Abstract
Biocementation, i.e., the production of biomimetic cement through the metabolic activity of microorganisms, offers exciting new prospects for various civil and environmental engineering applications. This paper presents a systematic literature review on a biocementation pathway, which uses the carbonic anhydrase (CA) activity of microorganisms that sequester CO2 to produce biocement. The aim is the future development of this technique for civil and (geo-)environmental engineering applications towards CO2-neutral or negative processes. After screening 248 potentially relevant peer-reviewed journal papers published between 2002 and 2023, 38 publications studying CA-biocementation were considered in the review. Some of these studies used pure CA enzyme rather than bacteria-produced CA. Of these studies, 7 used biocementation for self-healing concrete, 6 for CO2 sequestration, 10 for geotechnical applications, and 15 for (geo-)environmental applications. A total of 34 bacterial strains were studied, and optimal conditions for their growth and enzymatic activity were identified. The review concluded that the topic is little researched; more studies are required both in the laboratory and field (particularly long-term field experiments, which are totally lacking). No studies on the numerical modelling of CA-biocementation and the required kinetic parameters were found. The paper thus consulted the more widely researched field of CO2 sequestration using the CA-pathway, to identify other microorganisms recommended for further research and reaction kinetic parameters for numerical modelling. Finally, challenges to be addressed and future research needs were discussed.
Highlights
-
A review of biocementation via carbonic anhydrase (CA) was conducted.
-
The role of CA in the biocementation mechanism was discussed.
-
The application of CA in civil and geo-environmental engineering was reviewed and discussed.
-
Challenges, research gaps and prospects of CA application were identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to recent estimates (United Nations Environment Programme 2022) 15% of global carbon emissions are due to construction operations and the manufacture of construction materials. Historically, the most significant of these construction materials has been cement which has been reckoned to contribute 7% or 8% of global carbon dioxide emissions (Mavroulidou et al. 2015). While cement is primarily used in making concrete it has also been used for other purposes in civil construction, e.g., ground improvement. Together with the other main chemical substance used in ground improvement (lime), cement is an aggressive chemical which has the potential for causing environmental pollution.
Natural soils such as peat play a significant role in the natural carbon cycle but are unsuitable as foundation soils and need improvement (Safdar et al. 2021a, 2022). With increasing environmental awareness and the mandate to improve sustainability in the industry, engineers are now striving to find ways of providing economical and environmentally responsible ways of improving the properties of such soils. Thus, it is unsurprising that intensive research worldwide focuses on producing low-carbon cement via microbially induced calcium carbonate precipitation (MICP). Biocements are emerging as the most promising and transformative nature-based material in the construction industry. Biocements are binding agents produced through the natural biological process of biomineralisation, i.e., the biological production of minerals due to metabolic processes of different types of microorganisms/plants. Worldwide research efforts have focused on the potential application of biocements in the civil and environmental engineering industry, and a few commercial products have started entering the market. Applications include surface treatment or crack repairs in concrete (De Muynck et al. 2008; Zheng and Qian 2020b, Li and Qu 2015; Van Tittelboom et al. 2010; Sharma et al. 2017; Wiktor and Jonkers 2011; Achal et al. 2013; Joshi et al. 2021), biobricks (Bu et al. 2018; Bernardi et al. 2014; Lambert and Randall 2019; Kumar et al. 2019), restoration of heritage buildings (Castanier et al. 2000; Jroundi et al. 2012; Ortega-Villamagua et al. 2020; Tiano et al. 1999), and soil stabilisation (Al Qabany and Soga 2013; Al-Thawadi 2011; DeJong et al. 2010; Jiang et al. 2020; Gomez et al. 2017; Keykha et al. 2019; Martinez et al. 2014; Moravej et al. 2018; Oliveira et al. 2017; Punnoi et al. 2021; Safdar et al. 2021a, b).
Other applications include wind and water erosion control and suppression of dust generated from natural processes or construction activities (Cheng et al. 2021; Clarà Saracho et al. 2021; Dubey et al. 2021; Jiang et al. 2019; Salifu et al. 2016), bioremediation (Achal et al. 2012; de Oliveira et al. 2021; Li et al. 2016; Mwandira et al. 2017; Song et al. 2022; Zhang et al. 2022), and water treatment (Duarte-Nass et al. 2020; Mitchell and Ferris 2005; Torres-Aravena et al. 2018). The introduction of biocement as a potentially environmentally friendly and sustainable material is a nature-based solution. Microorganisms offer the potential of natural self-healing of biocement (Botusharova et al. 2020; da Silva et al. 2015; Tziviloglou et al. 2016). Very importantly, several microorganisms offer the potential to capture CO2 to use it to produce biominerals via carbonic anhydrase enzyme (Yadav et al. 2014). Moreover, the diversity of microorganisms can offer vital enzymes, withstanding the extreme operating conditions of the process. Consequently, upon further development, bio-based CO2 mineralisation to produce biocement by capturing CO2 can offer economic and environmental benefits over other chemicals/physical-based mineralisation processes (Jansson and Northen 2010).
Biobased alternatives to conventional cements have surged in the past decades, with the use of MICP getting particular attention. However, no comprehensive literature review has examined the carbonic anhydrase pathway. This pathway has been proposed to produce novel CO2-absorbing biocements towards civil/(geo-)environmental engineering industry activities that are potentially neutral or carbon negative, and can play an important role in the net carbon zero strategies globally.
Therefore, the specific objectives of the current review were to: identify the evolution and trends in biocementation via carbonic anhydrase and discuss its merits and demerits; examine the different civil and geo-environmental engineering applications of the CA pathway and factors affecting its development; and finally, provide an outlook on applications, potential advantages, and limitations. The results of this study will significantly improve the understanding of the present status of biocementation via carbonic anhydrase and identify gaps for future research leading to new perspectives and options for future studies.
1.1 Biocementation Pathways and their Merits and Demerits
Biocementation can be achieved via several pathways, including ureolysis, carbonic anhydrase, denitrification, methane oxidation, photosynthesis, iron reduction, and sulphate reduction. These pathways have been discussed in a number of recent reviews (e.g., Castro-Alonso et al. 2019; He et al. 2020; Ivanov and Stabnikov 2020; Jain et al. 2021; Khodadadi et al. 2017; Lee and Park 2018). Therefore, with the exception of the carbonic anhydrase pathway (the focus of this review), the other pathways are only briefly presented in the subsections below and summarised in Table 1. Each figure in the table illustrates the chemical reaction for producing biominerals via MICP.
1.1.1 Ureolysis-driven Biocementation
Ureolytic-driven biocementation utilises urease enzyme which catalyses the hydrolysis of urea into ammonia and carbamate, subsequently leading to the formation of bicarbonate, ammonium, and hydroxyl ions. The local increase in pH due to the hydroxyl ions shifts the bicarbonate equilibrium, leading to the formation of carbonate ions. In the presence of soluble calcium ions, CaCO3 precipitation can thus occur (Safdar et al. 2022). The most commonly used bacteria for this pathway are Sporosarcina pasteurii because they produce high concentrations of urease enzyme and are non-pathogenic (Nasser et al. 2022; Hadi et al. 2022). Additionally, indigenous ureolytic strains have been isolated worldwide from: a) mine tailings (Mwandira et al. 2019); b) caves (Li et al. 2019); c) beach rock (Imran et al. 2019); and d) indigenous soils (Burbank et al. 2012; Safdar et al. 2022).
The ureolytic pathway is the one most commonly applied and developed. The process is fast and easy to control (Stabnikov et al. 2022; Dilrukshi et al. 2018). However, it produces ammonium byproducts which are undesirable from an environmental point of view (Parvathy et al. 2023; Insausti et al. 2020). Moreover, if ammonium is oxidised, it may create acidic conditions dissolving the CaCO3 precipitate over time (Khodadadi et al. 2017).
1.1.2 Denitrification Pathway
The denitrification pathway uses denitrifying microorganisms (i.e., denitrifiers), which transform nitrogen into different nitrogen-based oxides (Jain et al. 2021; O’Donnell et al. 2017). For geotechnical applications the process has been commonly proposed to desaturate soil for soil liquefaction mitigation (Azeiteiro et al. 2017). This is because of nitrogen gas formed in the subsurface when denitrifying microorganisms are provided with a solution containing nitrate and dissolved organic matter. Organic matter is oxidized to inorganic carbon and nitrate is reduced to nitrogen gas. MICP can thus also occur using this process, as dissolved inorganic carbon produced during denitrification, can lead to calcium carbonate precipitation in the presence of soluble calcium ions. To this effect, most studies used calcium salts such as calcium acetate and calcium nitrate as substrates, so that the inorganic carbon produced by the microbial metabolism can readily precipitate with the dissolved calcium to form calcium carbonate minerals (van Paassen et al. 2018; Pham et al. 2018). However, the required amount of substrates to biocement the soil is much higher than that required to desaturate the soil. Significant advantages of this pathway for civil and (geo-)environmental engineering applications, are that denitrifiers are ubiquitous in the subsurface, so that denitrification can be induced in most soils by stimulation of indigenous microorganisms (van Paassen et al. 2018), and that the process can occur under anoxic or anaerobic conditions, so that it can be applied at depth/low oxygen level (Martienssen and Schöps 1999). However, biocementation by denitrification is a slow process, considerably slower compared to biocementation by ureolysis. Other disadvantages are that incomplete reaction may lead to accumulation of nitrous oxide (N2O), a greenhouse gas, as well as nitrite (NO2−) and nitric oxide (NO) byproducts that are potentially toxic and harmful to the environment and additionally inhibit the MICP process (van Paassen et al. 2018; Castro-Alonso et al. 2019). It is also possible that the production of nitrogen gas may have undesirable effects for geotechnical applications if soil matrix stability is compromised before biocementation has been achieved. The pathway can also be associated with a high eutrophication potential due to the nitrogen gas production (Porter et al. 2021).
1.1.3 Methane Oxidation Pathway
This autotrophic pathway involves microorganisms of the methane cycle that produce enzymes that release and consume methane (Murrell and Jetten 2009; Ganena et al. 2014, 2015). In this process, methane is oxidised with molecular oxygen to carbon dioxide, then converted to carbonates that can be used to form biominerals. The advantage of this process is that it has the potential to capture methane, a well-known greenhouse gas, and store it in biocements/building materials. This way methane becomes benign and the process can contribute to climate change mitigation. Also the process can occur under aerobic, anoxic and anaerobic conditions (Jain et al. 2021). Still, it suffers from the emission of hydrogen sulphide as a byproduct (Murrell and Jetten 2009). Furthermore, studies on this metabolic pathway were carried out under laboratory conditions but it is yet to be studied how the poccess can be implemented in situ under different environmental conditions (Jain et al. 2021).
1.1.4 Photosynthetic Pathway
The photosynthetic pathway uses the photolithoautotrophic nature of (micro-)algae and cyanobacteria to facilitate colonisation and biomineral formation (Irfan et al. 2019). As stated in Jain et al. (2021), during this process, the alkalinity across the microbial cell increases during the exchange of HCO3− /OH− ions. The microorganisms use CO2 (gaseous or dissolved) to form organic matter via photosynthesis, while bicarbonate is converted into CO2 and OH−, eventually forming carbonate minerals. Photosynthesis can be used to realise MICP cheaply and straightforwardly. However, the main limitations are the need for sunlight, restricting its applicability, especially for geotechnical applications at depth. Moreover, it can potentially have a considerable carbon footprint due to the production of CO2 (Vecchi et al. 2020).
1.1.5 Iron-reduction Pathway
Under anaerobic conditions, anaerobic heterotrophic iron-reducing bacteria produce dissolved salts or chelates of Fe(II) that can be transformed to ferrous carbonate (Guo et al. 2010). A significant advantage for geo-environmental and geotechnical applications is the attainment of biocementation at depth under anoxic conditions as well as at low pH conditions. However, due to the aggressive nature of the environment in which the process occurs, its application is limited and potentially undesirable (Castro-Alonso et al. 2019). Only a few studies have used the process and have reported that the iron based biocement is not as strong as calcium based biocement but that the clogging effect is high, hence the process would be most suitable for bioclogging (Ivanov et al. 2015).
1.1.6 Sulphate-reduction Pathway
In the sulphur cycle, sulfate-reducing bacteria can oxidise organic carbon to bicarbonate (Alshalif et al. 2016; Baumgartner et al. 2006; Tambunan et al. 2019); at the same time, hydrogen sulphide production increases the pH, favouring the precipitation of calcium carbonate. The potential advantages of this pathway are the ability of sulfate-reducing bacteria to produce biocement under anaerobic conditions and the use of organic matter, which can be readily available from waste sources such as food waste (Chetty et al. 2023). However, sulfate-reduction is a slow process and hydrogen sulfide gases are odorous and toxic, which potentially limits the use of this process for large-scale (geo-)environmental and civil engineering applications. Another challenge is that anaerobic conditions must be maintained throughout the process, which is quite difficult to ensure under real-field conditions (Jain et al. 2021).
1.2 Carbonic Anhydrase Pathway
The carbonic anhydrase is an enzyme with an active site that contains a Zinc ion (Zn2+) (Kim et al. 2020). It has been of interest and a subject of study since its first discovery in 1933 by Meldrum and Roughton in mammalian red blood cells of cattle (Meldrum and Roughton 1933). Since then, CA has been found in plants (Bradfield 1947), algae (Moroney et al. 2001), and bacteria (Veitch and Blankenship 1963; Supuran and Capasso 2017). The past few decades have proposed the usage of CA for industrial applications such as carbon sequestration (Molina-Fernández and Luis 2021; Yadav et al. 2014; Zhan et al. 2021), and biofuel production (Boone et al. 2013; Thakur et al. 2018). In the medical field, CA has found application in dental health (Abou Neel et al. 2016), as a component of artificial blood (Bian et al. 2012), for the prevention of kidney stones (Ghorai et al. 2020) and eye therapy (Jansook et al. 2021).
Its numerous applications are due to its distinctive CO2-catalyzing properties. CA has been proposed for biocementation, as CA can hydrolyse 600,000 molecules of CO2 per CA per second (Trachtenberg et al. 1999). For applications of CA-biocementation in civil or (geo-)environmental engineering, bacterial-CA (Zheng and Qian 2020a, b), as well as purified enzymes (Sharma et al. 2022) were used to induce calcium carbonate precipitation. The biochemical process involves gaseous CO2 dissolving in water to form hydrated aqueous CO2 (aq) (Eq. 1), which reacts with water to form H2CO3 (Eq. 2), whose ionisation in water generates H+ and HCO3− (Eq. 2). Under alkaline conditions, the HCO3− further ionises to form CO32− and H2O (Eq. 3):
In the presence of a calcium ion source, CaCO3 precipitates are formed from the reaction of (Ca2+) with CO32− (Eq. 5). Whilst other divalent ions can be used (e.g., Mg2+ or Fe2+), Ca2+ remains the most widely ions used; therefore, CaCO3 is precipitated in most biocementation studies. If bacteria are used rather than the free enzyme, bacteria cells serve as nucleation sites (Kahani et al. 2020):
A typical setup for CA-biocement production for possible civil or (geo-)environmental engineering applications would thus involve combining CA-producing bacteria, CO2, nutrient media, and a calcium (or another metal ion) source mixed with soil or soil-like waste (e.g., mine tailings), as illustrated in Fig. 1. Typically, bacteria cells are negatively charged, and thus attract and bind to the provided metal ion (Mg2+, Ca2+, or Fe2+), forming crystals (Power et al. 2013). The potential applications take advantage of the precipitated carbonate (in most cases, CaCO3) that acts as a binding agent between soil or soil-like waste particles (Charpe et al. 2019). The biomineral formed creates a cement that can also fill the void spaces. As a result, soil engineering properties such as strength and stiffness are enhanced (Chen et al. 2021).
2 Literature Review Methodology
This paper adopted a systematic literature review (Xiao and Watson 2019), as illustrated in Fig. 2. The first step was to search for literature from the Web of Science (WOS), Scopus database, and other databases until 20th January 2023. An appropriate keyword combination described as (bacteria) AND (microbial calcium carbonate precipitation OR biocementation AND (carbonic anhydrase*) was employed in the search command of respective databases to identify the relevant publications (Swartz 2011). Step 2 involved screening the literature search results, excluding all languages except English and removing duplicates, and excluding magazines, conference proceedings, book chapters, series, and newsletters to have only full and reviewed papers. The categorisation performed in steps 3 and 4 involved reading through the title and abstract of documents to check whether the study is related to civil and (geo-)environmental engineering. Both "primary articles" and "review" papers were considered. "Primary articles" include those papers publishing original experimental data from laboratory or field studies. "Review" are papers that summarise and report on the past or recent progress in the study area. Only papers that had adopted the carbonic anhydrase route for microbial calcium carbonate precipitation (MICP) or biocementation were reviewed and included in the narration of the review paper (Step 5).
3 Results and Discussion
3.1 Number and Categorisation of Research Articles
The Web of Science and Scopus search results generated 91 and 157 studies, respectively, excluding identical studies, not applicable, and languages other than English. One hundred sixty-nine (169) studies were excluded based on the article's abstract-level screening. The remaining studies, 38 in total, were considered in the systematic review. The trend in the number of research publications from 2002 to 2022 is represented in Fig. 3a and b, showing the thematic application areas. A steady increase can be observed in the number of publications on the topic. The literature shows that CA has been applied for carbon capture, self-healing concrete, bioremediation, geo-environmental applications and other. The increased number is due to the recent interest in the MICP technique as a sustainable technology (Fukue et al. 2011; Bhattacharya et al. 2018). This increased interest has yielded more excellent knowledge of the mechanism and factors affecting MICP (Soon et al. 2014; Chek et al. 2021; Soon et al. 2014). However, despite the advances in MICP, it is estimated that less than 1% of publications on biocementation studied the CA pathway.
3.2 Factors Affecting CA-biocementation
Many factors influence the process of biocementation, such as microorganism type and environmental factors (e.g., temperature and pH affecting microorganism viability, microbial growth, and enzymatic activity), the concentration of CO2, and metal cation source and concentration. These factors are described in the subsections below, explicitly referring to the CA pathway.
3.2.1 Bacteria Type
Bacteria are the source of enzymes and provide nucleation sites for mineral precipitation (Zhao et al. 2014). Many researchers have confirmed that the CA-producing bacteria enzyme plays a vital role in promoting the conversion of CO2 to bicarbonate and calcite (Steger et al. 2022). The hydration of CO2 triggers the proton transfer initiated by the CA, as it attacks the carbon atom of CO2 by zinc-bound OH− to produce bicarbonate, as described earlier (Fu et al. 2021). Thus, the CA enzyme (whether from bacteria or purified enzyme) affects the biocementation process (Giri and Pant 2019; Mirjafari et al. 2007). Results have shown that purified CA enzymes could significantly enhance the carbonate/bicarbonate formation and deposition in solution, compared to control samples without CA enzyme (Fig. 4). Therefore, selecting a CA-producing bacterial strain is critical to the biocementation process using the CA metabolic pathway. Additionally, the bacteria serve as the nucleation site for the CaCO3 precipitation process during the CA-biocementation process. A small number of bacterial cells will yield a small number of nucleation sites; consequently, a small amount of biocement will form between soil particles. In a recent study by Jin et al. (2021) using Bacillus mucilaginosus, it was confirmed that under the action of CA secreted by microbes, carbon dioxide was captured, enriched, and converted into carbonate ions, which reacted with calcium ions to form calcite. Similar results were reported by Zhan et al. (2021) using Paenibacillus mucilaginosus to produce CA and provide nucleation sites for the bio-mineralisation reaction. Selected CA strains screened by this study are shown in Table 2.
Comparison of precipitation with and without carbonic anhydrase enzyme (Reproduced based on data from Mirjafari et al. 2007)
Generally, biocementation applications can harness the enzymatic activity of bacteria through bioaugmentation or biostimulation (Gomez et al. 2017). Bioaugmentation involves the introduction of microorganisms to soils. These can be imported non-native microorganisms or native microorganisms that have been isolated from the native soil and screened for the intended usage; they are then cultivated and introduced back into the soil. For the bioaugmentation process, various strains of CA-producing bacteria have been isolated from soils. These include but are not limited to Bacillus mucilaginous L3 (Qian et al. 2015), Bacillus cereus C1 (Dhami et al. 2017), and Bacillus altitudinis M8 strain (Nathan and Ammini 2019). However, many researchers argue that bioaugmentation is not sustainable and eco-friendly (Raveh-Amit and Tsesarsky 2020). So far, the source of reliable high CA-producing bacteria is achieved by cultivating pure cultures which is a major cost factor of MICP application. Borchert and Saunders (2011) have successfully demonstrated the production of CA from a recombinant E. coli bacterial strain. Still, a high capital cost is associated with the large-scale production of enzymes from native and recombinant E. coli cell lines.
An alternative process is the stimulation of existing microorganisms in the soils by providing them with suitable nutrients (Pan et al. 2019). Biocementation by biostimulation can be of interest for practical geotechnical and geo-environmental engineering applications as native bacteria are well-distributed spatially within the subsurface and this can lead to an improved biocementation treatment with spatial uniformity (Gomez et al. 2017; Behzadipour and Sadrekarimi 2021; Dubey et al. 2021). Moreover, native microorganisms are well-suited and adapted to their native subsurface environment. Although little studied, biocementation by biostimulation of native CA-producing bacteria is possible and would merit further research. Consequently, before any full-scale CA-biocementation application in the field, an economically viable production of CA or CA-producing bacteria is necessary.
3.2.2 pH
The pH plays a vital role in biocementation, affecting microbial growth, metabolism/enzymatic activity, calcite solubility and crystallization and nature of biomineral formed. Moreover, the pH changes throughout the mineral precipitation process. Generally, the enzymatic activity is consistent with the growth and reproduction of bacteria: the better the bacteria grow, the higher the observed enzymatic activity. The optimal pH for most studied microbial CA-producing bacteria ranges from 7.0 to 9.0 (see Table 2). A high or low pH value generally results in the denaturation of enzyme activity due to the structural alteration of the amino acids of the CA enzymes. The optimal pH values for various CA-producing enzymes reported in recent studies were as follows: pH 6.5 for Bacillus mucilaginosus K02; pH 6.0 for Lactobacillus delbrueckii (Li et al. 2015); pH 7.0 for Corynebacterium flavescens (Sharma and Kumar 2021), and pH 8 for Bacillus sp (Sundaram and Thakur 2018). A study by Zheng and Qian (2020a) showed that as pH increased due to CO32− and OH− from the hydration of CO2, a pH value of 9.5 inhibited the growth of bacteria and eventually resulted in no bacterial growth at a pH value above 11.0. The study postulated that higher pH values lead to increased permeability of microbial cell membranes, reducing the absorption of nutrients by microorganisms and leading to bacterial death. Lai et al. (2023) performed a microscopic analysis of EICP-treated sand specimens. This showed only a few small crystals forming for pH ≤ 4.5. Conversely, at a higher pH, large calcium carbonate crystals were observed on the sand particle surface and/or at the interparticle contacts. It was thus postulated that lowering pH would lead to a reduction in enzyme activity affecting biocementation.
The pH value of concrete can reach 13 which is extremely unfavourable to the growth of most microorganisms. Thus, for such applications researchers adopted spore forming techniques and ways of shielding the bacteria during the hydration process. This could be achieved for example by using gels (Wang et al. 2012). Of all CA-producing microbes studied, Bacillus sphaericus alkali-resistant and spore-forming bacteria are used widely for self-healing concrete purposes (Zhu et al. 2021; Jonkers and Schlangen 2008). The concrete's matrix capillary water is reported to have pH values between 11 and 13, and Bacillus sphaericus still survives and can thrive at these pH ranges (Jonkers et al. 2010; Jonkers and Schlangen 2008; Zhu et al. 2021). The fundamental strategy for implementing bacteria in concrete is to protect these, for example, in a hydrogel, enabling them to last from several to hundreds of years under extreme environments (Setlow 1994; Wang et al. 2018). Indeed, although alkaliphilic bacteria would normally tolerate the highly alkaline concrete environment, their metabolic activity could still be affected by the high pH and the dry environment of hardened concrete. This was evidenced by Jonkers et al. (2010) who observed only 10% of the directly incorporated Bacillus cohnii spores in concrete after 42 days. Therefore, encapsulation rather than direct application is recommended, using carriers such as capillaries, porous materials, microcapsules, and hydrogel, as well as graphite nanoplatelets (GNPs) and lightweight aggregates (LWAs) (He et al. 2020; Lee and Park 2018). Further research would be required to study the viability of CA-producing bacteria in extreme environments and the performance of spore-forming CA bacteria encapsulated using different carriers for possible application in the construction sector.
In addition to and also as a result of its effect on microbial growth, pH plays a significant role in calcium carbonate crystallisation. Different initial pH lead to different crystalline morphology of precipitates, as evidenced by microstructural analysis of CA-treated materials shown by Zheng and Qian 2020b) for initial pH ranging from 7.0 to 10.0. Namely, the SEM micrographs showed that the morphologies of precipitates evolved progressively from agglomerations of tiny spheres in lower pH of 7.0 to larger compact crystals when pH increased to 9.0, and bacteria acted as nucleation sites to induce more regular spherical morphology. The different morphologies can be attributed to the fact that at low pH value, excess H+ produced by CO2 dissolving in water, would further reduce the pH of the solution, gradually dissolving CaCO3 products; hence, smaller particle sizes were observed. Conversely, at pH = 9.0, which was found to be the optimum pH for mineralisation for the CA bacteria used in this study, precipitation was enhanced. The bacteria acted as the nucleation sites to induce a more regular spherical morphology, which resulted in increased sizes of precipitates. Finally, an initial pH of 10.0, would restrict bacterial growth leading to a decrease in enzymatic activity and to the inhibition of the hydration reaction of CO2. This would result in a limited precipitation, mainly caused by the dissolution of CO2 in the alkaline solution. Further increases in initial pH values could eventually lead to the death and dissolution of CA bacteria, thus impacting on the quantity and stability of enzyme produced by microorganisms activity (Zheng and Qian 2020a). It should be noted that the large variation in the size of the biominerals has an impact on the contact points forming between soil grains, and consequently, on the strength of biocemented geomaterial. Longer retention times of enzymatic activity, will yield larger crystal sizes, enhancing the bonding between the biomineral and the geomaterial particles, hence leading to higher strengths.
3.2.3 Temperature
In addition to pH changes, temperature changes also have a significant impact on biocementation as they affect the enzymatic activity of the microorganisms, as well as the chemical stability of the biominerals. Increased or decreased temperature affects microbial metabolism and growth, hence affecting enzymatic activity. Therefore temperature is a key factor for the success of CA-biocementation. Some literature has pointed out that the optimal temperature for microbially induced calcium carbonate precipitation is around 35 °C, which correlates well with the optimal growth temperature of most CA-producing bacteria. A recent soil microcosm study by Jaya et al. (2019) showed that marine Bacillus safenis had optimal CA activity at 40 °C. Another study found that Citrobacter freundii was active and optimal at 37 °C (Giri et al. 2018). Unlike the other CA-producing bacterial strains studied, Methanobacterium thermoautotrophicum had an optimal high temperature of 75 °C (Smith and Ferry 1999). Therefore, the temperature should be optimised in CA-biocementation to cement different geomaterials efficiently for different applications in natural environments (Justo-Reinoso et al. 2021). However, few studies have optimised this process for application in the field as most of the experiments were conducted at room temperature. Future investigations should mimic the ground conditions, as low temperature conditions significantly affect microbial activity. For example, according to Sun et al. (2019), microbial activity is low at temperatures of 10 °C, affecting the success of biocementation. On the other hand the study by Zhang et al. (2011) shows that the CA activity of Bacillus mucilaginosus K02 at temperatures of 10 °C is reduced by approximately 30% of the activity achieved at optimal temperatures (Fig. 5). Therefore, studies focusing on how to achieve sufficient for biocementation microbial activity at low temperatures are of most relevance. In particular for geotechnical and geo-envirronmental applications, it will be impractical to control or maintain a constant temperature in the field. The soil temperature in the field would vary with altitude, latitude, soil type and depth, water content, proximity to industrial or agricultural site (Jain 2021). Though the native CA bacteria will be acclimated and able to survive in a wide range of temperatures in their natural environment, the effect of different temperatures on bacteria growth and activity will need to be thoroughly studied before field application, as it will be a major factor affecting biocementation success.
Effect of different temperatures on carbonic anhydrase produced by Bacillus mucilaginosus K02 (Reproduced using data from Zhang et al. 2011)
3.2.4 Cementation Solution
The review of existing experimental studies shows that the cementation solution is vital in the CA-biocementation process. Previous biocementation studies showed that the composition and concentration of the cementation solution affect the crystal type, appearance, size, composition of pore fluid, and pH value. Typically, the cementation solutions in CA-biocementation are a mixture of metal ion salt, a CO2 source, and trace nutrients (Bozbeyoglu et al. 2020; Dhami et al. 2014; Pan et al. 2019). In calcite precipitation studies, three calcium sources have been used widely, namely calcium nitrate (Ca(NO3)2), calcium acetate (Ca(CH3COO)), and calcium chloride (CaCl2). Pan et al. (2019) used three calcium sources that demonstrated that CA-producing bacteria could precipitate calcite in a sand column and showed that the three sources of calcium systems produced different crystal types (see Fig. 6). The morphologies of the crystals were mapped against the strengths achieved. Namely, the sand column with Ca(NO3)2 had the highest shear strength of 62.33 kPa, followed by the CaCl2 group, while the Ca(CH3COO)2 group had the worst strength of only 11.19 kPa. The authors attributed the higher strengths of the CaCl2 and CaNO3 systems to the fact that CaCO3 crystals were closely packed, attached to the sand particles, and well distributed, as opposed to CaCO3 crystals from the Ca(CH3COO)2 systems, where precipitates were much more scattered and not attached to the sand particles. Still, in the context of biocementation for ground improvement these strengths are too low: when using the ureolytic route for sand biocementation strengths as high as 30 MPa have been reported (Whiffin 2004). An interesting observation made by the authors is that holes were formed on the surface of their specimens, which could justify the low strength. The authors attributed these holes to the continuous production of CO2 during the process, so that gas bubbles disrupted the soil matrix. It is also notable that XRD identified the CaCO3 crystals as vaterite, i.e., the least stable CaCO3 form. Vaterite formation instead of calcite impacts the mechanical properties of the soil and can explain the lower strengths. This was particularly the case for the Ca(CH3COO)2 system, which had the lowest strengths, consistently with the formation of vaterite. However there was generally a limited evidence of calcite forming (the most stable polymorph) even in the other two systems, which overall concurs with the low strengths achieved.
The morphology of CaCO3 formed with the three different calcium sources in the presence of Bacillus cereus ( +) or not ( −): (A) CaCl2 system ( −); (B) CaCl2 system ( +); (C) Ca(NO3)2 system ( −); (D) Ca(NO3)2 system ( +); (E) Ca(CH3COO)2 system ( −); (F) Ca(CH3COO)2 system ( +) (Figure reproduced from Pan et al. (2019)
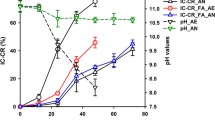
Zheng and Qian (2020a), investigated the effect of cementing solution concentration. Using cementing solutions of different molarities (0 to 160 mM) of calcium ions, they found that molarity also affects the growth and enzyme activity of CA bacteria, hence biocement production. Bacteria grew best, and the enzyme activity reached its maximum, when the Ca2+ concentration was 60 mM. An increased Ca2+ concentration inhibited the growth of CA-producing bacteria, and the enzyme activity of CA-producing bacteria decreased gradually. Once the Ca2+ concentration reached 160 mM, the bacteria hardly grew, and the activity of CA-producing bacteria almost disappeared. A possible explanation for this is that increased Ca2+ concentration affects the osmotic pressure around the membrane of bacteria cell. This triggers flow from the low Ca2+ concentration zone to the high Ca2+ concentration zone through the cell membrane, which leads to the dehydration of cells and the separation of plasma wall. This can cause bacteria to stop growing or even die (Zheng and Qian 2020a).
Finally, the source of CO2 is essential. CO2 used could be from the atmosphere or externally supplied; this could include captured industrial CO2. Most CO2 sequestration studies suggest using CO2 directly from point sources such as industrial sources (Leimbrink et al. 2017; Russo et al. 2013, 2016). However, this source of CO2 has impurities and, as such, needs to be purified before usage as other pollutants exist in the flue gas (Wattanaphan et al. 2013; Weber et al. 2000). This is plausible for CO2-capturing application purposes but could be less suitable for biocementation for soil improvement or bioremediation. For this purpose, the usage of NaHCO3 has been suggested by many researchers as a source of CO2 (Hanifa et al. 2023) although it is less attractive in terms of climate mitigation than the use of captured CO2. Alternatively, using CO2 directly from the atmosphere is plausible in the same fashion as when CO2 from the atmosphere is used for curing cement-based materials (Liu et al. 2021) but this has been little investigated. It should, however, be noted that CO2 addition can lead to a disruption of the soil matrix as observed in the experiments by Pan et al. (2019) and this requires further investigation especially for ground improvement applications.
To conclude, cementation solution composition/concentration variations can affect biocementation success. Investigators must carefully select the appropriate cementation solution type and concentration ratio before implementing CA-biocementation in various engineering applications. The limited evidence for the use of CA enzyme for ground improvement shows that strengths may be considerably lower compared to those achieved by the ureolytic route (see, e.g., Pan et al. 2019). To precipitate more calcium carbonate between sand particles, a higher cementation solution may be required; however, the MICP process may be retarded or even terminated with an increased solution concentration (Mahawish et al. 2018; Lai et al. 2021). Alternatively, multiple applications of cementing solutions of lower concentations may have to be adopted as a way of increasing precipitates and strength. However, this would reduce the efficiency of the process in large scale applications, increasing treatment duration and costs. This would be a major limitation for the CA based MICP soil treatment and needs to be studied thoroughly.
3.3 Applications of CA-biocementation in Civil and Environmental Engineering
3.3.1 Concrete Repair
As discussed earlier, biocementation can be applied for concrete crack repair and self-healing, soil/geomaterial stabilisation/ground improvement, carbon capture, and bioremediation. The CA-biocementation method has been used primarily for concrete crack repair or self-healing concrete (Ali et al. 2023). The conventional way of repairing concrete cracks involves injecting cement grout or epoxy into the concrete. However, environmental and health hazards such as allergies, asthma, and irritation of the eyes, nose, and throat have been reported when using these chemicals (Kapustka et al. 2020). CA-biocementation can be used instead for the self-healing of microcracks. In the latter method, restoration occurs by activating the biocementing components incorporated in the concrete when a gap appears. Namely, any seepage of water and air through cracks leads to the release of incorporated components in the concrete to initiate the CA-biocementation process which seals the microcracks. A recent study used Bacillus mucilaginous L3, a CA-producing strain that was shown to seal small cracks within seven days (Qian et al. 2015). This study predicted the depth of the CaCO3 precipitated layer based on the expected experimental results. Cracks in the concrete below 0.4 mm were almost entirely closed using this method. Chen et al. (2016) also repaired cement-based material damage by immobilising Bacillus sphaericus CA-bacteria and nutrients.
Free CA-enzyme was also introduced in the cement paste mix during the concrete preparation to develop a self-activated healing cement paste (Rosewitz et al. 2021). The results showed that after fracture, the hydration of samples containing CA promoted the formation of calcium carbonate crystals at ambient temperature. These results prove that using the CA enzyme to repair small cracks in concrete is practical, as reported using other pathways (Chuo et al. 2020).
3.3.2 Ground Improvement
To date, very few works have studied CA-biocementation for soil stabilisation. These include the recent study by Pan et al. (2019), who used different calcium sources to investigate sand biocementation by a CA-carbonic anhydrase-producing bacteria, as mentioned earlier. The study showed that CA-producing bacteria could precipitate calcite in a sand column of a diameter of 60 mm and a height of 50 mm using various calcium sources. Another study by Dhami et al. (2017) explored the CA-biocementation pathway by biostimulation. It showed that calcium carbonate could be precipitated by the synergy between ureolytic and CA-producing bacteria, as in the natural environment, no single process exists in isolation. The outcome was an improved biocementation efficiency as calcite precipitation increased by 50–72%.
3.3.3 Bioremediation
CA can also be applied for the bioremediation by either fixation or leaching of contaminants, as suggested by a recent study of steel slag carbonation using Bacillus mucilaginosus (Jin et al. 2021). The resulting inert material had a compressive strength of 11.2 MPa and could be used for contaminant encapsulation by adhering to the waste materials and resisting biodegradation. Further investigations of CA-biocementation for contaminant encapsulation are required as there is paucity of information regarding this application.
3.3.4 Future CA-biocementation Application
The CA-biocementation process could be used for other applications in the future, such as soil liquefaction mitigation, erosion protection, or heritage building restoration, in the same way as other pathways (e.g., denitrification or ureolytic pathways). However no studies were found using CA for these applications. This is a research gap that future studies can address (Fig. 7).
3.4 Modelling of CA-biocementation Processes
Although the biocementation technique has been extensively investigated in the recent past, to date, no predictive model has been formulated for the CA-biocementation pathway despite its various advantages. The vast majority of biocementation models are for the ureolytic route and are calibrated at laboratory scale (Dupraz et al. 2009; Fauriel and Laloui 2012; Kashizadeh et al. 2021; Martinez et al. 2014; Nassar et al. 2018; Matsubara and Yamada 2020; Sharma et al. 2021; van Wijngaarden et al. 2011; Wang and Nackenhorst 2020). Very few models are calibrated with field scale data (Minto et al. 2019; Cunningham et al. 2019). Such models are helpful for upscaled or field applications. It is costly and takes many years to carry out field experiments before the technology can be transferred from a laboratory-scale process to a practical field-scale deployable technology. Thus, it is necessary to have CA-biocementation models developed. Forward-looking, for CA-biocementation predictive modelling, the CO2 sequestration literature could be consulted as no modelling studies on CA-biocementation were found. This process could provide the required information (parameter values) for the CA enzyme kinetics. Figure 8 shows the step-by-step process of predictive model development.
The first step would be to understand and implement the general form of mass balance for chemical species transport by advection–dispersion-reaction relationship. CA-biocementation involves multi-component systems that require solving transport for many species (CO2, H2O, Ca2+, CO32−). Learning from previous models in the ureolytic pathway, all these species in the system are generally simulated using the advection–dispersion equation (Eq. 6) (Abbas et al. 2020; Cunningham et al. 2019; Fauriel and Laloui 2012; Martinez et al. 2014). The terms on the left-hand side express the advective transport, the first term on the right-hand side considers dispersion, and the second term considers reaction; in its simplest 1-D form, it is written as:
where
- C:
-
Constituent concentration (mol/m3),
- t:
-
time (s)
- u:
-
cross-sectional average flow velocity (m/s),
- x:
-
distance along the longitudinal axis (m),
- D:
-
Dispersion coefficient (m2/s), and
- K:
-
Reaction rate (1/s).
Secondly, during typical batch culture, the bacterial growth curve shows distinct stages of growth that describe the lag phase, exponential growth, and death phase, when conditions become unfavourable for growth and bacteria stop replicating (Balakrishnan et al. 2021). The microbial growth and enzyme activity can be expressed using the Monod equation. The Monod equation considers the number of microorganisms and the substrate concentration (Nežerka et al. 2022). This phenomenon has been modelled as chemical species, where the bacterium in suspension is considered irreversibly attached to solid surfaces in the soil profile and independent of velocity and bacteria growth; the Monod equation then simplifies into Eq. (7) (Minto et al. 2019). The rate of the forward reaction (rCA) for CO2 hydration rate (mol/(m3 s)) catalysed by generic carbonic anhydrase (CA) may be described according to the Michaelis–Menten model (Eq. 7) as follows:
where \({\mathrm{k}}_{\mathrm{cat}}\) is the turnover number (1/s), and \({\mathrm{K}}_{\mathrm{M}}\) is the Michaelis–Menten kinetic constant (mol/m3) and \(\left[{\mathrm{CO}}_{2}\right]\) and \([{\mathrm{CO}}_{2}]{ }_{e\mathrm{q}}\) the total CO2 concentration and the CO2 concentration in equilibrium respectively (mol/m3).
The reaction mechanism of CA has been extensively studied, and the reaction scheme is reported in the literature. Generally, the CA turnover numbers range between 104 and 106 s−1 for free CA and immobilised enzymes (Di Fiore et al. 2015). Russo et al. (2013, 2016) reported the kinetics of the recombinant CA from the thermophilic bacterium Sulfurihydrogenibium yellowstonens, namely that kcat/KM = 9.16 × 106 M−1 s−1. In another study, the kcat/KM assessed for the α-class human carbonic anhydrase HCAII was reported to be about 108 M−1 s−1 (Gaspar et al. 2017). Such parameters could be helpful for the models. The final step for CA-biocementation would be the precipitation of biominerals where CaCO3 is precipitated as an immobile mass. This can be incorporated into the CA-pathway models in the same way as in existing ureolytic models (e.g., Abbas et al. 2020; Cunningham et al. 2019; Martinez et al. 2011; Matsubara and Yamada 2020; van Wijngaarden et al. 2011).
Numerical models for the ureolytic pathway have been produced using software such as COMSOL-MULTIPHYSICS (Faeli et al. 2023), TOUGHREACT (Martinez et al. 2013), PHT3-D (Nassar et al. 2018), OpenFOAM (Minto et al. 2019) and others. These and other platforms could be useful for simulating the CA-biocementation with parameters obtained from CO2 sequestration studies. Once a model has been produced, the next step would be to determine the main influential parameters and calibrate the model, considering both aqueous and solid phase data from the experiments (Abbas et al. 2020). The stoichiometric constraints of individual reactions and the interconnectivity between the responses would be utilised in the calibration process (Martinez et al. 2011; Minto et al. 2019). However, most of the current mathematical models are limited to CO2 absorption into aqueous solutions along a biomimetic route. There are no models for CA-biocementation, which requires further study by researchers.
4 Discussion: Challenges and Future Directions
The CA pathway is an attractive nature-based method of producing environmentally friendly cement for civil and (geo-)environmental engineering applications. Combining CO2 capture with biocement production for different applications, is novel and exciting. It assists in climate change mitigation and adaptation. The carbonic anhydrase biocementation pathway is also free of undesirable byproducs. Due to its many advantages the pathway has excellent potential for large scale civil and environmental engineering applications, including concrete crack repair or self-healing, restoration of heritage buildings, bioremediation of contaminated construction sites, as well as problematic soil improvement, including preventing soil liquefaction and erosion problems. However, despite the proof of concept, several limitations still exist for commercialising CA-biocementation. Many research gaps were identified in this study and suggestions for future research to address these are listed below:
-
i.
Firstly, the different sources of CA (CA from bacteria or free/purified enzyme) need further investigation to improve the transition process from bench scale to field application. For this, it is also necessary to identify robust and site-specific microorganisms that can operate under a broad range of environmental parameters such as pH or temperature. The ability of the CA-biocementation route to achieve design requirements also needs to be addressed. For example, the soil strength gains reported currently, are generally lower than those achieved when the ureolytic route with Sporosarcina pasteurii was used. Moreover, to realise CA-bocementaton at field scale it is essential to find ways of producing biominerals cheaply. Overall sustainability assessments of the proposed processes through Life Cycle Analysis will also be required and these are currently lacking.
-
ii.
Secondly, immobilising CA enzymes using nanostructured materials could be investigated. This technique was proven to be helpful in the carbon capture process. Future studies can thus adapt the technique to increase biocementation efficiency (Al-Maqdi et al. 2021; Shende et al. 2018). A critical anticipated advantage of CA enzyme immobilisation is an improved stability.
-
iii.
Thirdly, CA-biocementation studies are currently limited to a few applications. Further research could broaden the use of the technique to other civil and (geo-)environmental engineering applications such as liquefaction and heritage building restoration.
-
iv.
Another aspect that has not been discussed in the literature but it is of most relevance for industrial scale implementation is finding practical ways of implementing CO2 for large scale projects, especially for geo-environmental and geotechnical applications. It should be considered for example how the CO2 can be implemented in the ground using currently available equipment (e.g., air sparging or electrokinetic setups), without disrupting the soil matrix, especially under existing infrastructure. Moreover, the literature review has shown that the type of the cementing solution and its purity may affect the biocementation success. Whilst the effect of impurities on the success of biocementation should be the focus of future laboratory work, the logistics of supplying CO2, especially captured industry waste CO2, free of potentially deleterious impurities would need to be considered for large scale projects. The possibility and practicality (in terms, for example, of rates of calcite precitation, and whether these would be practical in terms of timeframes required) of using atmospheric CO2 should be considered, so that CO2 biocementation becomes a carbon sink in the natural environment, but no such studies were found.
-
v.
Finally, the formulation of predictive models of the CA-biocementation pathway, which are currently lacking, needs to be addressed in future work, to support uplscaling towards field applications.
5 Conclusions
This study provided a comprehensive literature review on the use of the carbonic anhydrase biocementation pathway for environmental and geo-environmental engineering applications. The review covered the period from 1st January 2002 to 20th January 2023. The study of this pathway in comparison to other biocementation pathways revealed that the carbonic anhydrase biocementation pathway could be the net-zero biocementation solution for the construction sector. However this study also showed that although very promising, this pathway remains little researched. It was, therefore, concluded that more studies are required both in the laboratory and in the field for the different possible applications of the process. In particular long-term field experiments, which are lacking, are essential to assess the feasibility of this exciting route and to overcome barriers towards uptake by industry.
The study also showed that studies on the numerical modelling of the processes and information on the required kinetic parameters are also lacking; yet, these are important for industrial implementation. To complement the information and as an outlook for future research in this direction, it is relevant to consult the field of CO2 sequestration using CA-producing bacteria, to fill the knowledge gaps.
Finally, several issues common to other biocementation pathways need to be further investigated to overcome barriers to industrial-scale applications. These include the high cost of raw materials and culture media. Sustainability analyses of the proposed processes are of primary importance for the application of the technique at an industrial scale. Practical considerations regarding capturing CO2 for field scale applications must also be addressed towards industry uptake of the technique.
Data Availability
The research did not generate primary data. All collected literature sources can be found in the list of references.
References
Abbas M, Kunhappan D, Dadda A et al (2020) Discrete Element Modelling for biocemented sand: effect of calcite distribution at the microscopic scale. In: 4th European Conference on Unsaturated Soils (E-UNSAT 2020) E3S Web of Conferences, vol 195:05005. https://doi.org/10.1051/e3sconf/202019505005
Abdelsamad R, Al Disi Z, Abu-Dieyeh M et al (2022) Evidencing the role of carbonic anhydrase in the formation of carbonate minerals by bacterial strains isolated from extreme environments in Qatar. Heliyon 8(10):e11151. https://doi.org/10.1016/j.heliyon.2022.e11151
Abou Neel EA, Aljabo A, Strange A et al (2016) Demineralization-remineralization dynamics in teeth and bone. Int J Nanomed 11:4743–4763. https://doi.org/10.2147/IJN.S107624
Achal V, Pan X, Zhang D (2012) Bioremediation of strontium (Sr) contaminated aquifer quartz sand based on carbonate precipitation induced by Sr resistant Halomonas sp. Chemosphere (Oxford) 89(6):764–768. https://doi.org/10.1016/j.chemosphere.2012.06.064
Achal V, Mukerjee A, Sudhakara Reddy M (2013) Biogenic treatment improves the durability and remediates the cracks of concrete structures. Constr Build Mater 48:1–5. https://doi.org/10.1016/j.conbuildmat.2013.06.061
Al Qabany A, Soga K (2013) Effect of chemical treatment used in MICP on engineering properties of cemented soils. Géotechnique 63(4):331–339. https://doi.org/10.1680/geot.SIP13.P.022
Ali MF, Mukhtar H, Dufossé L (2023) Microbial calcite induction: a magic that fortifies and heals concrete. Int J Environ Sci Technol 20(1):1113–1134. https://doi.org/10.1007/s13762-022-03941-2
Al-Maqdi K, Bilal M, Alzamly A et al (2021) Enzyme-loaded flower-shaped nanomaterials: a versatile platform with biosensing, biocatalytic, and environmental promise. Nanomaterials 11(6):1460. https://doi.org/10.3390/nano11061460
Alshalif AF, Irwan JM, Othman N et al (2016) Isolation of sulphate reduction bacteria (SRB) to improve compress strength and water penetration of bio-concrete. In: The 3rd International Conference on Civil and Environmental Engineering for Sustainability (IConCEES 2015) MATEC web of conferences, 47:01016. https://doi.org/10.1051/matecconf/20164701016
Al-Thawadi SM (2011) Ureolytic bacteria and calcium carbonate formation as a mechanism of strength enhancement of sand. J Adv Sci Eng Res 1(1):98–114
Azeiteiro RJN, Coelho PALF, Taborda DMG et al (2017) Energy-based evaluation of liquefaction potential under non-uniform cyclic loading. Soil Dyn Earthq Eng (1984) 92:650–665. https://doi.org/10.1016/j.soildyn.2016.11.005
Bains A, Dhami NK, Mukherjee A et al (2015) Influence of exopolymeric materials on bacterially induced mineralization of carbonates. Appl Biochem Biotechnol 175(7):3531–3541. https://doi.org/10.1007/s12010-015-1524-3
Balakrishnan R, Silva RT, Hwa T et al (2021) Suboptimal resource allocation in changing environments constrains response and growth in bacteria. Molecular systems biology 17:12 e10597-n/a. https://doi.org/10.15252/msb.202110597
Bansal R, Dhami NK, Mukherjee A et al (2016) Biocalcification by halophilic bacteria for remediation of concrete structures in marine environment. J Ind Microbiol Biotechnol 43(11):1497–1505. https://doi.org/10.1007/s10295-016-1835-6
Baumgartner LK, Reid RP, Dupraz C et al (2006) Sulfate reducing bacteria in microbial mats: changing paradigms, new discoveries. Sed Geol 185(3):131–145. https://doi.org/10.1016/j.sedgeo.2005.12.008
Behzadipour H, Sadrekarimi A (2021) Biochar-assisted bio-cementation of a sand using native bacteria. Bull Eng Geol Environ 80(6):4967–4984. https://doi.org/10.1007/s10064-021-02235-0
Bernardi D, DeJong JT, Montoya BM et al (2014) Bio-bricks: biologically cemented sandstone bricks. Constr Build Mater 55:462–469. https://doi.org/10.1016/j.conbuildmat.2014.01.019
Bhattacharya A, Naik SN, Khare SK (2018) Harnessing the bio-mineralization ability of urease producing Serratia marcescens and Enterobacter cloacae EMB19 for remediation of heavy metal cadmium (II). J Environ Manage 215:143–152. https://doi.org/10.1016/j.jenvman.2018.03.055
Bian Y, Rong Z, Chang TMS (2012) Polyhemoglobin-superoxide dismutase-catalase-carbonic anhydrase: a novel biotechnology-based blood substitute that transports both oxygen and carbon dioxide and also acts as an antioxidant. Artif Cells Blood Substitutes Biotechnol 40(1–2):28–37. https://doi.org/10.3109/10731199.2011.582041
Bibi S, Oualha M, Ashfaq MY et al (2018) Isolation, differentiation and biodiversity of ureolytic bacteria of Qatari soil and their potential in microbially induced calcite precipitation (MICP) for soil stabilization. RSC Adv 8(11):5854–5863. https://doi.org/10.1039/c7ra12758h
Boone CD, Gill S, Habibzadegan A et al (2013) Carbonic anhydrase: an efficient enzyme with possible global implications. Int J Chem Eng 2013:813931. https://doi.org/10.1155/2013/813931
Borchert M, Saunders P (2011) U.S. Patent No. 7,892,814. Washington, DC: U.S. Patent and Trademark Office.
Botusharova S, Gardner D, Harbottle M (2020) Augmenting microbially induced carbonate precipitation of soil with the capability to self-heal. J Geotech Geoenviron Eng 146(4):04020010. https://doi.org/10.1061/(ASCE)GT.1943-5606.0002214
Bozbeyoglu NN, Şensoy Candoğan T, Arslan S et al (2020) Calcium carbonate precipitation by urease and carbonic anhydrase positive bacteria. Mühendislik Bilimleri Dergisi 26(3):513–518. https://doi.org/10.5505/pajes.2019.73848
Bradfield J (1947) Plant carbonic anhydrase. Nature 159(4040):467–468. https://doi.org/10.1038/159467a0
Bu C, Dong Q, Wen K et al (2018) Development of Innovative Bio-beam Using Microbial Induced Calcite Precipitation Technology. 27–30 May 2018 China , Shanghai. In: Proceedings of GeoShanghai 2018 International Conference: Geoenvironment and Geohazard. Springer Singapore, p 491–498
Burbank MB, Weaver TJ, Williams BC et al (2012) Urease activity of ureolytic bacteria isolated from six soils in which calcite was precipitated by indigenous bacteria. Geomicrobiol J 29(4):389–395. https://doi.org/10.1080/01490451.2011.575913
Castanier S, Le Métayer-Levrel G, Orial G, Loubière JF, Perthuisot JP (2000) Bacterial Carbonatogenesis and Applications to Preservation and Restoration of Historic Property. In: Ciferri O, Tiano P, Mastromei G (eds) Of Microbes and Art. Springer, Boston. https://doi.org/10.1007/978-1-4615-4239-1_14
Castro-Alonso MJ, Montañez-Hernandez LE, Sanchez-Muñoz MA et al (2019) Microbially Induced Calcium Carbonate Precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Front Mater 6:126. https://doi.org/10.3389/fmats.2019.00126
Charpe AU, Latkar MV, Chakrabarti T (2019) Biocementation: an eco-friendly approach to strengthen concrete. Proc Inst Civ Eng Eng Sustain 172(8):438–449. https://doi.org/10.1680/jensu.18.00019
Chek A, Crowley R, Ellis TN et al (2021) Evaluation of factors affecting erodibility improvement for MICP-treated beach sand. J Geotech Geoenviron Eng 147(3):04021001. https://doi.org/10.1061/(ASCE)GT.1943-5606.0002481
Chen H, Qian C, Huang H (2016) Self-healing cementitious materials based on bacteria and nutrients immobilized respectively. Constr Build Mater 126:297–303. https://doi.org/10.1016/j.conbuildmat.2016.09.023
Chen M, Gowthaman S, Nakashima K et al (2021) Evaluating mechanical strength of peat soil treated by fiber incorporated bio-cementation. Int J GEOMATE 20(78):2186–2990. https://doi.org/10.21660/2021.78.Gx162
Cheng Y, Tang C, Pan X et al (2021) Application of microbial induced carbonate precipitation for loess surface erosion control. Eng Geol 294:106387. https://doi.org/10.1016/j.enggeo.2021.106387
Chetty K, Garbe U, Wang Z et al (2023) Bioconcrete based on sulfate-reducing bacteria granules: cultivation, mechanical properties, and self-healing performance. J Sustain Cement-Based Mater 12(9):1049–1060. https://doi.org/10.1080/21650373.2022.2153389
Chuo SC, Mohamed SF, Mohd Setapar SH et al (2020) Insights into the current trends in the utilization of bacteria for microbially induced calcium carbonate precipitation. Materials 13(21):4993. https://doi.org/10.3390/ma13214993
Clarà Saracho A, Haigh SK, Ehsan Jorat M (2021) Flume study on the effects of microbial induced calcium carbonate precipitation (MICP) on the erosional behaviour of fine sand. Géotechnique 71(12):1135–1149. https://doi.org/10.1680/jgeot.19.P.350
Cunningham AB, Class H, Ebigbo A et al (2019) Field-scale modeling of microbially induced calcite precipitation. Comput Geosci 23(2):399–414. https://doi.org/10.1007/s10596-018-9797-6
da Silva FB, De Belie N, Boon N et al (2015) Production of non-axenic ureolytic spores for self-healing concrete applications. Constr Build Mater 93:1034–1041. https://doi.org/10.1016/j.conbuildmat.2015.05.049
De Muynck W, Cox K, Belie ND et al (2008) Bacterial carbonate precipitation as an alternative surface treatment for concrete. Constr Build Mater 22(5):875–885. https://doi.org/10.1016/j.conbuildmat.2006.12.011
De Oliveira D, Horn EJ, Randall DG (2021) Copper mine tailings valorization using microbial induced calcium carbonate precipitation. J Environ Manag 298:113440. https://doi.org/10.1016/j.jenvman.2021.113440
DeJong JT, Mortensen BM, Martinez BC, Nelson DC (2010) Bio-mediated soil improvement. Ecol Eng 36:197–210. https://doi.org/10.1016/J.ECOLENG.2008.12.029
Deng X, Li Y, Liu H et al (2021) Examining energy consumption and carbon emissions of microbial induced carbonate precipitation using the life cycle assessment method. Sustainability 13(9):4856. https://doi.org/10.3390/su13094856
Dhami NK, Reddy MS, Mukherjee A (2014) Synergistic role of bacterial urease and carbonic anhydrase in carbonate mineralization. Appl Biochem Biotechnol 172(5):2552–2561. https://doi.org/10.1007/s12010-013-0694-0
Dhami NK, Mukherjee A, Reddy M (2016) Micrographical, minerological and nano-mechanical characterisation of microbial carbonates from urease and carbonic anhydrase producing bacteria. Ecol Eng 94:443–454. https://doi.org/10.1016/j.ecoleng.2016.06.013
Dhami N, Alsubhi W, Watkin E et al (2017) Bacterial community dynamics and biocement formation during stimulation and augmentation: implications for soil consolidation. Front Microbiol 8:1267. https://doi.org/10.3389/fmicb.2017.01267
Di Fiore A, Alterio V, Monti SM et al (2015) Thermostable carbonic anhydrases in biotechnological applications. Int J Mol Sci 16(7):15456–15480. https://doi.org/10.3390/ijms160715456
Dilrukshi RAN, Nakashima K, Kawasaki S (2018) Soil improvement using plant-derived urease-induced calcium carbonate precipitation. Soils Found 58(4):894–910. https://doi.org/10.1016/j.sandf.2018.04.003
Duarte-Nass C, Rebolledo K, Valenzuela T et al (2020) Application of microbe-induced carbonate precipitation for copper removal from copper-enriched waters: challenges to future industrial application. J Environ Manag 256:109938. https://doi.org/10.1016/j.jenvman.2019.109938
Dubey AA, Ravi K, Mukherjee A et al (2021) Biocementation mediated by native microbes from Brahmaputra riverbank for mitigation of soil erodibility. Sci Rep 11(1):15250. https://doi.org/10.1038/s41598-021-94614-6
Dupraz S, Parmentier M, Ménez B et al (2009) Experimental and numerical modeling of bacterially induced pH increase and calcite precipitation in saline aquifers. Chem Geol 265(1):44–53. https://doi.org/10.1016/j.chemgeo.2009.05.003
Erşan YÇ, Belie Nd, Boon N (2015) Microbially induced CaCO3 precipitation through denitrification: an optimization study in minimal nutrient environment. Biochem Eng J 101:108–118. https://doi.org/10.1016/j.bej.2015.05.006
Faeli Z, Montoya BM, Gabr MA (2023) Elucidating factors governing MICP biogeochemical processes at macro-scale: a reactive transport model development. Comput Geotech 160:105514. https://doi.org/10.1016/j.compgeo.2023.105514
Fauriel S, Laloui L (2012) A bio-chemo-hydro-mechanical model for microbially induced calcite precipitation in soils. Comput Geotech 46:104–120. https://doi.org/10.1016/j.compgeo.2012.05.017
Feng J, Chen B, Sun W et al (2021) Microbial induced calcium carbonate precipitation study using Bacillus subtilis with application to self-healing concrete preparation and characterization. Construct Build Mater 280:122460. https://doi.org/10.1016/j.conbuildmat.2021.122460
Fu Y, Fan F, Zhang Y et al (2021) Conformational change of H64 and substrate transportation: insight into a full picture of enzymatic hydration of CO2 by carbonic anhydrase. Front Chem 9:706959. https://doi.org/10.3389/fchem.2021.706959
Fukue M, Ono S, Sato Y (2011) Cementation of sands due to microbiologically-induced carbonate precipitation. Soils Found 51(1):83–93. https://doi.org/10.3208/sandf.51.83
Ganena G, De Muynck W, Ho A et al (2014) Formate oxidation driven calcium carbonate precipitation by Methylocystis parvus OBBP. Appl Environ Microbiol 80(15):4659–4667. https://doi.org/10.1128/AEM.01349-14
Ganena G, Wang J, Ramos JA et al (2015) Biogenic concrete protection driven by the formate oxidation by Methylocystis parvus OBBP. Front Microbiol 6:786. https://doi.org/10.3389/fmicb.2015.00786
Gaspar J, Gladis A, Woodley JM et al (2017) Rate-based modelling and validation of a pilot absorber using MDEA enhanced with Carbonic Anhydrase (CA). Energy Procedia 114:707–718. https://doi.org/10.1016/j.egypro.2017.03.1213
Ghorai S, Pulya S, Ghosh K et al (2020) Structure-activity relationship of human carbonic anhydrase-II inhibitors: detailed insight for future development as anti-glaucoma agents. Bioorg Chem 95:103557. https://doi.org/10.1016/j.bioorg.2019.103557
Giri A, Pant D (2019) CO2 management using carbonic anhydrase producing microbes from western Indian Himalaya. Bioresour Technol Rep 8:100320. https://doi.org/10.1016/j.biteb.2019.100320
Giri A, Banerjee UC, Kumar M et al (2018) Intracellular carbonic anhydrase from Citrobacter freundii and its role in bio-sequestration. Biores Technol 267:789–792. https://doi.org/10.1016/j.biortech.2018.07.089
Gomez MG, Anderson CM, Graddy CMR et al (2017) Large-scale comparison of bioaugmentation and biostimulation approaches for biocementation of sands. J Geotech Geoenviron Eng 143(5):1–13. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001640
Guo CH, Stabnikov V, Ivanov V (2010) The removal of nitrogen and phosphorus from reject water of municipal wastewater treatment plant using ferric and nitrate bioreductions. Biores Technol 101(11):3992–3999. https://doi.org/10.1016/j.biortech.2010.01.039
Hadi S, Abbas H, Almajed A et al (2022) Biocementation by Sporosarcina pasteurii ATCC6453 under simulated conditions in sand columns. J Market Res 18:4375–4384. https://doi.org/10.1016/j.jmrt.2022.04.105
Hanifa M, Agarwal R, Sharma U et al (2023) A review on CO2 capture and sequestration in the construction industry: Emerging approaches and commercialised technologies. J CO2 Util 67:102292.https://doi.org/10.1016/j.jcou.2022.102292
Harnpicharnchai P, Mayteeworakoon S, Kitikhun S et al (2022) High level of calcium carbonate precipitation achieved by mixed culture containing ureolytic and nonureolytic bacterial strains. Lett Appl Microbiol 75(4):888–898. https://doi.org/10.1111/lam.13748
He J, Gray K, Norris A et al (2020) Use of biological additives in concrete pavements: a review of opportunities and challenges. J Transp Eng Part B: Pavements 146(3):04020036. https://doi.org/10.1061/JPEODX.0000188
Hu Y, Liu W, Wang W et al (2020) Biomineralization Performance of Bacillus sphaericus under the Action of Bacillus mucilaginosus. Adv Mater Sci Eng 2020:6483803. https://doi.org/10.1155/2020/6483803
Huang L, Li F, Ji C et al (2022) Carbon isotope fractionation and its tracer significance to carbon source during precipitation of calcium carbonate in the presence of Bacillus cereus LV-1. Chem Geol 609:121029. https://doi.org/10.1016/j.chemgeo.2022.121029
Imran M, Kimura S, Nakashima K et al (2019) Feasibility study of native ureolytic bacteria for biocementation towards coastal erosion protection by MICP method. Appl Sci 9(20):4462. https://doi.org/10.3390/app9204462
Insausti M, Timmis R, Kinnersley R et al (2020) Advances in sensing ammonia from agricultural sources. Sci Total Environ 706:135124. https://doi.org/10.1016/j.scitotenv.2019.135124
Irfan MF, Hossain SMZ, Khalid H et al (2019) Optimization of bio-cement production from cement kiln dust using microalgae. Biotechnol Rep 23:e00356. https://doi.org/10.1016/j.btre.2019.e00356
Ivanov V, Stabnikov V (2020) Environmental safety of biotechnological materials and processes. In: Pacheco-Torgal et al (eds) Bio-based Materials and Biotechnologies for Eco-efficient Construction. p 359–375. https://doi.org/10.1016/B978-0-12-819481-2.00017-9
Ivanov V., Chu J. Stabnikov V (2015) Basics of Construction Microbial Biotechnology. In: Pacheco Torgal F.et al. (eds.), Biotechnologies and Biomimetics for Civil Engineering, Springer, pp 21–56 https://doi.org/10.1007/978-3-319-09287-4_2
Jain S (2021) An Overview of Factors Influencing Microbially Induced Carbonate Precipitation for Its Field Implementation. In: Achal V, Chin CS (eds) Building Materials for Sustainable and Ecological Environment. Springer, Singapore, pp 73–99. https://doi.org/10.1007/978-981-16-1706-5_5
Jain S, Fang C, Achal V (2021) A critical review on microbial carbonate precipitation via denitrification process in building materials. Bioengineered 12(1):7529–7551. https://doi.org/10.1080/21655979.2021.1979862
Jansook P, Hnin HM, Loftsson T et al (2021) Cyclodextrin-based formulation of carbonic anhydrase inhibitors for ocular delivery – a review. Int J Pharm 606:120955. https://doi.org/10.1016/j.ijpharm.2021.120955
Jansson C, Northen T (2010) Calcifying cyanobacteria—the potential of biomineralization for carbon capture and storage. Curr Opin Biotechnol 21(3):365–371. https://doi.org/10.1016/j.copbio.2010.03.017
Jaya P, Nathan VK, Ammini P (2019) Characterization of marine bacterial carbonic anhydrase and their CO2 sequestration abilities based on a soil microcosm. Prep Biochem Biotechnol 49(9):891–899. https://doi.org/10.1080/10826068.2019.1633669
Jiang N, Tang C, Hata T et al (2020) Bio-mediated soil improvement: the way forward. Soil Use Manag 36(2):185–188. https://doi.org/10.1111/sum.12571
Jiang N, Tang C, Yin L et al (2019) Applicability of microbial calcification method for sandy-slope surface erosion control. J Mater Civ Eng 31(11). https://doi.org/10.1061/(ASCE)MT.1943-5533.0002897
Jin P, Zhang S, Liu Y et al (2021) Application of Bacillus mucilaginosus in the carbonation of steel slag. Appl Microbiol Biotechnol 105(23):8663–8674. https://doi.org/10.1007/s00253-021-11641-z
Jonkers HM, Thijssen A, Muyzer G et al (2010) Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol Eng 36(2):230–235. https://doi.org/10.1016/j.ecoleng.2008.12.036
Jonkers HM, Schlangen E (2008) Development of a bacteria-based self healing concrete. Tailor Made Concrete Structures – Walraven & Stoelhorst (eds) Taylor & Francis Group, London, ISBN 978–0–415–47535–8:425–430. https://doi.org/10.1201/9781439828410
Joshi S, Goyal S, Sudhakara Reddy M (2021) Bio-consolidation of cracks with fly ash amended biogrouting in concrete structures. Constr Build Mater 300(124044):1–11. https://doi.org/10.1016/j.conbuildmat.2021.124044
Jroundi F, Gómez-Suaga P, Jimenez-Lopez C et al (2012) Stone-isolated carbonatogenic bacteria as inoculants in bioconsolidation treatments for historical limestone. Sci Total Environ 425:89–98. https://doi.org/10.1016/j.scitotenv.2012.02.059
Justo-Reinoso I, Heath A, Gebhard S et al (2021) Aerobic non-ureolytic bacteria-based self-healing cementitious composites: a comprehensive review. J Build Eng 42:102834. https://doi.org/10.1016/j.jobe.2021.102834
Kahani M, Kalantary F, Soudi MR et al (2020) Optimization of cost effective culture medium for Sporosarcina pasteurii as biocementing agent using response surface methodology: up cycling dairy waste and seawater. J Clean Prod 253:120022. https://doi.org/10.1016/j.jclepro.2020.120022
Kapustka K, Ziegmann G, Klimecka-Tatar D et al (2020) Identification of health risks from harmful chemical agents – review concerning bisphenol A in workplace. Prod Eng Arch 26(2):45–49. https://doi.org/10.30657/pea.2020.26.10
Kashizadeh E, Mukherjee A, Tordesillas A (2021) Experimental and numerical investigations on confined granular systems stabilized by bacterial cementation. Int J Geomech 21(1):04020244. https://doi.org/10.1061/(ASCE)GM.1943-5622.0001891
Kaur G, Dhami NK, Goyal S et al (2016) Utilization of carbon dioxide as an alternative to urea in biocementation. Constr Build Mater 123:527–533. https://doi.org/10.1016/j.conbuildmat.2016.07.036
Keykha HA, Mohamadzadeh H, Asadi A et al (2019) Ammonium-free carbonate-producing bacteria as an ecofriendly soil biostabilizer. Geotech Test J 42(1):19–29. https://doi.org/10.1520/GTJ20170353
Khodadadi HT, Kavazanjian E, van Paassen L, DeJong J (2017) Bio-grout Materials: A Review. In: Byle MJ et al (eds) Grouting 2017: Grouting, Drilling, and Verification. p 1–12 https://doi.org/10.1061/9780784480793
Kim JK, Lee C, Lim SW et al (2020) Elucidating the role of metal ions in carbonic anhydrase catalysis. Nat Commun 11(1):4557. https://doi.org/10.1038/s41467-020-18425-5
Krause S, Liebetrau V, Löscher CR et al (2018) Marine ammonification and carbonic anhydrase activity induce rapid calcium carbonate precipitation. Geochim Cosmochim Acta 243:116–132. https://doi.org/10.1016/j.gca.2018.09.018
Kumar PPJ, Rajan Babu J, Nandhagopal B et al (2019) In vitro synthesis of bio-brick using locally isolated marine ureolytic bacteria, a comparison with natural calcareous rock. Ecol Eng 138:97–105. https://doi.org/10.1016/J.ECOLENG.2019.07.017
Lai HJ, Cui MJ, Wu SF et al (2021) Retarding effect of concentration of cementation solution on biocementation of soil. Acta Geotech 16:1457–1472. https://doi.org/10.1007/s11440-021-01149-1
Lai HJ, Cui MJ, Chu J (2023) Effect of pH on soil improvement using one-phase-low-pH MICP or EICP biocementation method. Acta Geotech 18:3259–3272. https://doi.org/10.1007/s11440-022-01759-3
Lambert SE, Randall DG (2019) Manufacturing bio-bricks using microbial induced calcium carbonate precipitation and human urine. Water Res 160:158–166. https://doi.org/10.1016/j.watres.2019.05.069
Lee YS, Park W (2018) Current challenges and future directions forbacterial self-healing concrete. Appl Microbiol Biotechnol 102(7):3059–3070. https://doi.org/10.1007/s00253-018-8830-y
Leimbrink M, Tlatlik S, Salmon S et al (2017) Pilot scale testing and modeling of enzymatic reactive absorption in packed columns for CO2 capture. Int J Greenhouse Gas Control 62:100–112. https://doi.org/10.1016/j.ijggc.2017.04.010
Li P, Qu W (2015) Bacteria for Concrete Surface Treatment. In: Pacheco Torgal F, Labrincha J, Diamanti M, Yu CP, Lee H (eds) Biotechnologies and Biomimetics for Civil Engineering. Springer, Cham, pp 325–358. https://doi.org/10.1007/978-3-319-09287-4_15
Li W, Chen W, Zhou P et al (2013) Influence of initial pH on the precipitation and crystal morphology of calcium carbonate induced by microbial carbonic anhydrase. Colloids Surf B 102:281–287. https://doi.org/10.1016/j.colsurfb.2012.08.042
Li C, Jiang X, Qiu Y et al (2015) Identification of a new thermostable and alkali-tolerant α-carbonic anhydrase from Lactobacillus delbrueckii as a biocatalyst for CO2 biomineralization. Bioresour Bioprocess 2:44. https://doi.org/10.1186/s40643-015-0074-4
Li M, Cheng X, Guo H et al (2016) Biomineralization of carbonate by terrabacter tumescens for heavy metal removal and biogrouting applications. J Environ Eng 142(9):C4015005. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000970
Li M, Fang C, Kawasaki S et al (2019) Bio-consolidation of cracks in masonry cement mortars by Acinetobacter sp. SC4 isolated from a karst cave. Int Biodeterior Biodegrad 141:94–100. https://doi.org/10.1016/j.ibiod.2018.03.008
Li D, Zhao H, Li G et al (2022) Calcium ion biorecovery from industrial wastewater by Bacillus amyloliquefaciens DMS6. Chemosphere 298:134328. https://doi.org/10.1016/j.chemosphere.2022.134328
Liu B, Qin J, Shi J et al (2021) New perspectives on utilization of CO2 sequestration technologies in cement-based materials. Construct Build Mater 272:121660. https://doi.org/10.1016/j.conbuildmat.2020.121660
Ludwig R, Al-Horani F, de Beer D et al (2005) Photosynthesis-controlled calcification in a hypersaline microbial mat. Limnol Oceanogr 50(6):1836–1843. https://doi.org/10.4319/lo.2005.50.6.1836
Mahawish A, Bouazza A, Gates WP (2018) Effect of particle size distribution on the bio-cementation of coarse aggregates. Acta Geotech 13:1019–1025. https://doi.org/10.1007/s11440-017-0604-7
Martienssen M, Schöps R (1999) Population dynamics of denitrifying bacteria in a model biocommunity. Water Res 33(3):639–646. https://doi.org/10.1016/S0043-1354(98)00222-X
Martinez BC, Barkouki TH, DeJong JD, Ginn TR (2011) Upscaling microbial induced calcite precipitation in 0.5 m columns: experimental and modeling results. In: Geo-frontiers 2011: advances in geotechnical engineering, pp 4049–4059. https://doi.org/10.1061/41165(397)414
Martinez BC, DeJong JT, Ginn TR et al (2013) Experimental Optimization of Microbial-Induced Carbonate Precipitation for Soil Improvement. J Geotech Geoenviron Eng 139(4):587–598. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000787
Martinez BC, DeJong JT, Ginn TR (2014) Bio-geochemical reactive transport modeling of microbial induced calcite precipitation to predict the treatment of sand in one-dimensional flow. Comput Geotech 58:1–13. https://doi.org/10.1016/j.compgeo.2014.01.013
Matsubara H, Yamada T (2020) Mathematical modelling and simulation of microbial carbonate precipitation: the urea hydrolysis reaction. Acta Geotech 15(1):29–38. https://doi.org/10.1007/s11440-019-00896-6
Mavroulidou M, Morrison T, Unsworth C, Gunn MJ (2015) Properties of concrete made of multicomponent mixes of low-energy demanding binders. Constr Build Mater 101:1122–1141. https://doi.org/10.1016/J.CONBUILDMAT.2015.10.091
Meldrum NU, Roughton FJW (1933) Carbonic anhydrase. Its preparation and properties. J Physiol (Lond) 80(2):113–142. https://doi.org/10.1113/jphysiol.1933.sp003077
Minto JM, Lunn RJ, El Mountassir G (2019) Development of a reactive transport model for field-scale simulation of microbially induced carbonate precipitation. Water Resour Res 55(8):7229–7245. https://doi.org/10.1029/2019WR025153
Mirjafari P, Asghari K, Mahinpey N (2007) Investigating the application of enzyme carbonic anhydrase for CO2 sequestration purposes. Ind Eng Chem Res 46(3):921–926. https://doi.org/10.1021/ie060287u
Mitchell AC, Ferris FG (2005) The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: temperature and kinetic dependence. Geochim Cosmochim Acta 69(17):4199–4210. https://doi.org/10.1016/j.gca.2005.03.014
Molina-Fernández C, Luis P (2021) Immobilization of carbonic anhydrase for CO2 capture and its industrial implementation: a review. J CO2 Util 47:101475. https://doi.org/10.1016/j.jcou.2021.101475
Moravej S, Habibagahi G, Nikooee E et al (2018) Stabilization of dispersive soils by means of biological calcite precipitation. Geoderma 315:130–137. https://doi.org/10.1016/j.geoderma.2017.11.037
Moroney JV, Bartlett SG, Samuelsson G (2001) Carbonic anhydrases in plants and algae. Plant Cell Environ 24(2):141–153. https://doi.org/10.1111/j.1365-3040.2001.00669.x
Murrell JC, Jetten MSM (2009) The microbial methane cycle. Environ Microbiol Rep 1(5):279–284. https://doi.org/10.1111/j.1758-2229.2009.00089.x
Mwandira W, Nakashima K, Kawasaki S (2017) Bioremediation of lead-contaminated mine waste by Pararhodobacter sp. based on the microbially induced calcium carbonate precipitation technique and its effects on strength of coarse and fine grained sand. Ecol Eng 109:57–64. https://doi.org/10.1016/j.ecoleng.2017.09.011
Mwandira W, Nakashima K, Kawasaki S et al (2019) Solidification of sand by Pb(II)-tolerant bacteria for capping mine waste to control metallic dust: case of the abandoned Kabwe Mine, Zambia. Chemosphere 228:17–25. https://doi.org/10.1016/j.chemosphere.2019.04.107
Nassar MK, Gurung D, Bastani M et al (2018) Large-scale experiments in Microbially Induced Calcite Precipitation (MICP): reactive transport model development and prediction. Water Resour Res 54(1):480–500. https://doi.org/10.1002/2017WR021488
Nasser AA, Sorour NM, Saafan MA et al (2022) Microbially-Induced-Calcite-Precipitation (MICP): a biotechnological approach to enhance the durability of concrete using Bacillus pasteurii and Bacillus sphaericus. Heliyon 8(7):e09879. https://doi.org/10.1016/j.heliyon.2022.e09879
Nathan VK, Ammini P (2019) Carbon dioxide sequestering ability of bacterial carbonic anhydrase in a mangrove soil microcosm and its bio-mineralization properties. Water Air Soil Pollut 230(8):1–12. https://doi.org/10.1007/s11270-019-4229-3
Nežerka V, Demo P, Schreiberová H et al (2022) Self-healing concrete: application of monod’s approach for modeling Bacillus pseudofirmus growth curves. Eur J Environ Civ Eng 26(16):8229–8241. https://doi.org/10.1080/19648189.2021.2021996
O’Donnell ST, Kavazanjian E, Rittmann BE (2017) MIDP: liquefaction mitigation via microbial denitrification as a two-stage process. II: MICP. J Geotech Geoenviron Eng 143(12). https://doi.org/10.1061/(ASCE)GT.1943-5606.0001806
Oliveira PJV, Freitas LD, Carmona JPSF (2017) Effect of soil type on the enzymatic calcium carbonate precipitation process used for soil improvement. J Mater Civ Eng 29(4):04016263. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001804
Ortega-Villamagua E, Gudiño-Gomezjurado M, Palma-Cando A (2020) Microbiologically induced carbonate precipitation in the restoration and conservation of cultural heritage materials. Molecules 25(23):1–23. https://doi.org/10.3390/molecules25235499
Pan L, Li Q, Zhou Y et al (2019) Effects of different calcium sources on the mineralization and sand curing of CaCO3 by carbonic anhydrase-producing bacteria. RSC Adv 9(70):40827–40834. https://doi.org/10.1039/c9ra09025h
Parvathy AJ, Das BC, Jifiriya MJ et al (2023) Ammonia induced toxico-physiological responses in fish and management interventions. Rev Aquac 15(2):452–479. https://doi.org/10.1111/raq.12730
Pham VP, van Paassen LA, van der Star WRL et al (2018) Evaluating strategies to improve process efficiency of denitrification-based MICP. J Geotech Geoenviron Eng 144(8):04018049. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001909
Porter H, Mukherjee A, Tuladhar R et al (2021) Life cycle assessment of biocement: an emerging sustainable solution? Sustainability 13(24):13878. https://doi.org/10.3390/su132413878
Power IM, Harrison AL, Dipple GM et al (2013) Carbon sequestration via carbonic anhydrase facilitated magnesium carbonate precipitation. Int J Greenhouse Gas Control 16:145–155. https://doi.org/10.1016/j.ijggc.2013.03.011
Punnoi B, Arpajirakul S, Pungrasmi W, Chompoorat T, Likitlersuang S (2021) Use of microbially induced calcite precipitation for soil improvement in compacted clays. Int J Geosynth Ground Eng 7:86. https://doi.org/10.1007/S40891-021-00327-1/TABLES/6
Qian C, Chen H, Ren L et al (2015) Self-healing of early age cracks in cement-based materials by mineralization of carbonic anhydrase microorganism. Front Microbiol 6:1225. https://doi.org/10.3389/fmicb.2015.01225
Qian C, Zheng T, Zhang X et al (2021) Application of microbial self-healing concrete: case study. Construct Build Mater 290:123226. https://doi.org/10.1016/j.conbuildmat.2021.123226
Qian C, Zhang X, Chen Y et al (2022) Microbial mineralization at the surface layer of cement-based materials and its effect on efflorescence performance. J Build Eng 52:104480. https://doi.org/10.1016/j.jobe.2022.104480
Raveh-Amit H, Tsesarsky M (2020) Biostimulation in desert soils for microbial-induced calcite precipitation. Appl Sci 10(8):2905. https://doi.org/10.3390/app10082905
Rosewitz JA, Wang S, Scarlata SF et al (2021) An enzymatic self-healing cementitious material. Appl Mater Today 23:101035. https://doi.org/10.1016/j.apmt.2021.101035
Russo ME, Olivieri G, Marzocchella A et al (2013) Post-combustion carbon capture mediated by carbonic anhydrase. Sep Purif Technol 107:331–339. https://doi.org/10.1016/j.seppur.2012.06.022
Russo ME, Bareschino P, Olivieri G et al (2016) Modeling of slurry staged bubble column for biomimetic CO2 capture. Int J Greenhouse Gas Control 47:200–209. https://doi.org/10.1016/j.ijggc.2016.01.045
Safdar MU, Mavroulidou M, Gunn MJ et al (2021a) Innovative methods of ground improvement for railway embankment peat fens foundation soil. Géotechnique 71(11):985–998. https://doi.org/10.1680/jgeot.19.SiP.030
Safdar MU, Mavroulidou M, Gunn MJ et al (2021b) Electrokinetic biocementation of an organic soil. Sustain Chem Pharm 71(11):985–998. https://doi.org/10.1016/j.scp.2021.100405
Safdar MU, Mavroulidou M, Gunn MJ et al (2022) Towards the development of sustainable ground improvement techniques—biocementation study of an organic soil. Circ Econ Sustain 2:1589–1614. https://doi.org/10.1007/s43615-021-00071-8
Salifu E, MacLachlan E, Iyer KR, Knapp CW, Tarantino A (2016) Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: a preliminary investigation. Eng Geol 201:96–105. https://doi.org/10.1016/j.enggeo.2015.12.027
Seifan M, Samani AK, Berenjian A (2016) Bioconcrete: next generation of self-healing concrete. Appl Microbiol Biotechnol 100(6):2591–2602. https://doi.org/10.1007/s00253-016-7316-z
Setlow P (1994) Mechanisms which contribute to the long-term survival of spores of Bacillus species. J Appl Bacteriol 76:49S-60S. https://doi.org/10.1111/j.1365-2672.1994.tb04357.x
Sharma T, Kumar A (2021) Efficient reduction of CO2 using a novel carbonic anhydrase producing Corynebacterium flavescens. Environ Eng Res 26(3):183–191. https://doi.org/10.4491/eer.2020.191
Sharma TK, Alazhari M, Heath A et al (2017) Alkaliphilic Bacillus species show potential application in concrete crack repair by virtue of rapid spore production and germination then extracellular calcite formation. J Appl Microbiol 122(5):1233–1244. https://doi.org/10.1111/jam.13421
Sharma M, Satyam N, Tiwari N et al (2021) Simplified biogeochemical numerical model to predict pore fluid chemistry and calcite precipitation during biocementation of soil. Arab J Geosci 14:807. https://doi.org/10.1007/s12517-021-07151-x
Sharma T, Sharma A, Cl Xia et al (2022) Enzyme mediated transformation of CO2 into calcium carbonate using purified microbial carbonic anhydrase. Environ Res 212:113538. https://doi.org/10.1016/j.envres.2022.113538
Shende P, Kasture P, Gaud RS (2018) Nanoflowers: the future trend of nanotechnology for multi-applications. Artif Cells Nanomed Biotechnol 46:413–422. https://doi.org/10.1080/21691401.2018.1428812
Silva-Castro G, Uad I, Gonzalez-Martinez A et al (2015) Bioprecipitation of calcium carbonate crystals by bacteria isolated from saline environments grown in culture media amended with seawater and real brine. Biomed Res Int 2015:816102–12. https://doi.org/10.1155/2015/816102
Smith KS, Ferry JG (1999) A plant-type (β-Class) carbonic anhydrase in the thermophilic methanoarchaeon methanobacterium thermoautotrophicum. J Bacteriol 181(20):6247–6253. https://doi.org/10.1128/JB.181.20.6247-6253.1999
Song H, Kumar A, Ding Y et al (2022) Removal of Cd2+ from wastewater by microorganism induced carbonate precipitation (MICP): an economic bioremediation approach. Sep Purif Technol 297:121540. https://doi.org/10.1016/j.seppur.2022.121540
Soon NW, Lee LM, Khun TC et al (2014) Factors affecting improvement in engineering properties of residual soil through microbial-induced calcite precipitation. J Geotech Geoenviron Eng 140(5):04014006. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001089
Stabnikov V, Stabnikov D, Udymovych V (2022) Increase the ecological safety of the soil biogrouting using plant urease. Ukrainian Food J 11(2):302–314. https://doi.org/10.24263/2304-974X-2022-11-2-10
Steger F, Reich J, Fuchs W et al (2022) Comparison of carbonic anhydrases for CO2 sequestration. Int J Mol Sci 23(2):957. https://doi.org/10.3390/ijms23020957
Su F, Yang Y, Qi Y et al (2022) Combining microbially induced calcite precipitation (MICP) with zeolite: a new technique to reduce ammonia emission and enhance soil treatment ability of MICP technology. J Environ Chem Eng 10(3):107770. https://doi.org/10.1016/j.jece.2022.107770
Sun X, Miao L, Tong T et al (2019) Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech 14:627–638. https://doi.org/10.1007/s11440-018-0758-y
Sundaram S, Thakur IS (2018) Induction of calcite precipitation through heightened production of extracellular carbonic anhydrase by CO2 sequestering bacteria. Bioresour Technol 253:368–371. https://doi.org/10.1016/j.biortech.2018.01.081
Supuran CT, Capasso C (2017) An overview of the bacterial carbonic anhydrases. Metabolites 7(4):56. https://doi.org/10.3390/metabo7040056
Swartz MK (2011) The PRISMA statement: a guideline for systematic reviews and meta-analyses. J Pediatr Health Care 25(1):1–2. https://doi.org/10.1016/j.pedhc.2010.09.006
Tambunan T, Juki MI, Othman N (2019) Mechanical properties of sulphate reduction bacteria on the durability of concrete in chloride condition. In: International Conference on Sustainable Civil Engineering Structures and Construction Materials (SCESCM 2018), MATEC Web of Conferences 258:1024. https://doi.org/10.1051/matecconf/201925801024.
Thakur IS, Kumar M, Varjani SJ et al (2018) Sequestration and utilization of carbon dioxide by chemical and biological methods for biofuels and biomaterials by chemoautotrophs: opportunities and challenges. Bioresour Technol 256:478–490. https://doi.org/10.1016/j.biortech.2018.02.039
Tiano P, Biagiotti L, Mastromei G (1999) Bacterial bio-mediated calcite precipitation for monumental stones conservation: methods of evaluation. J Microbiol Methods 36(1):139–145. https://doi.org/10.1016/S0167-7012(99)00019-6
Torres-Aravena Á, Duarte-Nass C, Azócar L, Mella-Herrera R, Rivas M, Jeison D (2018) Can microbially induced calcite precipitation (MICP) through a ueolytic pathway be successfully applied for removing heavy metals from wastewaters? Crystals 8(11):438. https://doi.org/10.3390/cryst8110438
Trachtenberg MC, Tu CK, Landers RA et al (1999) Carbon dioxide transport by proteic and facilitated transport membranes. Life Support Biosph Sci 6(4):293–302
Tziviloglou E, Wiktor V, Jonkers HM, Schlangen E (2016) Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr Build Mater 122:118–125. https://doi.org/10.1016/J.CONBUILDMAT.2016.06.080
Uad I, Gonzalez-Lopez J, Silva-Castro G et al (2014) Precipitation of carbonates crystals by bacteria isolated from a submerged fixed-film bioreactor used for the treatment of urban wastewater. Int J Environ Res 8(2):435–446
United Nations Environment Programme (2022) 2022 Global Status Report for Buildings and Construction: Towards a Zero-emission, Efficient and Resilient Buildings and Construction Sector. Nairobi. https://globalabc.org/our-work/tracking-progress-global-status-report. Accessed 6 June 2023
Van Paassen L, Pham V; Mahabadi N et al (2018) Desaturation via Biogenic Gas Formation as a Ground Improvement Technique. Second Pan-American Conference on Unsaturated Soils PanAm Unsaturated Soils 2017 GSP 300. https://doi.org/10.1061/9780784481677.013
Van Tittelboom K, De Belie N, De Muynck W et al (2010) Use of bacteria to repair cracks in concrete. Cem Concr Res 40(1):157–166. https://doi.org/10.1016/j.cemconres.2009.08.025
van Wijngaarden WK, Vermolen FJ, van Meurs GAM et al (2011) Modelling biogrout: a new ground improvement method based on microbial-induced carbonate precipitation. Transp Porous Med 87(2):397–420. https://doi.org/10.1007/s11242-010-9691-8
Vecchi V, Barera S, Bassi R et al (2020) Potential and challenges of improving photosynthesis in algae. Plants 9(1):67. https://doi.org/10.3390/plants9010067
Veitch FP, Blankenship LC (1963) Carbonic anhydrase in bacteria. Nature 197(4862):76–77. https://doi.org/10.1038/197076a0
Vincent J, Sabot R, Lanneluc I et al (2020) Biomineralization of calcium carbonate by marine bacterial strains isolated from calcareous deposits. Matér Tech 108(3):302. https://doi.org/10.1051/mattech/2020027
Wang X, Nackenhorst U (2020) A coupled bio-chemo-hydraulic model to predict porosity and permeability reduction during microbially induced calcite precipitation. Adv Water Resour 140:103563. https://doi.org/10.1016/j.advwatres.2020.103563
Wang J, Van Tittelboom K, De Belie N, Verstraete W (2012) Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr Build Mater 26(1):532–540. https://doi.org/10.1016/j.conbuildmat.2011.06.054
Wang J, Mignon A, Trenson G et al (2018) A chitosan based pH-responsive hydrogel for encapsulation of bacteria for self-sealing concrete. Cement Concr Compos 93:309–322. https://doi.org/10.1016/j.cemconcomp.2018.08.007
Wattanaphan P, Sema T, Idem R et al (2013) Effects of flue gas composition on carbon steel (1020) corrosion in MEA-based CO2 capture process. Int J Greenhouse Gas Control 19:340–349. https://doi.org/10.1016/j.ijggc.2013.08.021
Weber R, Orsino S, Lallemant N et al (2000) Combustion of natural gas with high-temperature air and large quantities of flue gas. Proc Combust Inst 28(1):1315–1321. https://doi.org/10.1016/S0082-0784(00)80345-8
Whiffin VS (2004) Microbial CaCO3 Precipitation for the Production of Biocement, PhD Thesis, Murdoch University, Perth, Western Australia
Wiktor V, Jonkers HM (2011) Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem Concr Compos 33:763–770. https://doi.org/10.1016/j.cemconcomp.2011.03.012
Xiao Y, Watson M (2019) Guidance on conducting a systematic literature review. J Plan Educ Res 39(1):93–112. https://doi.org/10.1177/0739456X17723971
Xu Y, Yu C, Yu X (2021) Microbial mineralization and carbonation consolidation of dredger fill and its mechanical properties. J Mater Civ Eng 33(7):04021144. https://doi.org/10.1061/(ASCE)MT.1943-5533.0003769
Yadav RR, Krishnamurthi K, Mudliar SN et al (2014) Carbonic anhydrase mediated carbon dioxide sequestration: promises, challenges and future prospects: carbonic anhydrase mediated carbon dioxide sequestration. J Basic Microbiol 54(6):472–481. https://doi.org/10.1002/jobm.201300849
Yang G, Li L, Li F et al (2021) Mechanism of carbonate mineralization induced by microbes: taking Curvibacter lanceolatus strain HJ-1 as an example. Micron 140:102980. https://doi.org/10.1016/j.micron.2020.102980
Yi H, Zhan Q, Yu X (2022) Optimization of mineralization curing system for efficient and safe utilization of steel slag wastes. J Wuhan Univ Technol-Mater Sci Ed 37(4):595–602
Zhan Q, Yu X, Pan Z et al (2021) Microbial-induced synthesis of calcite based on carbon dioxide capture and its cementing mechanism. J Clean Prod 278:123398. https://doi.org/10.1016/j.jclepro.2020.123398
Zhang C, Li F, Li X et al (2018) The roles of Mg over the precipitation of carbonate and morphological formation in the presence of arthrobacter sp. strain MF-2. Geomicrobiol J 35(7):545–554. https://doi.org/10.1080/01490451.2017.1421727
Zhang J, Su P, Li L (2022) Bioremediation of stainless steel pickling sludge through microbially induced carbonate precipitation. Chemosphere 298:134213. https://doi.org/10.1016/j.chemosphere.2022.134213
Zhao Q, Li L, Li C et al (2014) Factors affecting improvement of engineering properties of MICP-treated soil catalyzed by bacteria and urease. J Mater Civ Eng 26(12):4014094. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001013
Zhao Y, Yan H, Zhou J et al (2019) Bio-precipitation of calcium and magnesium ions through extracellular and intracellular process induced by bacillus licheniformis SRB2. Minerals 9(9):526. https://doi.org/10.3390/min9090526
Zhang S, Zhang Z, Lu Y, Rostam-Abadi M, Jones A (2011) Activity and stability of immobilized carbonic anhydrase for promoting CO2 absorption into a carbonate solution for post-combustion CO2 capture. Bioresour Technol 102(22):10194–10201. https://doi.org/10.1016/j.biortech.2011.09.043
Zheng T (2021) Bacteria-induced facile biotic calcium carbonate precipitation. J Cryst Growth 563:126096. https://doi.org/10.1016/j.jcrysgro.2021.126096
Zheng T, Qian C (2020a) Influencing factors and formation mechanism of CaCO3 precipitation induced by microbial carbonic anhydrase. Process Biochem (1991) 91:271–281. https://doi.org/10.1016/j.procbio.2019.12.018
Zheng T, Qian C (2020b) Self-healing of later-age cracks in cement-based materials by encapsulation-based bacteria. J Mater Civ Eng 32(11):04020341. https://doi.org/10.1061/(ASCE)MT.1943-5533.0003437
Zheng T, Su Y, Zhang X et al (2020) Effect and mechanism of encapsulation-based spores on self-healing concrete at different curing ages. ACS Appl Mater Interfaces 12(47):52415–52432. https://doi.org/10.1021/acsami.0c16343
Zheng T, Qian C, Su Y (2021) Influences of different calcium sources on the early age cracks of self-healing cementitious mortar. Biochem Eng J 166:107849. https://doi.org/10.1016/j.bej.2020.107849
Zheng L, Dong Y, Li B et al (2022) Simultaneous removal of high concentrations of ammonia nitrogen and calcium by the novel strain Paracoccus denitrificans AC-3 with good environmental adaptability. Bioresour Technol 359:127457. https://doi.org/10.1016/j.biortech.2022.127457
Zhu Y, Ma N, Jin W et al (2017) Genomic and transcriptomic insights into Calcium Carbonate biomineralization by marine Actinobacterium Brevibacterium linens BS258. Front Microbiol 8:602. https://doi.org/10.3389/fmicb.2017.00602
Zhu X, Mignon A, Nielsen SD et al (2021) Viability determination of Bacillus sphaericus after encapsulation in hydrogel for self-healing concrete via microcalorimetry and in situ oxygen concentration measurements. Cem Concr Compos 119:104006. https://doi.org/10.1016/j.cemconcomp.2021.104006
Zhuang D, Yan H, Tucker ME et al (2018) Calcite precipitation induced by Bacillus cereus MRR2 cultured at different Ca2+ concentrations: further insights into biotic and abiotic calcite. Chem Geol 500:64–87. https://doi.org/10.1016/j.chemgeo.2018.09.018
Funding
The European Commission has funded this work under the Horizon 2020 Marie Skłodowska-Curie Individual Fellowships, project NOBILIS, Grant Number 101025184 (Grant holder: London South Bank University). Additional support for the research has been received from Network Rail, UK, which partner with project NOBILIS.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception. Maria Mavroulidou and Michael J. Gunn are the LSBU supervisors of Dr Mwandira, a Fellow of MSCA-IF NOBILIS. Diane Purchase, Hemda Garelick and Jonathan Garelick are the secondment supervisors/mentors of Dr Mwandira from Middlesex University and Network Rail, UK, respectively, partnering with project NOBILIS. Wilson Mwandira performed literature collection and analysis. Wilson Mwandira wrote the first draft of the manuscript, and Maria Mavroulidou produced the second draft. All authors commented on the latter version of the manuscript, suggested amendments and approved the submission of the final manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mwandira, W., Mavroulidou, M., Gunn, M.J. et al. Concurrent Carbon Capture and Biocementation through the Carbonic Anhydrase (CA) Activity of Microorganisms -a Review and Outlook. Environ. Process. 10, 56 (2023). https://doi.org/10.1007/s40710-023-00667-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40710-023-00667-2