Abstract
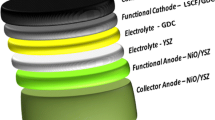
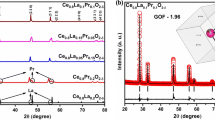
Solid oxide fuel cells (SOFCs) have attracted significant attention as a highly efficient type of fuel cell. Recent research proposes the use of co-doped scandium-stabilized zirconia with Gd and Ce (denoted as 10Sc0.5Gd0.5CeSZ) and Yb and Bi co-doped gadolinium-doped ceria (denoted as GYBC) as promising materials for the electrolyte and buffer layers, respectively. 10Sc0.5Gd0.5CeSZ exhibits excellent structural stability and ionic conductivity, which can be attributed to the doping of Ce for enhanced stability and Gd for improved ionic conductivity. On the other hand, GYBC demonstrates good sinterability and ionic conductivity due to the ability of Bi to lower the sintering temperature and the high ionic conductivity of Yb. To evaluate the feasibility of 10Sc0.5Gd0.5CeSZ and GYBC at the single cell level. X-ray diffraction (XRD) peaks and Rietveld refinements show good structural stability with slight increase in the lattice parameter by doping. The particle morphologies, size distributions, and BET surface areas are evaluated for the basic material characterizations. Then, lanthanum strontium cobalt ferrite (LSCF)–gadolinium-doped ceria (GDC) was selected as cathode material with 10Sc0.5Gd0.5CeSZ and GYBC. Finally, a single cell composed of Ni-Yttria stabilized zirconia (YSZ)/10Sc0.5Gd0.5CeSZ/GYBC/LSCF-GDC (6.5:3.5) is fabricated by sequential 3-layer co-tape casting technique, and it shows good open circuit voltage of > 1.0 V, high electrochemical performance of 0.73 W/cm2 and low ohmic resistance of 0.17 Ωcm2 at 750 °C. Then, the electrochemical characteristics and long-term durability of this single cell are evaluated over 500 h without degradation issues. Based on these results, it is concluded that 10Sc0.5Gd0.5CeSZ and GYBC are promising candidate materials for SOFCs.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdalla, A. M., Hossain, S., Azad, A. T., Petra, P. M. I., Begum, F., Eriksson, S. G., & Azad, A. K. (2018). Nanomaterials for solid oxide fuel cells: A review. Renewable and Sustainable Energy Reviews, 82, 353–368. https://doi.org/10.1016/j.rser.2017.09.046
Jaiswal, N., Tanwar, K., Suman, R., Kumar, D., Upadhyay, S., & Parkash, O. (2019). A brief review on ceria based solid electrolytes for solid oxide fuel cells. Journal of Alloys and Compounds, 781, 984–1005. https://doi.org/10.1016/j.jallcom.2018.12.015
Lee, K., Jin, S., Kang, J., Lee, S., & Bae, J. (2015). Development of metal supported solid oxide fuel cell based on interconnect coating. ECS Transactions, 68, 1721. https://doi.org/10.1149/06801.1721ecst
Shin, J. W., Lee, S., Go, D., Yang, B. C., Kim, T., Jo, S. E., Su, P. C., & An, J. (2023). Nanometer Yttria-doped ceria shell by atomic layer deposition over porous pt for improved oxygen reduction reactions. International Journal of Precision Engineering and Manufacturing—Green Technology, 10, 773–781. https://doi.org/10.1007/s40684-023-00506-7
Jo, M., Kim, S., & Lee, C. (2022). Morphology engineering for compact electrolyte layer of solid oxide fuel cell with roll-to-roll eco-production. International Journal of Precision Engineering and Manufacturing—Green Technology, 9, 431–441. https://doi.org/10.1007/s40684-022-00425-z
Ji, S. H., & Kim, W. J. (2022). Is coating oxide on porous metal thin-film for low-temperature solid oxide fuel cell cathode a panacea for performance enhancement? International Journal of Precision Engineering and Manufacturing, 23, 445–451. https://doi.org/10.1007/s12541-022-00628-z
Hussain, S., & Yangping, L. (2020). Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transitions, 4, 113–126. https://doi.org/10.1007/s41825-020-00029-8
Dwivedi, S. (2020). Solid oxide fuel cell: Materials for anode, cathode and electrolyte. International Journal of Hydrogen Energy, 45, 23988–24013. https://doi.org/10.1016/j.ijhydene.2019.11.234
Sharaf, O. Z., & Orhan, M. F. (2014). An overview of fuel cell technology: Fundamentals and applications. Renewable and Sustainable Energy Reviews, 32, 810–853. https://doi.org/10.1016/j.rser.2014.01.012
Jo, S., Sharma, B., Park, D.-H., & Myung, J.-H. (2020). Materials and nano-structural processes for use in solid oxide fuel cells: A review. Journal of the Korean Ceramic Society, 57, 135–151. https://doi.org/10.1007/s43207-020-00022-3
Ji, S., Kim, W., Han, S., Jeong, S., & Park, T. (2023). Stability enhancement of reformate-fueled, low-temperature solid oxide fuel cell with nickel thin-film anode by water bubbling. International Journal of Precision Engineering and Manufacturing— Green Technology, 10, 999–1006. https://doi.org/10.1007/s40684-022-00484-2
Lee, S., Jang, Y.-H., Shin, H. Y., Lee, K., Bae, M., Kang, J., & Bae, J. (2019). Reliable sealing design of metal-based solid oxide fuel cell stacks for transportation applications. International Journal of Hydrogen Energy, 44, 30280–30292. https://doi.org/10.1016/j.ijhydene.2019.09.087
Jang, Y.-H., Lee, S., Shin, H. Y., & Bae, J. (2018). Development and evaluation of a 3-cell stack of metal-based solid oxide fuel cells fabricated via a sinter-joining method for auxiliary power unit applications. International Journal of Hydrogen Energy, 43, 16215–16229. https://doi.org/10.1016/j.ijhydene.2018.06.141
Azizi, M. A., & Brouwer, J. (2018). Progress in solid oxide fuel cell-gas turbine hybrid power systems: System design and analysis, transient operation, controls and optimization. Applied energy, 215, 237–289. https://doi.org/10.1016/j.apenergy.2018.01.098
Lee, K., Yoon, B., Kang, J., Lee, S., & Bae, J. (2017). Evaluation of Ag-doped (MnCo)3O4 spinel as a solid oxide fuel cell metallic interconnect coating material. International Journal of Hydrogen Energy, 42, 29511–29517. https://doi.org/10.1016/j.ijhydene.2017.10.017
Simwonis, D., Tietz, F., & Stöver, D. (2000). Nickel coarsening in annealed Ni/8YSZ anode substrates for solid oxide fuel cells. Solid State Ionics, 132, 241–251. https://doi.org/10.1016/S0167-2738(00)00650-0
Fan, L., Zhu, B., Su, P.-C., & He, C. (2018). Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy, 45, 148–176. https://doi.org/10.1016/j.nanoen.2017.12.044
Yoon, B. Y., & Bae, J. (2013). Characteristics of nano La0. 6Sr0. 4Co0. 2Fe0. 8O3− δ-infiltrated La0. 8Sr0. 2Ga0. 8Mg0. 2O3− δ scaffold cathode for enhanced oxygen reduction. International Journal of Hydrogen Energy, 38, 13399–13407. https://doi.org/10.1016/j.ijhydene.2013.07.087
Yoon, B. Y., Kim, J. H., & Bae, J. (2013). Effects of infiltrated Sr and Mn doped LaCrO3 on porous La0. 8Sr0. 2Ga0. 8Mg0. 2O3-δ scaffolds used as anodes in solid oxide fuel cells. Solid State Ionics, 249, 26–33. https://doi.org/10.1016/j.ssi.2013.07.007
Kim, J., Sengodan, S., Kim, S., Kwon, O., Bu, Y., & Kim, G. (2019). Proton conducting oxides: A review of materials and applications for renewable energy conversion and storage. Renewable and Sustainable Energy Reviews, 109, 606–618. https://doi.org/10.1016/j.rser.2019.04.042
Zakaria, Z., & Kamarudin, S. K. (2021). Advanced modification of Scandia-stabilized zirconia electrolytes for solid oxide fuel cells application—a review. International Journal of Energy Research, 45, 4871–4887. https://doi.org/10.1002/er.6206
Zhigachev, A. O., Rodaev, V. V., Zhigacheva, D. V., Lyskov, N. V., & Shchukina, M. A. (2021). Doping of scandia-stabilized zirconia electrolytes for intermediate-temperature solid oxide fuel cell: A review. Ceramics International, 47, 32490–32504. https://doi.org/10.1016/j.ceramint.2021.08.285
Mahmood, A., Bano, S., Yu, J. H., & Lee, K.-H. (2015). High-performance solid oxide electrolysis cell based on ScSZ/GDC (scandia-stabilized zirconia/gadolinium-doped ceria) bi-layered electrolyte and LSCF (lanthanum strontium cobalt ferrite) oxygen electrode. Energy, 90, 344–350. https://doi.org/10.1016/j.energy.2015.06.109
Bai, B., McPhee, W. A., Smirnova, A. L., & Sammes, N. M. (2007). A comparison and characterization of CeO2-doped and Bi2O3-doped scandia stabilized zirconia as IT-SOFC electrolytes. ECS Transactions, 7, 2213. https://doi.org/10.1149/1.2729337
Lakshmi, V. V., & Bauri, R. (2011). Phase formation and ionic conductivity studies on ytterbia co-doped scandia stabilized zirconia (0.9 ZrO2–0.09 Sc2O3–0.01 Yb2O3) electrolyte for SOFCs. Solid state sciences, 13, 1520–1525. https://doi.org/10.1016/j.solidstatesciences.2011.05.014
Shin, H. C., Yu, J. H., Lim, K. T., Lee, H. L., & Baik, K. H. (2016). Effects of Partial Substitution of CeO 2 with M 2 O 3 (M= Yb, Gd, Sm) on Electrical Degradation of Sc 2 O 3 and CeO 2 Co-doped ZrO 2. Journal of the Korean Ceramic Society, 53, 500–505. https://doi.org/10.4191/kcers.2016.53.5.500
Arachi, Y., Asai, T., Yamamoto, O., Takeda, Y., Imanishi, N., Kawate, K., & Tamakoshi, C. (2001). Electrical conductivity of ZrO2 Sc2 O 3 doped with HfO2, CeO2, and Ga2 O 3. Journal of the Electrochemical Society, 148, A520. https://doi.org/10.1149/1.1366622
Wang, Z., Cheng, M., Bi, Z., Dong, Y., Zhang, H., Zhang, J., Feng, Z., & Li, C. (2005). Structure and impedance of ZrO2 doped with Sc2O3 and CeO2. Materials Letters, 59, 2579–2582. https://doi.org/10.1016/j.matlet.2004.07.065
Shimazu, M., Isobe, T., Ando, S., Hiwatashi, K.-I., Ueno, A., Yamaji, K., Kishimoto, H., Yokokawa, H., Nakajima, A., & Okada, K. (2011). Stability of Sc2O3 and CeO2 co-doped ZrO2 electrolyte during the operation of solid oxide fuel cells. Solid State Ionics, 182, 120–126. https://doi.org/10.1016/j.ssi.2010.08.030
Zhigachev, A. O., Zhigacheva, D. V., & Lyskov, N. V. (2019). Influence of yttria and ytterbia doping on phase stability and ionic conductivity of ScSZ solid electrolytes. Materials Research Express, 6, 105534. https://doi.org/10.1088/2053-1591/ab3ed0
Haering, C., Roosen, A., Schichl, H., & Schnöller, M. (2005). Degradation of the electrical conductivity in stabilised zirconia system: Part II: Scandia-stabilised zirconia. Solid State Ionics, 176, 261–268. https://doi.org/10.1016/j.ssi.2004.07.039
Yamaji, K., Kishimoto, H., Brito, M. E., Horita, T., Yokokawa, H., Shimazu, M., Yashiro, K., Kawada, T., & Mizusaki, J. (2013). Effect of Mn-doping on stability of Scandia stabilized zirconia electrolyte under dual atmosphere of solid oxide fuel cells. Solid State Ionics, 247, 102–107. https://doi.org/10.1016/j.ssi.2013.05.019
Lim, K. T., Lee, H. L., Shin, H. C., Lee, C. H., & Kim, B. S. (2017). Highly ionic conductive zirconia electrolyte for high-efficiency solid oxide fuel cell. US patent, 9(847), 545.
Lee, S., Lee, K., Jang, Y.-H., & Bae, J. (2017). Fabrication of solid oxide fuel cells (SOFCs) by solvent-controlled co-tape casting technique. International Journal of Hydrogen Energy, 42, 1648–1660. https://doi.org/10.1016/j.ijhydene.2016.07.066
Kang, S., Lee, J., Cho, G. Y., Kim, Y., Lee, S., Cha, S. W., & Bae, J. (2020). Scalable fabrication process of thin-film solid oxide fuel cells with an anode functional layer design and a sputtered electrolyte. International Journal of Hydrogen Energy, 45, 33980–33992. https://doi.org/10.1016/j.ijhydene.2020.09.033
Lee, K., Kang, J., Lee, J., Lee, S., & Bae, J. (2018). Evaluation of metal-supported solid oxide fuel cells (MS-SOFCs) fabricated at low temperature (∼ 1000 °C) using wet chemical coating processes and a catalyst wet impregnation method. International Journal of Hydrogen Energy, 43, 3786–3796. https://doi.org/10.1016/j.ijhydene.2018.01.027
Lim, Y. H., Lee, J., Yoon, J. S., Kim, C. E., & Hwang, H. J. (2007). Electrochemical performance of Ba0. 5Sr0. 5CoxFe1−xO3−δ (x= 0.2–0.8) cathode on a ScSZ electrolyte for intermediate temperature SOFCs. Journal of power sources, 171, 79–85. https://doi.org/10.1016/j.jpowsour.2007.05.050
Loureiro, F. J., Yang, T., Stroppa, D. G., & Fagg, D. P. (2015). Pr 2 O 2 SO 4–La 0.6 Sr 0.4 Co 0.2 Fe 0.8 O 3−δ: A new category of composite cathode for intermediate temperature-solid oxide fuel cells. Journal of Materials Chemistry A, 3, 12636–12641. https://doi.org/10.1039/C4TA06640E
Kim, W.-H., Song, H.-S., Moon, J., & Lee, H.-W. (2006). Intermediate temperature solid oxide fuel cell using (La, Sr)(Co, Fe) O3-based cathodes. Solid State Ionics, 177, 3211–3216. https://doi.org/10.1016/j.ssi.2006.07.049
Hsieh, W.-S., Lin, P., & Wang, S.-F. (2014). Characteristics of electrolyte supported micro-tubular solid oxide fuel cells with GDC-ScSZ bilayer electrolyte. International Journal of Hydrogen Energy, 39, 17267–17274. https://doi.org/10.1016/j.ijhydene.2014.08.060
Rehman, S. U., Shaur, A., Kim, H. S., Joh, D. W., Song, R. H., Lim, T. H., Hong, J. E., Park, S. J., & Lee, S. B. (2021). Effect of transition metal doping on the sintering and electrochemical properties of GDC buffer layer in SOFCs. International Journal of Applied Ceramic Technology, 18, 511–524. https://doi.org/10.1111/ijac.13650
Accardo, G., Frattini, D., Ham, H., Han, J., & Yoon, S. (2018). Improved microstructure and sintering temperature of bismuth nano-doped GDC powders synthesized by direct sol-gel combustion. Ceramics International, 44, 3800–3809. https://doi.org/10.1016/j.ceramint.2017.11.165
Gil, V., Tartaj, J., Moure, C., & Duran, P. (2007). Rapid densification by using Bi2O3 as an aid for sintering of gadolinia-doped ceria ceramics. Ceramics International, 33, 471–475. https://doi.org/10.1016/j.ceramint.2005.10.012
Lim, K. T., Lee, H. L., Shin, H. C., Lee, C. H., Kim, B. S., Choi, J. H., & Lee, S. J. (2020). Ceria electrolyte for low-temperature sintering and solid oxide fuel cell using the same. US patent, 10(581), 102.
Fujimori, H., Yashima, M., Kakihana, M., & Yoshimura, M. (1998). Structural changes of scandia-doped zirconia solid solutions: Rietveld analysis and Raman scattering. Journal of the American Ceramic Society, 81, 2885–2893. https://doi.org/10.1111/j.1151-2916.1998.tb02710.x
Li, J., Li, J.-G., Liu, S., Li, X., Sun, X., & Sakka, Y. (2013). The development of Ce3+-activated (Gd, Lu) 3Al5O12 garnet solid solutions as efficient yellow-emitting phosphors. Science and Technology of Advanced Materials. https://doi.org/10.1088/1468-6996/14/5/054201
Vinothkumar, G., Rengaraj, S., Arunkumar, P., Cha, S. W., & Suresh Babu, K. (2018). Ionic radii and concentration dependency of RE3+ (Eu3+, Nd3+, Pr3+, and La3+)-doped cerium oxide nanoparticles for enhanced multienzyme-mimetic and hydroxyl radical scavenging activity. The Journal of Physical Chemistry C, 123, 541–553. https://doi.org/10.1021/acs.jpcc.8b10108
Shannon, R. D. (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta crystallographica section A: Crystal physics, diffraction, theoretical and general crystallography, 32, 751–767. https://doi.org/10.1107/S0567739476001551
Shojaie, A. F., & Loghmani, M. H. (2010). La3+ and Zr4+ co-doped anatase nano TiO2 by sol-microwave method. Chemical Engineering Journal, 157, 263–269. https://doi.org/10.1016/j.cej.2009.12.025
Ahuja, A., Gautam, M., Sinha, A., Sharma, J., Patro, P., & Venkatasubramanian, A. (2020). Effect of processing route on the properties of LSCF-based composite cathode for IT-SOFC. Bulletin of Materials Science, 43, 1–9. https://doi.org/10.1007/s12034-020-2075-y
Raju, K., Kim, S., Yu, J. H., Kim, S.-H., Seong, Y.-H., & Han, I.-S. (2018). Rietveld refinement and estimation of residual stress in GDC–LSCF oxygen transport membrane ceramic composites. Ceramics International, 44, 10293–10298. https://doi.org/10.1016/j.ceramint.2018.03.036
Kim, Y.-M., Kim-Lohsoontorn, P., Baek, S.-W., & Bae, J. (2011). Electrochemical performance of unsintered Ba0.5Sr0.5Co0.8Fe0.2O3−δ, La0.6Sr0.4Co0.8Fe0.2O3−δ, and La0.8Sr0.2MnO3−δ cathodes for metal-supported solid oxide fuel cells. International Journal of Hydrogen Energy, 36, 3138–3146. https://doi.org/10.1016/j.ijhydene.2010.10.065
Zhang, J., Ji, Y., Gao, H., He, T., & Liu, J. (2005). Composite cathode La0.6Sr0.4Co0.2Fe0.8O3–Sm0.1Ce0.9O1.95–Ag for intermediate-temperature solid oxide fuel cells. Journal of Alloys and Compounds, 395, 322–325. https://doi.org/10.1016/j.jallcom.2004.11.056
Li, N., Verma, A., Singh, P., & Kim, J.-H. (2013). Characterization of La0.58Sr0.4Co0.2Fe0.8O3−δ–Ce0.8Gd0.2O2 composite cathode for intermediate temperature solid oxide fuel cells. Ceramics International, 39, 529–538. https://doi.org/10.1016/j.ceramint.2012.06.059
Khurana, S., Johnson, S., Karimaghaloo, A., & Lee, M. H. (2018). Effect of Sintering process with Co 3 O 4 on the performance of LSCF-based cathodes for solid oxide fuel cells. International Journal of Precision Engineering and Manufacturing-Green Technology, 5, 637–642. https://doi.org/10.1007/s40684-018-0066-x
Jiang, S. P. (2019). Development of lanthanum strontium cobalt ferrite perovskite electrodes of solid oxide fuel cells-A review. International Journal of Hydrogen Energy, 44, 7448–7493. https://doi.org/10.1016/j.ijhydene.2019.01.212
Abd Aziz, A. J., Baharuddin, N. A., Somalu, M. R., & Muchtar, A. (2020). Review of composite cathodes for intermediate-temperature solid oxide fuel cell applications. Ceramics International, 46, 23314–23325. https://doi.org/10.1016/j.ceramint.2020.06.176
Shearing, P., Bradley, R., Gelb, J., Tariq, F., Withers, P., & Brandon, N. (2012). Exploring microstructural changes associated with oxidation in Ni–YSZ SOFC electrodes using high resolution X-ray computed tomography. Solid State Ionics, 216, 69–72. https://doi.org/10.1016/j.ssi.2011.10.015
Sarikaya, A., Petrovsky, V., & Dogan, F. (2013). Development of the anode pore structure and its effects on the performance of solid oxide fuel cells. International Journal of Hydrogen Energy, 38, 10081–10091. https://doi.org/10.1016/j.ijhydene.2013.05.160
Wright, G. J., & Yeomans, J. A. (2008). Constrained sintering of yttria-stabilized zirconia electrolytes: The influence of two-step sintering profiles on microstructure and gas permeance. International Journal of Applied Ceramic Technology, 5, 589–596. https://doi.org/10.1111/j.1744-7402.2008.02263.x
Teocoli, F., Ni, D. W., Brodersen, K., Foghmoes, S. P. V., Ramousse, S., & Esposito, V. (2014). Effects of co-sintering in self-standing CGO/YSZ and CGO/ScYSZ dense bi-layers. Journal of Materials science, 49, 5324–5333. https://doi.org/10.1007/s10853-014-8235-y
Haanappel, V. A. C., Mai, A., & Mertens, J. (2006). Electrode activation of anode-supported SOFCs with LSM- or LSCF-type cathodes. Solid State Ionics, 177, 2033–2037. https://doi.org/10.1016/j.ssi.2005.12.038
Simner, S. P., Anderson, M. D., Pederson, L. R., & Stevenson, J. W. (2005). Performance variability of La (Sr) FeO3 SOFC cathode with Pt, Ag, and Au current collectors. Journal of the Electrochemical Society, 152, A1851. https://doi.org/10.1149/1.1995687
Shin, S. M., Yoon, B. Y., Kim, J. H., & Bae, J. M. (2013). Performance improvement by metal deposition at the cathode active site in solid oxide fuel cells. International Journal of Hydrogen Energy, 38, 8954–8964. https://doi.org/10.1016/j.ijhydene.2013.04.115
Song, J.-H., Jung, M. G., Park, H. W., & Lim, H.-T. (2013). The effect of fabrication conditions for GDC buffer layer on electrochemical performance of solid oxide fuel cells. Nano-Micro Letters, 5, 151–158. https://doi.org/10.1007/BF03353744
Khan, M. Z., Mehran, M. T., Song, R.-H., Lee, J.-W., Lee, S.-B., Lim, T.-H., & Park, S.-J. (2016). Effect of GDC interlayer thickness on durability of solid oxide fuel cell cathode. Ceramics International, 42, 6978–6984. https://doi.org/10.1016/j.ceramint.2016.01.085
Acknowledgements
This Research was funded and conducted under 「the Competency Development Program for Industry Specialists of Korean Ministry of Trade, Industry and Energy (MOTIE), operated by Korea Institute for Advancement of Technology(KIAT). (No. P0017120, HRD program for Foster R&D specialist of parts for eco-friendly vehicle(xEV)). This research was supported by National R&D Program through the National Research Foundation of Korea(NRF) funded by Ministry of Science and ICT(2021M3H4A3A02086497)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S., Lee, K., Lee, J. et al. Evaluation of Electrolyte Materials of Gd- and Ce-Doped Scandia-Stabilized Zirconia and Yb- and Bi-Doped Gadolinium-Doped Ceria for Highly Durable Solid Oxide Fuel Cells. Int. J. of Precis. Eng. and Manuf.-Green Tech. (2023). https://doi.org/10.1007/s40684-023-00577-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40684-023-00577-6