in the Rhea mother-child cohort study. BMC Med. 2014. https://doi.org/10.1186/1741-7015-12-110.
Bell ML, Belanger K, Ebisu K, Gent JF, Leaderer BP. Relationship between birth weight and exposure to airborne fine particulate potassium and titanium during gestation. Environ Res. 2012;117:83–9. https://doi.org/10.1016/j.envres.2012.05.004.
Liu C, Luo D, Wang Q, et al. Serum homocysteine and folate concentrations in early pregnancy and subsequent events of adverse pregnancy outcome: the Sichuan Homocysteine study. BMC Pregnancy Childbirth. 2020;20(1):176. https://doi.org/10.1186/s12884-020-02860-9.
Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol. 2006;40(11):3463–73. https://doi.org/10.1021/es052580b.
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–94. https://doi.org/10.1093/toxsci/kfm128.
Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol. 2015;45(1):53–67. https://doi.org/10.3109/10408444.2014.952400.
Lam J, Koustas E, Sutton P, et al. The Navigation Guide - evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122(10):1040–51. https://doi.org/10.1289/ehp.1307923.
Johnson PI, Sutton P, Atchley DS, et al. The Navigation Guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122(10):1028–39. https://doi.org/10.1289/ehp.1307893.
Souza MCO, Saraiva MCP, Honda M, et al. Exposure to per- and polyfluorinated alkyl substances in pregnant Brazilian women and its association with fetal growth. Environ Res. 2020;187: 109585. https://doi.org/10.1016/j.envres.2020.109585.
Chang CJ, Barr DB, Ryan PB, et al. Per- and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: a meet-in-the-middle approach. Environ Int. 2022;158: 106964. https://doi.org/10.1016/j.envint.2021.106964.
Taibl KR, Dunlop AL, Barr DB, et al. Newborn metabolomic signatures of maternal per- and polyfluoroalkyl substance exposure and reduced length of gestation. Nat Commun. 2023;14(1):3120. https://doi.org/10.1038/s41467-023-38710-3.
Gao H. Amino acids in reproductive nutrition and health. Adv Exp Med Biol. 2020;1265:111–31. https://doi.org/10.1007/978-3-030-45328-2_7.
Manta-Vogli PD, Schulpis KH, Dotsikas Y, Loukas YL. The significant role of amino acids during pregnancy: nutritional support. J Matern Fetal Neonatal Med. 2020;33(2):334–40. https://doi.org/10.1080/14767058.2018.1489795.
Matthews DE. Review of lysine metabolism with a focus on humans. J Nutr. 2020;150(Suppl 1):2548s–55s. https://doi.org/10.1093/jn/nxaa224.
Xu K, Liu G, Fu C. The tryptophan pathway targeting antioxidant capacity in the placenta. Oxid Med Cell Longev. 2018;2018:1054797. https://doi.org/10.1155/2018/1054797.
Hood RB, Liang D, Tang Z, et al. Length of PM(25) exposure and alterations in the serum metabolome among women undergoing infertility treatment. Environ Epidemiol. 2022;6(1):191. https://doi.org/10.1097/ee9.0000000000000191.
Zhong J, Karlsson O, Wang G, et al. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc Natl Acad Sci USA. 2017;114(13):3503–8. https://doi.org/10.1073/pnas.1618545114.
Rohart F, Gautier B, Singh A, Ka LC. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13(11):e1005752. https://doi.org/10.1371/journal.pcbi.1005752.
Acknowledgements
We would like to thank all members from the Environmental Metabolomics and Exposomics Research Group at Emory (EMERGE) for their valuable input and feedback on this project.
Funding
Support for this project was provided by the National Institute of Health (NIH) research grant (R21ES032117). We also acknowledge the tremendous support from the HERCULES Exposome Research Center, supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (P30ES019776). Additionally, RBH, SH, and KS were funded by a NIEHS T32 training grant (T32ES012870).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
None to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hood, R.B., Moyd, S., Hoffman, S. et al. Metabolomics Application in Understanding the Link Between Air Pollution and Infant Health Outcomes: A Narrative Review. Curr Pollution Rep (2024). https://doi.org/10.1007/s40726-024-00313-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s40726-024-00313-x