Abstract
Purpose of Review
Major waterborne viruses comprise numerous variants rather than only a master sequence and form a genetically diverse population. High genetic diversity is advantageous for adaptation to environmental changes because the highly diverse population likely includes variants resistant to an adverse effect. Disinfection is a broadly employed tool to inactivate pathogens, but due to virus evolvability, waterborne viruses may not be inactivated sufficiently in currently applied disinfection conditions. Here, by focusing on virus population genetics, we explore possibility and factor of emergence of disinfection sensitivity change.
Recent Findings
To test whether virus population obtains disinfection resistance, the evolutionary experiment developed in the field of population genetics has been applied, indicating the change in disinfection sensitivity. It has been also confirmed that the sensitivity of environmental strains is lower than that of laboratory strains. In some of these studies, genetic diversity within a population less sensitive to disinfection is higher. Researches in virus population genetics have shown the contribution of intra-population genetic diversity to virus population phenotype, so disinfection sensitivity change may attribute to the genetic diversity.
Summary
The research elucidating a relationship between virus evolution and disinfection has only recently begun, but significant information about the relationship has been accumulated. To develop an effective disinfection strategy for the control of waterborne virus spread, we need to clarify whether disinfection practice truly affects virus outbreaks by refining both laboratory and field experiments related to virus evolution in the disinfection-exerted environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disinfection is broadly employed to inactivate pathogens in water and on environmental surfaces and foods. To ensure human health in the use of water and the consumption of foods, appropriate disinfection condition, which can be determined by mathematical approaches and bears the constant inactivation efficiency [1,2,3], needs to be sustained. Variability of environmental condition has been incorporated into the framework in determination of disinfection condition in previous studies [1,2,3]. However, the uncertainty related to virus evolvability also needs to be taken into account for sufficiently inactivating viruses since the rapidly evolved virus population comprises numerous variant sequences distinct from the master sequence (i.e., genetically diverse population). High genetic diversity affects virus phenotype due to the existence of variants (see “Effect of genetic diversity on virus phenotype”) and may increase the probability of an appearance of new phenotypes that decrease disinfection sensitivity. Therefore, it is difficult to conclude that the genetically diverse virus population is always inactivated at constant rate by disinfection. The studies related to recent viral outbreaks have indicated that the originally infrequent genotypes become dominant and the genetic diversity within a genotype increases compared to past cases (e.g., several point mutations occur in the well-known genotype) [4,5,6,7,8,9,10,11,12,13]. If genetic and population structure changes truly affect the efficiency of disinfection, unraveling the mechanism of disinfection resistance of a virus population is significant to establish proper disinfection approaches, and the change in disinfection sensitivity can be associated with current outbreaks.
Virus population genetics, especially for an RNA virus, has been progressed rapidly. Many waterborne viruses (e.g., norovirus, rotavirus and enteroviruses) are also RNA viruses, which typically lack proofreading activity and postreplicative repair during genome replication. As a result, they exhibit high mutation rates compared to DNA-based viruses and organisms. Thus, after replication, an RNA virus population forms a mutant swarm including one (or a few) master sequence (s) and minor variant sequences even in a genotype (such dynamic distribution of a mutant swarm is termed “quasispecies”) [14]. Lab-scale experiments have indicated that the genetic diversity within a population (termed “intra-population genetic diversity”) greatly affects virus phenotypes such as pathogenesis and growth ability (see “Virus Population Genetics”). The genetically diverse population has the potential to survive in hostile environment because the probability that adaptive variants exist in the population becomes high (natural selection) [15]. Also, owing to the heterotypic cooperation between variants within a highly diverse population [16], the population can include many aggregates which may keep the inner part of an aggregate intact when disinfection is conducted. Developing the population genetics of waterborne viruses must contribute to the establishment of more effective disinfection strategy that sufficiently inactivates genetically diverse populations. The problem is that the effect of population structure change on disinfection efficiency has not been encapsulated well at present.
Aim and Scope
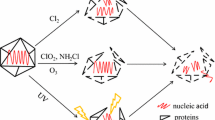
This review will address possible strategies of disinfection for waterborne viruses, in which the lability of virus population structure and its effect on disinfection efficacy are considered (Fig. 1). Before suggesting the strategy, firstly, general information of virus disinfection and some cases of different disinfection sensitivity of closely-related virus samples are summarized (see “Disinfection sensitivity of virus”). In addition, the latest researches of virus population genetics (e.g., effect of population diversity on viral phenotypes, social-like behavior of virus population) are collected to find knowledge plausible to explain variability of disinfection sensitivity (see “Virus Population Genetics”). Finally, current researches about virus evolution with disinfection are introduced in the section “Evolution Versus Disinfection,” and here, the virus evolvability links to the variability of disinfection sensitivity. Implications shown in this review can encourage researchers in water engineering to conduct disinfection experiments that take the effect of heterogeneity of virus population into account. When a lot of dataset related to virus disinfection with information of variety of the population structure is accumulated, a novel framework more accurately predicting virus inactivation efficiency can be constructed by previously suggested methods [1,2,3] in the future. Also, it can be unfolded whether the change in disinfection sensitivity is associated with occurrence of waterborne virus outbreaks.
Disinfection Sensitivity of Virus
Water Intervention and Disinfection
Water intervention is an action to clean water environments surrounding humans and keep human body parts, such as hands, sanitary. Water treatment including membrane filtration and chemical and physical disinfectants is one of the water interventions employed at water and wastewater treatment plants. To make environments clean enough to reduce health risks, the water, sanitation, and hygiene (WASH) program has recommended preparing safe water, building a basic toilet and disseminating the habit of hand washing with soap [17]. A disinfectant is useful to inactivate pathogenic viruses in water [18, 19] and is implemented at various sites, such as environmental surfaces, food processing areas, and water and wastewater treatment plants. However, it has remained unclear whether disinfection practice practically reduces the number of people infected with waterborne viruses. The WASH practice in Malawi only temporally reduced the mortality rate relative to rotavirus infection [20]. In Zimbabwe, the effect of the household-level WASH interventions on a decrease in diarrhea cases was estimated to be small [21].
Variability of Disinfection Sensitivity
The inactivation mechanisms are different among disinfectants and virus species. Usually, disinfectants decrease virus infectivity by damaging capsid proteins or nucleic acid. Free chlorine denatures the spike protein of the rotavirus OSU strain, which results in loss of binding ability to the receptor [22]. Whereas adenovirus type 2 does not lose the binding ability to the receptor and its genome is not damaged by free chlorine, the expression of the protein governing a life cycle process within a cell is inhibited [23]. Wigginton et al. investigated the inactivation mechanisms and found that free chlorine, singlet oxygen, and UV disinfection damaged the viral genome, which induced loss of the replication ability of MS2 phage [24]. Free chlorine degraded capsid proteins and the protein assembly, which resulted in loss in binding or injection abilities as well as chlorine dioxide (ClO2). In the experiment with free chlorine disinfection using hepatitis A virus (HAV), the most sensitive region to chlorine was estimated to be the 5’ non-translated region of the genome [25]. A microplasma UV, which has higher inactivation rate constant than a conventional mercury-based UV lamp, damaged the adenovirus capsid protein rather than the nucleic acid and the fiber protein [26]. Torrey et al. developed a novel method for testing whether the impairing genome functionality was one of factors of virus inactivation by transfection of a viral genome into a host cell [27]. As a result, free chlorine damaged the protein of echovirus type 11, ozone reduced genome functionality, and UV impaired both the genome and proteins. The disinfection technology such as photocatalytic disinfection has been also developed, which generates some reactive species and is expected to be disinfection byproduct-free with the capacity of virus inactivation [28]. Tong et al. confirmed that the disinfection using photocatalyzed TiO2 damaged viral RNA rather than viral proteins [29]. The metal-free photocatalytic disinfection in a membrane reactor has been also developed and has inactivated adenovirus by reacting its capsid protein [30].
Disinfection efficacy may be affected by the diversity of the nucleotide and amino acid sequences. The reaction rate of disinfectants differs according to the type of amino acids or the specific sites on a capsid protein [24, 31]. If one amino acid replacement occurs on the capsid protein-coding gene, for example, the rotavirus outer capsid consists of 260 trimers per a virion, so that 780 amino acids will be replaced by the mutation. In this case, the structural or electrostatic potential of a virion can be changed significantly by only one amino acid mutation. In addition, if the mutation dominates in the virus population, the reaction rate of disinfectants for the protein can be changed, which eventually alters the disinfection efficacy as a population. Sigstam et al. confirmed differences of disinfection sensitivity of the closely related phage strains (MS2 and fr phages shared 87% identical capsid protein sequence) [32]. Additionally, the responses of laboratory and environmental strains of coxsackievirus to disinfection differed despite the same serotype [33, 34•, 35••].
Viruses classified as the same type (genotype/serotype) appear to have a spectrum of disinfection sensitivity. Generally, a virus population includes not only a master sequence but also many variants because of higher mutation rate and forms a genetically diverse population (i.e., quasispecies). Immune response is different among individuals, which may form the individual-specific structure of a virus population. Feces, which contain individual-specific virus populations, are flown into a WWTP. The virus genetic diversity temporally increases at a WWTP by collecting and mixing such individual-specific virus populations. The disinfection sensitivity can fluctuate according to the level of genetic diversity due to slight changes in viral capsid proteins. The finding that environmental strains are less sensitive to disinfectants than laboratory strains [33•, 34••, 35] can be explained by such distinct population structures between them. To understand the behavior of waterborne viruses under processes of disinfection and establish more appropriate intervention strategy, we need to incorporate knowledge about virus population genetics with water engineering.
Virus Population Genetics
Evolution of Viral Quasispecies
Intra-population genetic diversity increases when a mutation occurs during the genome replication within a host cell. However, mutated genes are not always fixed in a virus population since some variants generated by mutation are often defective [36, 37], in which natural selection can work to eliminate such defective variants [38]. In the theory of natural selection, the frequency of strains resistant to the selection increases [38]. On the other hand, bottleneck effect randomly changes the population structure due to sudden decrease in the size of population. The change in virus population structure based on randomness is called genetic drift [15].
The hierarchy in a quasispecies (master vs minor sequences) is explained by “fitness,” which represents the ability of an individual to reproduce and survive in current environment [39]. A subpopulation has a master sequence that is likely to be selectively superior to other minor variants. When environment changes rapidly, a portion of the minor variants advantageous for the selection occurring in new environment can increase their frequency and may become a master sequence in a population.
Selection and bottleneck effect carry the quasispecies from the present to a new sequence space (a theoretical space capable of accommodating all genome sequences [38]) and alter the degree of occupation in the sequence space by the subpopulation. The movement of a quasispecies across the sequence space also changes the fitness distribution of a viral population. The model representing the relationship between sequence space and fitness is called the “fitness landscape,” in which the x-y plane is a sequence space and the z-axis is fitness (Fig. 2) [39]. Each quasispecies has coordinates on the sequence space and fitness distributions. If a beneficial mutation appears in a quasispecies, the variant begins to spread in the fitness landscape. Afterward, several variants derived from or strongly linked to the beneficial mutation (they may show lower fitness than the initial strain) gather around the coordinate of the beneficial subpopulation. The movement of such variants can form new fitness hills (or distribution). The hill initiated from a higher fitness subpopulation (or individual) is tall and sharp, whereas that initiated from a lower one is short and broad. The sharp hill may accept new mutations with difficulty since the quasispecies is already suited to a given environment [40]. Since the fitness levels are similar among individuals but the distribution spreads equally (i.e., genetically diverse and selectively almost neutral population), the flat quasispecies includes various sequences and possibly survives from the change in environment [40,41,42,43,44,45]. Furthermore, the flat quasispecies is capable of exploring the broader range of sequence space in the fitness landscape [40].
A schematic overview of fitness landscape based on ref [36]. The x-y plane and z-axis indicate the sequence space and fitness values, respectively. A major subpopulation (red) has highest fitness at the situation. Two subpopulations (blue and green) are genetically diverse and capable of expressing new phenotypes when environment changes (red allows imply the opportunity of getting new phenotype) although fitness values are lower
Effect of Genetic Diversity on Virus Phenotype
The effect of intra-population genetic diversity on virus phenotypes has been investigated by in vitro experiments. Combe et al. analyzed viral single nucleotide polymorphisms (SNPs; sequence variation having a certain frequency at a position of sequence) within a single cell [46]. They expected the occurrence of bottleneck effect under the assumption that only a small fraction of virus infectious units infected a cell. However, as a result, multiple SNPs pre-existing in the inoculum and those newly generating were detected within a single cell. This result implies that several sequence variants can exist, even in the identical infectious unit, and single cell harbors numerous variants by the cell-specific spontaneous mutation rate of virus. Combe et al. also gave suggestive result in which viral phenotype was determined not by a single major subpopulation but by a collective infectious unit including multiple variants [46]. The virus behavior within a single cell has also been investigated by both experiments and simulations, which have indicated that just a few viral genomes selected through the bottleneck contribute to infection within a cell [47]. In addition, some progenies succeed in entering the repeated infection cycle, whereas others are eliminated stochastically, which results in heterogeneous accumulation of variants among cells. As another example, when coinfection of multiple variants in a single cell is found, the drug treatment for a cure of cells that virus infected can promote the selection of drug-resistant variants [48]. These studies imply that virus transmits to a cell along with multifarious variants (collective infectious unit), which contributes to having diverse virus populations and modifies a phenotype of a virus population. Understanding the effect of intra-population genetic diversity on the pathogenesis and the survival ability is of importance to establish the strategy for inhibiting virus spread because a minor variant and/or the intra-population genetic diversity enhanced by such minorities greatly impact viral phenotypes.
Social-Like Behavior of Viruses
In a genetically diverse population, it can be imagined that an adaptive variant has the superior ability to reproduce compared to less adaptive and defective variants (i.e., competition). Additionally, some variants likely help inferior ones replicate within a cell when coinfection occurs (i.e., cooperation). Recently, remarkable interactions between variants have been reported. Sociovirology aims to elucidate the virus evolution mechanism with respect to virus social-like interactions such as competition and cooperation [16, 49]. Important research concerning virus cooperation has been done by Vignuzzi et al. [50]. They first confirmed that the genetically stable poliovirus population lost the ability to grow in unfavorable conditions, neurotropism, and virulence for animals. Then, by forcibly increasing the diversity using a chemical mutagen, the pathogenesis and the virulence were restored despite the same consensus sequence. In addition, the non-neurotropic variant was successfully isolated from the host brain after it was simultaneously inoculated with the neurotropic one. The study by Vignuzzi et al. implies that positive interaction between multiple variants enables a defective variant to enter or replicate in the host cell.
Some waterborne viruses transmit as multiple rather than single virions. Clusters of rotavirus, norovirus, and enterovirus particles are enclosed in a vesicle provided by their host cells and transmit among hosts with enhancing pathogenesis [51•, 52, 53]. The transmission unit and form of a virus cluster, including multiple particles, are termed a collective infectious unit (e.g., plaque-forming unit constitutes multiple variants rather than clonal ones) and a bloc transmission carrying multiple genomes into the host, respectively. Both styles of the collective infectious unit and bloc transmission are advantageous for maintaining a diverse mutant spectrum [54, 55]. Following the report by Combe et al. [46], the insight into the effect of cellular-level quasispecies on viral phenotypes has been refined by applying the cooperation concept [56••, 57, 58•]. Andreu-Moreno and Sanjuan illustrated whether and how viral aggregates modify the population phenotype by using monodisperse and aggregated vesicular stomatitis viruses [56••]. In this study, the aggregated virus made its replication initiation faster and enlarged the population size at the early step of replication compared to the monodisperse population because of an increase in the cellular multiplicity of infection. Also, they suggested that virus aggregate has the priority of virus replication and contributes to the mitigation of the stochastic loss of the population size. Xue et al. exhibited another example of cooperation, in which the mixed population of two influenza variants promoted the growth ability under the higher multiplicity of infection (about 0.2) [57]. A simulation study supported the benefit of bloc transmission by which the fitness of even rare variants increased and the population diversity was maintained [58•]. If the aggregation of virus frequently occurs in environment, multiple variants are able to infect simultaneously and may bear new lineages by recombination within a host. Thus, transmission of collective infectious units, including both defective and beneficial variants, is thought to give a virus population the chance of obtaining new phenotypes (e.g., escape from immune response and resistance to drugs).
Collective infection also causes competition within a population. Turner and Chao showed that, at first, a virus population grew rapidly under high multiplicity of infection (i.e., co-infection with various mutants) [59, 60]. However, they then found that the fitness decreased due to the domination of the selfishness variant (it is usually defective if intact individuals are absent in the population) monopolizing intracellular products. A defective variant, which cannot replicate alone but frequently emerges in a population (called a cheater virus), had higher fitness when coinfecting with intact individuals [61]. When another type of cheater variant coinfected, either variant was excluded from the population [61]. Relationships between other types of variants such as a colonizer (it is more efficient in killing cells when infecting alone) and a competitor (it suppresses the propagation of the colonizer when collective infection occurs) have been also explored [62]. As a result of the collective infection of colonizer and competitor, the frequency of cell killing decreased in a density-dependent manner. This study suggests that high virulence is beneficial for not only the host but also the virus.
Sociovirology is the recently developed research field, so that many mysteries remain that should be solved to develop an understanding of the nature of quasispecies and to help us establish efficient intervention frameworks. In addition, the associations among sociovirological knowledge, epidemiology and clinical virology, and a response to disinfectant are still unclear. Hence, true behavior of a genetically diverse virus population by taking social interactions in an environment in which disinfection is exerted into account needs to be identified.
Evolution Versus Disinfection
Evolutional Experiments with Disinfection
Studies related to virus evolution in the water-engineering field have been conducted recently. To validate whether and how virus population adapts to disinfection, the serial passage experiment (virus population is cultured repeatedly) developed by virus population geneticist has been applied to the water-engineering field. Zhong et al. established an experimental evolution assay based on serial passages of MS2 phage with/without ClO2 [63]. In the study, they confirmed the appearance of ClO2 less sensitive populations derived from some lineages (Table 1). Several fixed mutations on the consensus sequence, which replaced the amino acids with ClO2 less reactive ones and changed the host binding ability, were identified, although only some of the mutations were shared among less sensitive populations [63]. The research group also observed the appearance of echovirus populations less sensitive to UV and ClO2, and most of these populations had higher genetic diversity than sensitive populations (Table 1) [64••, 65]. In other words, minor variants in the quasispecies might contribute to a decrease in the disinfectant sensitivities. The ClO2 less sensitive populations likewise had less sensitivity to free chlorine (Table 1) [66]. Compared to UV less sensitive populations, the intra-population genetic diversity of ClO2 populations is likely to be higher. Rachmadi et al. also confirmed the generation of chlorine-resistant populations of murine norovirus through serial passage experiments (Table 1), and one unique amino acid replacement on the gene coding a capsid protein was shared among less sensitive populations [67•]. Less sensitive populations that experienced five passages with free chlorine showed higher intra-population genetic diversity, but at ten serial passages, intra-genetic diversity decreased due to the dominance of several mutations. Recently, an important study about virus resistance to disinfection has been reported, in which the environmental condition of global warming was taken into account [68]. In the experiment, the thermotolerant population of echovirus appeared through serial passages under higher temperatures and also exhibited less sensitivity to chlorine. This finding implies that the climate or water temperature variation may result in the site-specific population structure of waterborne virus.
Disinfection Strategy for the Disinfection-Resistant Virus Strain
Even at the quasispecies level, disinfection sensitivity decreases when specific variants that are less sensitive to disinfection emerge or when intra-population genetic diversity is higher. In the case that specific mutations are dominant in a less sensitive population, disinfection possibly acts as a form of selection, and a virus population evolves toward acquiring disinfection resistance. However, the probability that such mutation generates and then dominates in a population can be very low because mutation occurs stochastically within a host cell and then the mutated gene is exposed to various types of selection and bottleneck events. Unless the disinfection-resistant mutation is defective (e.g., low replicative ability) and lost by bottleneck effect (e.g., dilution with river or seawater), the virus population retains the mutation. Zhong et al. and Rachmadi et al. used common ancestral populations for their serial passage experiments and identified mutations that were likely to be associated with the disinfection sensitivity [63, 67•]. Confirming whether the mutations are observed in lineages derived from other ancestral populations and in environmental samples is difficult. The universality of disinfection-resistant mutations needs to be investigated by using other virus populations that have different passage histories. If disinfection works as a form of natural selection, we suggest that water engineers consider introducing multiple disinfectants and drugs at each WWTP to prevent waterborne viruses from gaining disinfection resistance (cross-resistance also needs to be investigated) (Fig. 1).
Disinfection Strategy for the Genetically Diverse Virus Population
An increase of the intra-population genetic diversity in less sensitive virus populations has also been observed in previous studies [64••, 65, 67•]. Why does the diversity increase in spite of the exposure to disinfectants? According to the study using serial passages of rotavirus populations, a bottleneck effect enables minor variants to obtain sequence spaces that were originally occupied by the major sequence and created by the bottleneck [69•]. Then, minor variants increase their frequency in the quasispecies, and consequently the intra-population genetic diversity becomes higher [69•]. If this previous research can be applied to virus evolution with disinfection, disinfection has worked as the bottleneck effect in previous evolutionary experiments with disinfection, and the intra-population genetic diversity is stochastically enhanced. In this case, one of the possible factors determining disinfection sensitivity is likely to be cooperation among variants in a genetically diverse population. Also, waterborne viruses are given off from their host cells as a vesicle cloaked-cluster [51•, 52, 53], which has more protection from a disinfectant since not all virions or not all virion surfaces are exposed to environment. Based on Fig. 2, the flat quasispecies is likely to achieve the area related to disinfection resistance according to the stochastic event (e.g., mutation and bottleneck effect) because of higher intra-population genetic diversity. Note that virus population does not adapt to disinfectants when a bottleneck effect is a major driver of population structure changes under disinfection. Therefore, all we have to do is to make the disinfectant concentration stronger or set the longer contact time because virus population does not always evolve toward gaining disinfection resistance via the bottleneck effect. To determine the appropriate disinfection intensity, the spectrum of disinfection sensitivity of waterborne viruses should be further investigated (Fig. 1).
Conclusions
The study of viruses in the water-engineering field has just begun to incorporate knowledge about virus population genetics and its experimental techniques. Nevertheless, significant information about virus evolution in disinfection-exerted environments has been accumulated, and we have the opportunity to develop an effective disinfection strategy for the control of waterborne virus spread. In the field of “Virus disinfection and population genetics,” the most important point that we need to clarify is whether all waterborne viruses evolve toward the disinfection resistance or just change its sensitivity temporally. The mechanism of virus resistance to disinfection may be hard to elucidate because of high mutation rate, but it should be deeply understood: Does a dominant subpopulation or a swarm of minor variants within a population determine the disinfection sensitivity? How do population genetic factors contribute to disinfection resistance? In the next step, to gain more accurate insight into virus evolution in human society, the lability of disinfection efficiency, which has been confirmed experimentally, even in virus samples isolated from human excreta and environmental water including drinking water and wastewater needs to be investigated. Also, how the population structure of environmental samples differs from laboratory strains and less sensitive strains generated by evolutionary experiments needs to be clarified. Virus aggregation can be formed by physicochemical interaction between variants (e.g., electrostatic potential distinct from each variant) and reduce disinfection sensitivity by protecting an inner part of the aggregate from exposure to a disinfectant, which maintains the infectivity of virions within the aggregate or those partially damaged. Therefore, future research focused on the disinfection of waterborne viruses needs to take sociovirological knowledge into account. In addition, the range of disinfection sensitivity change needs to be identified, which then enables us to establish the predictive inactivation model taking virus evolvability into account in the future (Fig 1). According to the population-genetic role of disinfection, which may vary among virus species, the most effective strategy of disinfection has to be constructed. Finally, to test one possibility that the disinfection resistance is truly related to virus outbreaks, a research framework that enables us to collect more data about virus evolution under disinfection-exerted environment should be elaborated.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kadoya S, Nishimura O, Kato H, Sano D. Predictive water virology: hierarchical Bayesian modeling for estimating virus inactivation curve. Water. 2019;11:2187.
Kadoya S, Nishimura O, Kato H, Sano D. Regularized regression analysis for the prediction of virus inactivation efficiency by chlorine disinfection. Environ Sci Wat Res Technol. 2020;6:3341–50.
Kadoya S, Nishimura O, Kato H, Sano D. Predictive water virology using regularized regression analyses for projecting virus inactivation efficiency in ozone disinfection. Wat Res X. 2021;11:100093.
Mori K, Nakazawa H, Hase S, Nagano M, Kimoto K, Oda M, et al. Whole genomic analysis of human G8P[14] group A rotavirus detected from community gastroenteritis outbreak. J Med Virol. 2018;90:1411–7.
Alleman MM, Jorba J, Greene SA, Diop OM, Iber J, Tallis G, et al. Update on vaccine-derived poliovirus outbreaks – Worldwide, July 2019 – February 2020. Morb Mortal Wkly Rep. 2020;69:489–95.
Macklin GR, O’Reilly KM, Grassly NC, Edmunds WJ, Mach O, Krishnan RSG, et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science. 2020;368:401–5.
Hassel C, Mirand A, Farkas A, Diedrich S, Huemer HP, Peigue-Lafeuille H, et al. Phylogeography of coxsackievirus A16 reveals global transmission pathways and recent emergence and spread of a recombinant genogroup. J Virol. 2017;91:e00630–17.
Monge S, Benschop K, Soetens L, Pijnacker R, Hahne S, Wallinga J, et al. Echovirus type 6 transmission clusters and the role of environmental surveillance in early warning, the Netherlands, 2007 to 2016. Eurosurveill. 2018;23:1800288.
Li J, Zhu R, Huo D, Du Y, Yan Y, Liang Z, et al. An outbreak of coxsackievirus A6-associated hand, foot, and mouth disease in kindergarten in Beijing in 2015. BMC Pediatrics. 2018;18:277.
Burr SE, Sillah A, Joof H, Bailey RL, Holland MJ. An outbreak of acute haemorrhagic conjunctivitis associated with coxsackievirus A24 variant in The Gambia. West Africa. BMC Res Notes. 2017;10:692.
Fragoso-Fonseca DE, Escobar-Escamilla N, Rodriguez-Maldonado AP, Barrera-Badillo G, Garces-Ayala F, Mendieta-Condado E, et al. Complete genome sequence of a coxsackievirus type A24 variant causing an outbreak of acute haemorrhagic conjunctivitis in southeastern Mexico in 2017. Arch Virol. 2020;165:1015–8.
Lema C, Torres C, Van der Sanden S, Cisterna D, Freire MC, Gomez RM. Global phylodynamics of echovirus 30 revealed behavior among viral lineages. Virol. 2019;531:79–92.
Chen Y, Sun Y, Yan J, Miao Z, Xu C, Zhang Y, et al. Molecular epidemiology and prevalence of echovirus 30 in Zhejiang province, China, from 2012 to 2015. J Microbiol Biotechnol. 2017;27:2221–7.
Domingo E, Escarmis C, Sevilla N, Moya A, Elena SF, Quer J, et al. Basic concepts in RNA virus evolution. FASEB. 1996;10:859–64.
Domingo E. Virus as populations: compositions, complexity, dynamics, and biological implications. USA: Elsevier Inc.; 2016.
Diaz-Munoz SL, Sanjuan R, West S. Sociovirology: Conflict, cooperation, and communication among viruses. Cell Host Microbes. 2017;22:437–41.
United Nations Children’s Fund. Title of subordinate document. In: Water, Sanitation and Hygiene (WASH). https://www.unicef.org/wash/3942_3952.html. Accessed on 14 Nov 2020.
Centers for Disease Control and Prevention. Title of subordinate document. In: Cleaning and disinfecting your home. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/disinfecting-your-home.html. Accessed on 14 Nov 2020.
Centers for Disease Control and Prevention. Title of subordinate document. In: Rotavirus and drinking water from private wells. https://www.cdc.gov/healthywater/drinking/private/wells/disease/rotavirus.html. Accessed on 14 Nov 2020.
Bar-Zeev N, King C, Phiri T, Beard J, Mvula H, Crampin AC, et al. Impact of monovalent rotavirus vaccine on diarrhoea-associated post-neonatal infant mortality in rural communities in Malawi: a population-based birth cohort study. Lancet Glob Health. 2018;6:e1036–44.
Humphrey JH, Mbuya MNN, Ntozini R, Moulton LH, Stoltzfus RJ, Tavengwa NV, et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob Health. 2019;7:e132–47.
Fuzawa M, Araud E, Li J, Shisler JL, Nguyen TH. Free chlorine disinfection mechanisms of rotaviruses and human norovirus surrogate Tulane virus attached to fresh produce surfaces. Environ Sci Technol. 2019;53:11999–2006.
Page MA, Shisler JL, Marinas BJ. Mechanistic aspects of adenovirus serotype 2 inactivation with free chlorine. Appl Environ Microbiol. 2010;76:2946–54.
Wigginton KR, Pescon BM, Sigstam T, Bosshard F, Tamar K. Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ Sci Technol. 2012;46:12069–78.
Li JW, Xin ZT, Wang XW, Zheng JL, Chao FH. Mechanisms of inactivation of hepatitis A virus by chlorine. Appl Environ Microbiol. 2002;68:4951–5.
Oh C, Sun PP, Araud E, Nguyen TH. Mechanism and efficacy of virus inactivation by a microplasma UV lamp generating monochromatic UV irradiation at 222 nm. Wat Res. 2020;186:116386.
Torrey J, von Gunten U, Kohn T. Differences in viral disinfection mechanisms as revealed by quantitative transfection of echovirus 11 genomes. Appl Environ Microbiol. 2019;85:e00961–19.
Zhang C, Li Y, Shuai D, Shen Y, Wang D. Progress and challenges in photocatalytic disinfection of waterborne viruses: a review to fill current knowledge gaps. Chem Eng J. 2019;355:399–415.
Tong Y, Shi G, Hu G, Hu X, Han L, Xie X, et al. Photo-catalyzed TiO2 inactivates pathogenic viruses by attacking viral genome. Chem Eng J. 2021;414:128788.
Zhang C, Li Y, Li J. Improved disinfection performance towards human adenovirus using an efficient metal-free heterojunction in a vis-LED photocatalytic membrane reactor: operation analysis and optimization. Chem Eng J. 2021;392:123687.
Wigginton KR, Kohn T. Virus disinfection mechanisms: the role of virus composition, structure, and function. Curr Opin Virol. 2012;2:84–9.
Sigstam T, Gannon G, Cascella M, Pescon BM, Wigginton KR, Kohn T. Subtle differences in virus composition affect disinfection kinetics and mechanisms. Appl Environ Microbiol. 2013;79:3455–67.
Wolf C, von Gunten U, Kohn T. Kinetics of inactivation of waterborne enteric viruses by ozone. Environ Sci Technol. 2018;52:2170–7.
• Torii S, Itamochi M, Katayama H. Inactivation kinetics of waterborne virus by ozone determined by a continuous quench flow system. Wat Res. 2020;186:116291 This article indicated that virus strains isolated from environmental water showed less sensitivity to ozone than laboratory strains.
•• Meister S, Verbyla ME, Klinger M, Kohn T. Variability in disinfection resistance between currently circulating enterovirus B serotypes and strains. Environ Sci Technol. 2018;52:3696–705 This article showed the difference of disinfection sensitivity between environmental and laboratory strains.
Muller HJ. The relation of recombination of mutational advance. Mut res. 1964;1:2–9.
Muller HJ. Some genetic aspects of sex. The American Naturalist. 1932;66:118–38.
Domingo E, Sheldon J, Perales C. Viral quasispecies evolution. Microbiol Mol Biol Rev. 2012;76:159–216.
Lauring AS, Frydman J, Andino R. The role of mutational robustness in RNA virus evolution. Nature Rev Microbiol. 2013;11:327–36.
Fernandez G, Clotet B, Martinez MA. Fitness landscape of human immunodeficiency virus type 1 protease quasispecies. J Virol. 2007;81:2485–96.
Lauring AD, Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010;6:e1001005.
Wilke CO. Quasispecies theory in the context of population genetics. BMC Evol Biol. 2005;5:44.
Burch CL, Chao L. Evolvability of an RNA virus is determined by its neighborhood. Nature. 2000;406:625–8.
Codoner FM, Daros J-A, Sole RV, Elena SF. The fittest versus the flattest: experimental confirmation of the quasispecies effect with subviral pathogens. PLoS Pathog. 2006;2:e136.
Sanjuan R, Cuevas JM, Furio V, Holmes EC, Moya A. Selection for robustness in mutagenized RNA virus. PLoS Genet. 2007;3:e93.
Combe M, Garijo R, Geller R, Cuevas JM, Sanjuan R. Single-cell analysis of RNA virus infection identifies multiple genetically diverse viral genomes within single infectious units. Cell Host Microbes. 2015;18:424–32.
Miyashita S, Ishibashi K, Kishino H, Ishikawa M. Viruses roll the dice: the stochastic behavior of viral genome molecules accelerates viral adaptation at the cell and tissue levels. PLoS Biol. 2015;13:e1002094.
Ke R, Li H, Wang S, Ding W, Ribeiro RM, Giorgi EE, et al. Superinfection and cure of infected cells as mechanisms for hepatitis C virus adaptation and persistence. Proc Natl Acad Sci. 2018;115:7139–48.
Diaz-Munoz SL. Uncovering virus-virus interactions by unifying approaches and harnessing high-throughput tools. mSystems. 2019;4:e00121-19.
Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439:344–8.
• Sanitana M, Ghosh S, Ho BA, Rajasekaran V, Du W-L, Mutsafi Y, et al. Vesicle-cloaked virus clusters are optimal units for inter-organismal viral transmission. Cell Host Microbes. 2018;24:208–20 This article indicated that some waterborne viruses such as norovirus and rotavirus transmitted not as single but as multiple virions enclosed in a vesicle.
Chen Y-H, Du WL, Hagemeijer MC, Takvorian PM, Pau C, Cali AB, et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–30.
Robinson SM, Tsueng G, Sin J, Mangale V, Rahawi S, McIntyre LL, et al. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014;10:e1004045.
Sanjuan R. Collective infectious units in viruses. Trends Microbiol. 2017;25:402–12.
Shirogane Y, Watanabe S, Yanagi Y. Cooperation between different variants: a unique potential for virus evolution. Virus Res. 2019;264:68–73.
•• Andreu-Moreno I, Sanjuan R. Collective infection of cells by viral aggregates promotes early viral proliferation and reveals a cellular-level Allee effect. Curr Biol. 2018;28:3212–9 This article indicated that virus phenotype was improved by the transmission form of virus aggregate and proved the benefit of cooperative interaction.
Xue KS, Hooper KA, Ollodart AR, Dingens AS, Bloom JD. Cooperation between distinct viral variants promotes growth of H3N2 influenza in cell culture. eLife. 2016;5:e13974.
• Leeks A, Segredo-Otero EA, Sanjuan R, West SA. Beneficial coinfection can promote within-host viral diversity. Virus Evol. 2018;4:vey028 This article indicated that cooperation between rare variants enhanced the fitness and maintain the genetic diversity.
Turner PE, Chao L. Sex and the evolution of intrahost competition in RNA virus φ6. Genetics. 1998;150:523–32.
Turner PE, Chao L. Prisoner’s dilemma in an RNA virus. Nature. 1999;398:441–3.
Meir M, Harel N, Miller D, Gelbart M, Eldar A, Gophana U, et al. Competition between social cheater viruses is driven by mechanistically different cheating strategies. Sci Adv. 2020;6:eabb7990.
Ojosnegros S, Beerenwinkel N, Antal T, Nowak MA, Escarmis C, Domingo E. Competition-colonization dynamics in an RNA virus. Proc Natl Acad Sci. 2010;107:2108–12.
Zhong Q, Carratala A, Nazarov S, Guerrero-Ferreira RC, Piccinini L, Bachmann V, et al. Genetic, structural, and phenotypic properties of MS2 coliphage with resistance to ClO2 disinfection. Envrion Sci Technol. 2016;50:13520–8.
•• Carratala A, Shim H, Zhong Q, Bachmann V, Jensen JD, Kohn T. Experimental adaptation of human echovirus 11 to ultraviolet radiation leads to resistance to disinfection and ribavirin. Virus Evol. 2017;3:vex035 This article showed that the disinfection sensitivity of waterborne virus decreased through the serial passages experiment with ultraviolet and the less-sensitive population had cross-resistance to a drug.
Zhong Q, Carratala A, Shim H, Bachmann V, Jenesen JD, Kohn T. Resistance of echovirus 11 to ClO2 is associated with enhanced host receptor use, altered entry routes, and high fitness. Environ Sci Technol. 2017;51:10746–55.
Zhong Q, Carratal A, Ossola R, Bachmann V, Kohn T. Cross-resistance of UV- or chlorine dioxide-resistant echovirus 11 to other disinfectants. Front Microbiol. 2017;8:1928.
• Rachmadi AT, Kitajima M, Watanabe K, Yaegashi S, Serrana J, Nakamura A. Free-chlorine disinfection as a selection pressure on norovirus. Appl Environ Microbiol. 2018;84:e00244-18 This article showed that norovirus populations less-sensitive to chlorine emerged and shared an amino acid replacement.
Carratala A, Bachmann V, Julian TR, Kohn T. Adaptation of human enterovirus to warm environments leads to resistance against chlorine disinfection. Environ Sci Technol. 2020;54:11292–300.
• Kadoya S, Urayama S-I, Nunoura T, Hirai M, Takaki Y, Kitajima M, et al. Bottleneck size-dependent changes in the genetic diversity and specific growth rate of a rotavirus A strain. J Virol. 2020;94:e02083-19 This article indicated that intra-population genetic diversity significantly affected the population growth ability.
Acknowledgements
We acknowledge funding from JSPS KAKENHI (Grant Number 19J10800) and “The Sanitation Value Chain: Designing Sanitation Systems as Eco-Community Value System” Project, Research Institute for Humanity and Nature (RIHN, Project No. 14200107). We thank Prof. Tamar Kohn, Ecole Polytechnique Federale de Lausanne, Switzerland, for the precious comments that greatly improved this article.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Funding
This study is supported by JSPS KAKENHI (Grant Number 19J10800) and "The Sanitation Value Chain: Designing Sanitation Systems as Eco-Community Value System" Project, Research Institute for Humanity and Nature (RIHN, Project No. 14200107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
There authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biology and Pollution
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadoya, Ss., Katayama, H. & Sano, D. Virus Disinfection and Population Genetics: Toward the Control of Waterborne Virus Diseases by Water Engineering. Curr Pollution Rep 7, 407–416 (2021). https://doi.org/10.1007/s40726-021-00189-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-021-00189-1