Abstract
Purpose of Review
Holm oak is a relevant species, both for its distribution and ecological importance. Among the risks looming over this species, oak decline—influenced by extreme climatic events, and alien-invasive species—is considered the main factor causing the loss of holm oak in Mediterranean open woodlands. The aim of this review is to identify and summarize the effects of drought and pathogen root rot, focusing on tree physiology, and the relationship between the stressors (biotic and abiotic) and the tree response.
Recent Findings
Symptoms of root rot are often associated with drought. However, it has been shown the presence of a differential response to root rot and severe drought is related with general defence mechanisms triggered by the plant. Soil microbiota has also been shown to be a key factor influencing health status and soil pathogen abundance. The application of next-generation sequencing techniques to forest pathology allows us to study complex relationships between soil, plant and microorganisms.
Summary
Tolerance of holm oak against Phytophthora cinnamomi root rot is related to specific hydric and photosynthetic mechanisms that differ from those associated with drought. This response involves changes in the metabolism of the photosynthetic organs of the plant which can be linked with changes in functional traits. Studies of the soil microbiome have identified several pathogens, apart from P. cinnamomi, involved in holm oak decline, and the relevance of key fungal species in the management of this syndrome. In this regard, the presence of beneficial microorganisms such as Trichoderma spp. or ectomycorrhizae influences the physiological status of trees affected by root rot.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mabberley DJ. Mabberley’s plant-book: a portable dictionary of plants, their classification and uses. 4th ed. Cambridge University Press; 2017.
•• Gil-Pelegrín E, Peguero-Pina JJ, Sancho-Knapik D, editors. Oaks physiological ecology. Exploring the functional diversity of genus Quercus L. [Internet]. Cham: Springer International Publishing; 2017 [cited 2018 Jan 23]. Available from: http://link.springer.com/10.1007/978-3-319-69099-5. Accessed 15 March 2018. This book is the latest comprehensive review which summarizes the knowledge on physiology and ecology of the genus Quercus.
Cavender-Bares J. Diversity, distribution and ecosystem services of the north american oaks. Int Oaks. 2016;27:12.
Aschmann H. Distribution and peculiarity of mediterranean ecosystems. In: di Castri F, Mooney HA, editors. Mediterranean type ecosystems [Internet]. Berlin: Springer Berlin Heidelberg; 1973. [cited 2019 Apr 20]. p. 11–9. Available from: http://link.springer.com/10.1007/978-3-642-65520-3_2.
Terradas J. Holm oak and holm oak forests: an introduction. In: Rodà F, Retana J, Gracia CA, Bellot J, editors. Ecology of Mediterranean evergreen oak forests [Internet]. Berlin: Springer Berlin Heidelberg; 1999. [cited 2019 Apr 25]. p. 3–14. Available from: http://link.springer.com/10.1007/978-3-642-58618-7_1. Accessed 20 April 2019.
Peñuelas J, Sardans J, Filella I, Estiarte M, Llusià J, Ogaya R, et al. Assessment of the impacts of climate change on Mediterranean terrestrial ecosystems based on data from field experiments and long-term monitored field gradients in Catalonia. Environ Exp Bot. 2018;152:49–59.
Caudullo G, Welk E, San-Miguel-Ayanz J. Chorological maps for the main European woody species. Data in Brief. 2017;12:662–6. Accessed 25 April 2019.
• Moreno G, Pulido FJ. The functioning, management and persistence of dehesas. In: Rigueiro-Rodróguez A, McAdam J, Mosquera-Losada MR, editors. Agroforestry in Europe [Internet]. Dordrecht: Springer Netherlands; 2008 [cited 2019 May 30]. p. 127–60. Available from: http://link.springer.com/10.1007/978-1-4020-8272-6_7. This review summarizes the evolution ofdehesasystems focusing and integrating soil degradation and management factors at different study levels.
Díaz Esteban M, Pulido DF. 6310: Dehesas perennifolias de Quercus spp. Bases ecológicas preliminares para la conservación de los tipos de hábitat de interés comunitario en España. Madrid: Ministerio de Medio Ambiente y Medio Rural y Marino; 2009.
Costa Pérez JC, Martín Vicente Á, Fernández Alés R, Estirado Oliet M. de Andalucía D. Caracterización ambiental (2006) [Internet]. Consejería de Medio Ambiente. Sevilla: Junta de Andalucía. Consejería de Medio Ambiente; 2006 [cited 2017 Apr 4]. Available from: http://www.juntadeandalucia.es/medioambiente. Accessed 04 April 2017.
Olea L, San Miguel-Ayanz A. The Spanish dehesa. A traditional Mediterranean silvopastoral system linking production and nature conservation. Grassland Science in Europe [Internet]. Badajoz, Spain; 2006 [cited 2018 Aug 28]. p. 3–13. Available from: http://www.seepastos.es/docs%20auxiliares/Actas%20Reuniones%20escaneadas/Proceedings/sessions/Opening/openning.3.pdf. Accessed 28 Aug 2018.
Horta M, Caetano P, Medeira C, Maia I, Cravador A. Involvement of the β-cinnamomin elicitin in infection and colonisation of cork oak roots by Phytophthora cinnamomi. Eur J Plant Pathol. 2010;127:427–36.
• Gea-Izquierdo G, Fernández-de-Uña L, Cañellas I. Growth projections reveal local vulnerability of Mediterranean oaks with rising temperatures. For Ecol Manag. 2013;305:282–93 This paper is one of the first approaches which integrates functional responses and dendrochronology together with nonlinear relationships between growth and climate to assess the future vulnerability ofQuercusspp. in response to climate change.
Moreno G, Aviron S, Berg S, Crous-Duran J, Franca A, de Jalón SG, et al. Agroforestry systems of high nature and cultural value in Europe: provision of commercial goods and other ecosystem services. Agrofor Syst. 2018;92:877–91.
Sánchez M, Caetano P, Ferraz J, Trapero A. Phytophthora disease of Quercus ilex in south-western Spain. For Pathol. 2002;32:5–18.
Duque-Lazo J, Navarro-Cerrillo RM, van Gils H, Groen TA. Forecasting oak decline caused by Phytophthora cinnamomi in Andalusia: identification of priority areas for intervention. For Ecol Manag. 2018;417:122–36.
Frisullo S, Lima G, Magnano di San Lio G, Camele I, Melissano L, Puglisi I, et al. Phytophthora cinnamomi involved in the decline of holm oak (Quercus ilex) stands in Southern Italy. For Sci. 2018;64:290–8.
Pérez-Sierra A, López-García C, León M, García-Jiménez J, Abad-Campos P, Jung T. Previously unrecorded low-temperature Phytophthora species associated with Quercus decline in a Mediterranean forest in eastern Spain. For Pathol. 2013;43:331–9.
•• Oßwald W, Fleischmann F, Rigling D, Coelho AC, Cravador A, Diez J, et al. Strategies of attack and defence in woody plant–Phytophthora interactions. For Path. 2014;44:169–90 This work presents a very clear scheme of the different types of interaction between oomycete and woody plants. This review examines in depth some specific mechanisms of great relevance, paying attention to all the different views regarding the specific interaction.
Burgess TI, Scott JK, Mcdougall KL, Stukely MJC, Crane C, Dunstan WA, et al. Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Glob Chang Biol. 2017;23:1661–74.
Jorrin-Novo J, Navarro Cerrillo RM. Variabilidad y respuesta a distintos estreses en poblaciones de encina (Quercus ilex L.) en Andalucía mediante una aproximación proteómica. Ecosistemas. 2014;23:99–107.
Trumbore S, Brando P, Hartmann H. Forest health and global change. Science. 2015;349:814–8.
Jung T, Blaschke H, Osswald W. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathol. 2000;49:706–18.
Manion PD, Lachance D. Forest decline concepts. APS Press; 1992.
Allen CD, Breshears DD, McDowell NG. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere. 2015;6:art129.
Hernández-Lambraño RE, Rodríguez de la Cruz D, Sánchez-Agudo JA. Spatial oak decline models to inform conservation planning in the Central-Western Iberian Peninsula. For Ecol Manag. 2019;441:115–26.
Sales-Baptista E, d’Abreu MC, Ferraz-de-Oliveira MI. Overgrazing in the Montado? The need for monitoring grazing pressure at paddock scale. Agrofor Syst. 2016;90:57–68.
Duque-Lazo J, Navarro-Cerrillo RM. What to save, the host or the pest? The spatial distribution of xylophage insects within the Mediterranean oak woodlands of Southwestern Spain. For Ecol Manag. 2017;392:90–104.
Senado. Ponencia de Estudio Sobre la Protección del Ecosistema de la Dehesa. Senado; 2010 p. 26. Report No.: 543/000009.
Bourgeade P, Bourioug M, Macor S, Alaoui-Sossé L, Alaoui-Sossé B, Aleya L. Potential vulnerability of oak forests to climate change-induced flooding: effects of mild oxygen deficiency on Quercus robur and Quercus petraea seedling physiology. Environ Sci Pollut Res. 2018;25:5550–7.
de la Ruiz Torre J. Flora Mayor. ICONA (Organismo Autónomo de Parques Nacionales). 2006.
Robert EMR, Mencuccini M, Martínez-Vilalta J. The anatomy and functioning of the xylem in oaks. In: Gil-Pelegrín E, Peguero-Pina JJ, Sancho-Knapik D, editors. Oaks physiological ecology exploring the functional diversity of genus Quercus L [Internet]. Cham: Springer International Publishing; 2017 [cited 2019 May 16]. p. 261–302. Available from. https://doi.org/10.1007/978-3-319-69099-5_8.
Quero JL, Sterck FJ, Martínez-Vilalta J, Villar R. Water-use strategies of six co-existing Mediterranean woody species during a summer drought. Oecologia. 2011;166:45–57.
Lopez B, Sabate S, Gracia C. Fine roots dynamics in a Mediterranean forest: effects of drought and stem density. Tree Physiol. 1998;18:601–6.
Erwin DC, Ribeiro OK. Phytophthora diseases worldwide. APS Press; 1996.
Robin C, Capron G, Desprez-Loustau ML. Root infection by Phytophthora cinnamomi in seedlings of three oak species. Plant Pathol. 2001;50:708–16.
Corcobado T, Cubera E, Juárez E, Moreno G, Solla A. Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agric For Meteorol. 2014;192–193:1–8.
Ogaya R, Peñuelas J. Comparative field study of Quercus ilex and Phillyrea latifolia: photosynthetic response to experimental drought conditions. Environ Exp Bot. 2003;50:137–48.
Limousin JM, Rambal S, Ourcival JM, Rocheteau A, Joffre R, Rodriguez-Cortina R. Long-term transpiration change with rainfall decline in a Mediterranean Quercus ilex forest. Glob Chang Biol. 2009;15:2163–75.
IPCC. Climate Change 2014: mitigation of Climate Change: Working Group III Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Internet]. In: Edenhofer O, Pitchs-Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K, et al., editors. . New York: Cambridge University Press; 2014. [cited 2019 May 20]. Available from: https://archive.ipcc.ch/pdf/assessment-report/ar5/syr/AR5_SYR_FINAL_All_Topics.pdf. Accessed 20 May 2019.
Gea-Izquierdo G, Nicault A, Battipaglia G, Dorado-Liñán I, Gutiérrez E, Ribas M, et al. Risky future for Mediterranean forests unless they undergo extreme carbon fertilization. Glob Chang Biol. 2017;23:2915–27.
Vidal-Macua JJ, Ninyerola M, Zabala A, Domingo-Marimon C, Pons X. Factors affecting forest dynamics in the Iberian Peninsula from 1987 to 2012. The role of topography and drought. For Ecol Manag. 2017;406:290–306.
Peguero-Pina JJ, Mendoza-Herrer Ó, Gil-Pelegrín E, Sancho-Knapik D. Cavitation limits the recovery of gas exchange after severe drought stress in holm oak (Quercus ilex L.). Forests. 2018;9:443.
Natalini F, Alejano R, Vázquez-Piqué J, Cañellas I, Gea-Izquierdo G. The role of climate change in the widespread mortality of holm oak in open woodlands of southwestern Spain. Dendrochronologia. 2016;38:51–60.
• Moreno-Fernández D, Ledo A, Martín-Benito D, Cañellas I, Gea-Izquierdo G. Negative synergistic effects of land-use legacies and climate drive widespread oak decline in evergreen Mediterranean open woodlands. For Ecol Manag. 2019;432:884–94 This work introduces the role of land use legacy as a driver of tree decline in afforested areas ofQuercus ilexL., evidencing synergistic effects of different factors in the vulnerability assessment of forest species in front of global change.
Hernández-Lambraño RE, González-Moreno P, Sánchez-Agudo JÁ. Environmental factors associated with the spatial distribution of invasive plant pathogens in the Iberian Peninsula: the case of Phytophthora cinnamomi Rands. For Ecol Manag. 2018;419–420:101–9.
Ahanger RA, Bhat HA, Bhat TA, Ganie SA, Lone AA, Ganai SA, et al. Impact of climate change on plant diseases. Anim Sci. 2013;12.
Prospero S, Cleary M. Effects of host variability on the spread of invasive forest diseases. Forests. 2017;8:80.
• Sena K, Crocker E, Vincelli P, Barton C. Phytophthora cinnamomi as a driver of forest change: Implications for conservation and management. For Ecol Manag. 2018;409:799–807 This work reviews the impacts ofP. cinnamomion forest ecosystems around the world, focused on the disease diagnosis and symptoms and the management strategies to improve the control of theP. cinnamomi’s dispersion.
Liebhold AM, Brockerhoff EG, Kalisz S, Nuñez MA, Wardle DA, Wingfield MJ. Biological invasions in forest ecosystems. Biol Invasions. 2017;19:3437–58.
Rizzo DM, Garbelotto M. Sudden oak death: endangering California and Oregon forest ecosystems. Front Ecol Environ. 2003;1:197–204.
Vettraino AM, Morel O, Perlerou C, Robin C, Diamandis S, Vannini A. Occurrence and distribution of Phytophthora species in European chestnut stands, and their association with Ink Disease and crown decline. Eur J Plant Pathol. 2005;111:169.
Thomas FM, Blank R, Hartmann G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For Pathol. 2002;32:277–307.
Brasier CM. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann For Sci. 1996;53:347–58.
Vettraino AM, Barzanti GP, Bianco MC, Ragazzi A, Capretti P, Paoletti E, et al. Occurrence of Phytophthora species in oak stands in Italy and their association with declining oak trees. For Pathol. 2002;32:19–28.
Brasier CM, Scott JK. European oak declines and global warming: a theoretical assessment with special reference to the activity of Phytophthora cinnamomi. EPPO Bulletin. 1994;24:221–32.
Brasier CM, Robredo F, Ferraz JFP. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathol. 1993;42:140–5.
Moralejo E, García-Muñoz JA, Descals E. Susceptibility of Iberian trees to Phytophthora ramorum and P. cinnamomi. Plant Pathol. 2009;58:271–83.
Maurel M, Robin C, Capron G, Desprez-Loustau M-L. Effects of root damage associated with Phytophthora cinnamomi on water relations, biomass accumulation, mineral nutrition and vulnerability to water deficit of five oak and chestnut species. For Pathol. 2001;31:353–69.
De Rigo D, Cadullo G. Quercus ilex in Europe: distribution, habitat, usage and threats. In: European Atlas of Forest Tree Species [Internet]. Luxembourg: Publication Office of the European Union; 2016. p. 152–3. [cited 2018 Jan 18] Available from: http://forest.jrc.ec.europa.eu/european-atlas-of-forest-tree-species/. Accessed 15 March 2018.
• Hardham AR, Blackman LM. Phytophthora cinnamomi. Mol Plant Pathol. 2018;19:260–85 This paper summarizes the latest advances on the knowledge of molecular mechanisms of the plant-pathogen interaction forPhytophthora cinnamomi.
• Ruiz Gómez FJ, Navarro-Cerrillo RM, Sánchez-Cuesta R, Pérez-de-Luque A. Histopathology of infection and colonization of Quercus ilex fine roots by Phytophthora cinnamomi. Plant Pathol. 2015;64:605–16 This is the first specific work describing the interaction betweenP. cinnamomiandQ. ilexat histological level, identifying host responses, pathogen adaptation and proposing a conceptual model of pathogen development into the root.
Redondo MA, Pérez-Sierra A, Abad-Campos P, Torres L, Solla A, Reig-Armiñana J, et al. Histology of Quercus ilex roots during infection by Phytophthora cinnamomi. Trees. 2015;29:1943–57.
Ruiz Gómez FJ, Sanchez-Cuesta R, Navarro-Cerrillo RM, Perez-de-Luque A. A method to quantify infection and colonization of holm oak (Quercus ilex) roots by Phytophthora cinnamomi. Plant Methods. 2012;8:39.
Hardham AR. Phytophthora cinnamomi. Mol Plant Pathol. 2005;6:589–604.
Crone M, McComb JA, O’Brien PA, Hardy GESJ. Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biology. 2013;117:112–23.
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL. Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Aust J Bot. 2008;56:279–310.
• Català S, Berbegal M, Pérez-Sierra A, Abad-Campos P. Metabarcoding and development of new real-time specific assays reveal Phytophthora species diversity in holm oak forests in eastern Spain. Plant Pathol. 2017;66:115–23 This is the first work assessing oomycete diversity in the holm oak forests of the east of Spain, including the combination of 454 pyrosequencing and qPCR to quantify the concentration of inoculum in the soil.
• Ruiz Gómez FJR, Navarro-Cerrillo RM, Pérez-de-Luque A, Oβwald W, Vannini A, Morales-Rodríguez C. Assessment of functional and structural changes of soil fungal and oomycete communities in holm oak declined dehesas through metabarcoding analysis. Sci Rep. 2019;9:5315 This paper describes the structure and the biodiversity of the soil community in declined holm oak dehesas, and their relationship with the symptoms of holm oak decline. Also, it is the first work using NGS metabarcoding techniques to compare fungal and oomycete communities in forest soils.
Corcobado T, Cubera E, Pérez-Sierra A, Jung T, Solla A. First report of Phytophthora gonapodyides involved in the decline of Quercus ilex in xeric conditions in Spain. New Disease Rep. 2010;22:33.
Romero MA, Sánchez JE, Jiménez JJ, Belbahri L, Trapero A, Lefort F, et al. New Pythium taxa causing root rot on Mediterranean Quercus species in south-west Spain and Portugal. J Phytopathol. 2007;155:289–95.
Sánchez ME, Andicoberry S, Trapero A. Pathogenicity of three Phytophthora spp. causing late seedling rot of Quercus ilex ssp. ballota. For Pathol. 2005;35:115–25.
Mora-Sala B, Berbegal M, Abad-Campos P. The use of qPCR reveals a high frequency of Phytophthora quercina in two Spanish holm oak areas. Forests. 2018;9:697.
Mora-Sala B, Abad-Campos P, Berbegal M. Response of Quercus ilex seedlings to Phytophthora spp. root infection in a soil infestation test. Eur J Plant Pathol. 2018;154:215–25.
Corcobado T, Miranda-Torres JJ, Martín-García J, Jung T, Solla A. Early survival of Quercus ilex subspecies from different populations after infections and co-infections by multiple Phytophthora species. Plant Pathol. 2017;66:792–804.
Blanchette RA, Biggs AR. Defense mechanisms of woody plants against fungi. Springer Science & Business Media; 2013.
Jackson MB. Long-distance signalling from roots to shoots assessed: the flooding story. J Exp Bot. 2002;53:175–81.
Blaschke H. Decline symptoms on roots of Quercus robur. For Pathol. 1994;24:386–98.
Cahill D. Cellular and histological changes induced by Phytophthora cinnamomi in a group of plant species ranging from fully susceptible to fully resistant. Phytopathology. 1989;79:417.
Brummer M, Arend M, Fromm J, Schlenzig A, Oßwald WF. Ultrastructural changes and immunocytochemical localization of the elicitin quercinin in Quercus robur L. roots infected with Phytophthora quercina. Physiol Mol Plant Pathol. 2002;61:109–20.
Portz RL, Fleischmann F, Koehl J, Fromm J, Ernst D, Pascholati SF, et al. Histological, physiological and molecular investigations of Fagus sylvatica seedlings infected with Phytophthora citricola. For Pathol. 2011;41:202–11.
Kukowski KR, Schwinning S, Schwartz BF. Hydraulic responses to extreme drought conditions in three co-dominant tree species in shallow soil over bedrock. Oecologia. 2013;171:819–30.
Rodríguez-Calcerrada J, Sancho-Knapik D, Martin-StPaul NK, Limousin J-M, McDowell NG, Gil-Pelegrín E. Drought-induced oak decline—factors involved, physiological dysfunctions, and potential attenuation by forestry practices. In: Gil-Pelegrín E, Peguero-Pina JJ, Sancho-Knapik D, editors. Oaks physiological ecology exploring the functional diversity of genus Quercus L [Internet]. Cham: Springer International Publishing; 2017. p. 419–51. [cited 2019 May 21] Available from. https://doi.org/10.1007/8-3-319-69099-5_13.
Sghaier-Hammami B, Valero-Galvàn J, Romero-Rodríguez MC, Navarro-Cerrillo RM, Abdelly C, Jorrín-Novo J. Physiological and proteomics analyses of holm oak (Quercus ilex subsp. ballota [Desf.] Samp.) responses to Phytophthora cinnamomi. Plant Physiol Biochem. 2013;71:191–202.
Swiecki TJ, Berndhart EA. Testing and implementing methods for managing Phytophthora root diseases in California native habitats and restoration sites. San Francisco: Proceedings of the Sudden Oak Death Sixth Science Symposium; 2017. p. 53.
• Ruiz Gómez F, Pérez-de-Luque A, Sánchez-Cuesta R, Quero J, Navarro Cerrillo R. Differences in the response to acute drought and Phytophthora cinnamomi Rands Infection in Quercus ilex L. seedlings. Forests. 2018;9:634 This paper shows the results of the first experiment which assess the interaction between acute drought andP. cinnamomiroot rot inQ. ilexseedlings under controlled conditions. The statistical analysis of physiology variables showed the decouplement between hydric mechanisms and photosynthesis in inoculated seedlings.
León I, García J, Fernández M, Vázquez-Piqué J, Tapias R. Differences in root growth of Quercus ilex and Quercus suber seedlings infected with Phytophthora cinnamomi. Silva Fennica [Internet]. 2017 [cited 2017 Oct 9];51. Available from: https://www.silvafennica.fi/article/6991
• Corcobado T, Cubera E, Moreno G, Solla A. Quercus ilex forests are influenced by annual variations in water table, soil water deficit and fine root loss caused by Phytophthora cinnamomi. Agric For Meteorol. 2013;169:92–9 On this work, authors related the influence ofP. cinnamomiwith the hydric stress suffered by trees in the field, concluding that the relationship between both stressors is determinant in tree survival.
Turco E, Close TJ, Fenton RD, Ragazzi A. Synthesis of dehydrin-like proteins in Quercus ilex L. and Quercus cerris L. seedlings subjected to water stress and infection with Phytophthora cinnamomi. Physiol Mol Plant Pathol. 2004;65:137–44.
Ruiz Gómez FJ. Study of the interaction between root rot oomycetes and Quercus ilex L. [Internet]. [Idep - Universidad de Córdoba]: Universidad de Córdoba; 2018. Available from: http://helvia.uco.es/xmlui/handle/10396/17454. Accessed 05 Dec 2018.
Reeksting BJ, Taylor NJ, van den Berg N. Flooding and Phytophthora cinnamomi: Effects on photosynthesis and chlorophyll fluorescence in shoots of non-grafted Persea americana (Mill.) rootstocks differing in tolerance to Phytophthora root rot. S Afr J Bot. 2014;95:40–53.
Caretto S, Linsalata V, Colella G, Mita G, Lattanzio V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int J Mol Sci. 2015;16:26378–94.
Borghetti M, Grace J, Raschi A, editors. Water transport in plants under climatic stress: proceedings of an international workshop, held in Vallombrosa, Firenze, Italy. Cambridge ; New York: Cambridge University Press; 1993.
Canny M. Tyloses and the maintenance of transpiration. Ann Bot. 1997;80:565–70.
Carnicer J, Coll M, Pons X, Ninyerola M, Vayreda J, Peñuelas J. Large-scale recruitment limitation in Mediterranean pines: the role of Quercus ilex and forest successional advance as key regional drivers. Glob Ecol Biogeogr. 2014;23:371–84.
Cavender-Bares J, Ramírez-Valiente JA. Physiological evidence from common garden experiments for local adaptation and adaptive plasticity to climate in American live oaks (Quercus Section Virentes): implications for conservation under global change. In: Gil-Pelegrín E, Peguero-Pina JJ, Sancho-Knapik D, editors. Oaks physiological ecology exploring the functional diversity of genus Quercus L [Internet]. Cham: Springer International Publishing; 2017. p. 107–35. [cited 2019 May 21] Available from. https://doi.org/10.1007/978-3-319-69099-5_4.
• Navarro-Cerrillo RM, Ruiz Gómez FJ, Cabrera-Puerto RJ, Sánchez-Cuesta R, Palacios Rodriguez G, Quero Pérez JL. Growth and physiological sapling responses of eleven Quercus ilex ecotypes under identical environmental conditions. For Ecol Manag. 2018;415–416:58–69 This work studies the influence of genetic variability and environmental pressure in plant growth and physiology, in a common garden of holm oak progenies coming from selected seed sources of Andalusia.
Rutten G, Gómez-Aparicio L. Plant-soil feedbacks and root responses of two Mediterranean oaks along a precipitation gradient. Plant Soil. 2018;424:221–31.
Gómez-Aparicio L, Ibáñez B, Serrano MS, De Vita P, Ávila JM, Pérez-Ramos IM, et al. Spatial patterns of soil pathogens in declining Mediterranean forests: implications for tree species regeneration. New Phytol. 2012;194:1014–24.
Avila JM, Linares JC, García-Nogales A, Sánchez ME, Gómez-Aparicio L. Across-scale patterning of plant–soil–pathogen interactions in Quercus suber decline. Eur J Forest Res. 2017;136:677–88.
Cardillo E, Acedo A, Abad E. Topographic effects on dispersal patterns of Phytophthora cinnamomi at a stand scale in a Spanish heathland. PLoS One. 2018;13:e0195060.
Pérez-de-Luque A, Tille S, Johnson I, Pascual-Pardo D, Ton J, Cameron DD. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Scientific Reports [Internet]. 2017 [cited 2018 Jun 2];7. Available from: http://www.nature.com/articles/s41598-017-16697-4. Accessed 02 June 2018.
Tamayo-Vélez Á, Osorio NW. Soil fertility improvement by litter decomposition and inoculation with the fungus Mortierella sp. in avocado plantations of Colombia. Commun Soil Sci Plant Anal. 2018;49:139–47.
Dickie IA, Guza RC, Krazewski SE, Reich PB. Shared ectomycorrhizal fungi between a herbaceous perennial (Helianthemum bicknellii) and oak (Quercus) seedlings. New Phytol. 2004;164:375–82.
Mosca E, Montecchio L, Barion G, Dal Cortivo C, Vamerali T. Combined effects of thinning and decline on fine root dynamics in a Quercus robur L. forest adjoining the Italian Pre-Alps. Ann Bot. 2017;119:1235–46.
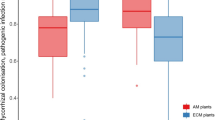
•• Corcobado T, Vivas M, Moreno G, Solla A. Ectomycorrhizal symbiosis in declining and non-declining Quercus ilex trees infected with or free of Phytophthora cinnamomi. For Ecol Manag. 2014;324:72–80 This study demonstrates the relationship between the presence and abundance of ectomycorrhizae with a lower severity of decline symptoms in holm oak trees.
Dunstan WA, Rudman T, Shearer BL, Moore NA, Paap T, Calver MC, et al. Containment and spot eradication of a highly destructive, invasive plant pathogen (Phytophthora cinnamomi) in natural ecosystems. Biol Invasions. 2010;12:913–25.
• Aleandri MP, Chilosi G, Bruni N, Tomassini A, Vettraino AM, Vannini A. Use of nursery potting mixes amended with local Trichoderma strains with multiple complementary mechanisms to control soil-borne diseases. Crop Prot. 2015;67:269–78. In this work, authors demonstrated the ability ofTrichodermato controlPhytophthorasoilborne pathogens which affectQuercusspecies.
Morales-Rodríguez C, Bastianelli G, Aleandri M, Chilosi G, Vannini A. Application of Trichoderma spp. complex and biofumigation to control damping-off of Pinus radiata D.Don caused by Fusarium circinatum Nirenberg and O’Donnell. Forests. 2018;9:421.
Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M. Trichoderma–plant–pathogen interactions. Soil Biol Biochem. 2008;40:1–10.
Ros M, Raut I, Santisima-Trinidad AB, Pascual JA. Relationship of microbial communities and suppressiveness of Trichoderma fortified composts for pepper seedlings infected by Phytophthora nicotianae. PLoS One. 2017;12:e0174069.
Chemeltorit PP, Mutaqin KH, Widodo W. Combining Trichoderma hamatum THSW13 and Pseudomonas aeruginosa BJ10–86: a synergistic chili pepper seed treatment for Phytophthora capsici infested soil. Eur J Plant Pathol. 2017;147:157–66.
Promwee A, Yenjit P, Issarakraisila M, Intana W, Chamswarng C. Efficacy of indigenous Trichoderma harzianum in controlling Phytophthora leaf fall (Phytophthora palmivora) in Thai rubber trees. J Plant Diseases Protect. 2017;124:41–50.
Siddaiah CN, Satyanarayana NR, Mudili V, Kumar Gupta V, Gurunathan S, Rangappa S, et al. Elicitation of resistance and associated defense responses in Trichoderma hamatum induced protection against pearl millet downy mildew pathogen. Sci Rep. 2017;7:43991.
Kong P, Hong C. Biocontrol of boxwood blight by Trichoderma koningiopsis Mb2. Crop Prot. 2017;98:124–7.
Haddad PE, Leite LG, Lucon CMM, Harakava R. Selection of Trichoderma spp. strains for the control of Sclerotinia sclerotiorum in soybean. Pesq Agrop Brasileira. 2017;52:1140–8.
Acknowledgements
The authors thank the “Campus de Excelencia Agroalimentaria” (CeiA3); the University of Córdoba and the “Agencia de Medio Ambiente y Agua”; “Red SEDA”; and “Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible” (Junta de Andalucía) for their continuous support of the study of holm oak decline in the Andalusian territory.
Funding
The first author is supported by the project ESPECTRAMED (CGL2017-86161-R), funded by the Spanish Ministry of Economy, Industry and Competitiveness. We also thank Prof. Maurizio Mencuccini for his suggestion that we write this review, and Mr. Rafael Sánchez-Cuesta for allowing us access to unpublished data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interests.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Physiological Processes
Rights and permissions
About this article
Cite this article
Ruiz-Gómez, F.J., Pérez-de-Luque, A. & Navarro-Cerrillo, R.M. The Involvement of Phytophthora Root Rot and Drought Stress in Holm Oak Decline: from Ecophysiology to Microbiome Influence. Curr Forestry Rep 5, 251–266 (2019). https://doi.org/10.1007/s40725-019-00105-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-019-00105-3