Abstract
Purpose of Review
The increasing demand for sleep diagnostic studies represents a challenge for many healthcare systems. Home polysomnography (hPSG), either set up by a technician or self-applied by the patient, provides comprehensive sleep signals and has the potential to replace in-lab sleep studies in a large number of cases.The aim of this study is to assess the existing evidence regarding the technical feasibility of hPSG in a systematic review. A systematic literature search was conducted in MEDLINE, PubMed, and Google Scholar to identify relevant research. Using a priori-defined inclusion criteria, studies were reviewed by three researchers, and a quality assessment was conducted. Relevant data were extracted, and the pooled failure rate with hPSG was computed. Additional subgroup analyses were conducted to further assess factors influencing technical feasibility.
Recent Findings
Thirty studies totaling 14,465 patients were included (mean sample size 482 ± 1289 participants). Common deployment models for hPSG were at-home application by a technician (58%) and technician-led in-hospital set-up (31%), followed by at-home self-application by the patient (11%). Technical failure rate across the studies ranged from 0 to 23.4%, with a pooled failure rate of 7.8% (95% CI 5.5–10.1%). Depending on deployment models, failure rates varied slightly. Failures of hPSG were largely related to signal acquisition. No studies reported adverse events from hPSG. Patient preferences were assessed by eleven studies, with 56% (range 22–95%) preferring hPSG over in-lab recording.
Summary
Based on the research identified for this review, home PSG is safe and technically feasible with relatively low failure rates. Further research is required to better understand decision-making with this tool in comparison to other sleep diagnostic procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep disorders have become a significant public health concern, affecting millions of individuals across the world [1,2,3]. Overnight sleep testing is commonly required to determine the type and severity of sleep disorders, and testing modalities are commonly categorized into four levels, according to the range of signals acquired by the measurement device [4] (Table 1). Based on this classification, a polysomnography (PSG) can be recorded in the sleep laboratory (level I test), which provides most diagnostic information with at least twelve channels, or unattended at home or in the laboratory with at least seven channels (level II test). For both test modalities, direct measures of sleep in the form of electroencephalography (EEG), electrooculography (EOG), and electromyography (EMG) are required, and can thus be used to diagnose a broad range of sleep disorders. Level III (home sleep apnea test or polygraphy) and level IV tests (screening), record cardiorespiratory signals and are indicated for identification and diagnosing sleep-related breathing disorders such as obstructive sleep apnea (OSA). In-laboratory PSG is considered the gold standard diagnostic tool for assessing sleep disorders, which allows to diagnose the entire spectrum of sleep conditions across all age groups [5, 6]. However, the limitations of in-lab PSG, including high costs, limited accessibility, and potential disruption of natural sleep patterns, drove the development and adoption of home-based tests as an alternative diagnostic approach. Recently, level II testing with unattended polysomnography at the patients’ home is increasingly used to overcome barriers present with in-lab PSG.
Home polysomnography (hPSG) involves the use of portable monitoring devices that enable the acquisition of physiological data during sleep in the patient’s own home environment [7]. Over the past decade, technological advancements have led to the miniaturization of sensors and the development of user-friendly devices, allowing for the unobtrusive measurement of key physiological parameters such as electroencephalography (EEG), electrooculography (EOG), electromyography (EMG), electrocardiography (ECG), and respiratory airflow [8•]. These advances have sparked growing interest in utilizing hPSG as a practical and accurate method for diagnosing a broad range of sleep disorders, including obstructive sleep apnea, insomnia, periodic limb movement disorder, and narcolepsy. Until recently, hPSG systems had to be set up by trained technicians either in the hospital or at the patient’s home, which implies certain logistical challenges and limited scalability of this method. With new technological developments and further miniaturization of measurement devices, self-appliable hPSG systems are now available which could increase utilization of this method for diagnostics of a broad range of sleep disorders at scale.
Though this technology has the potential to improve diagnostic pathways for patients with suspected sleep disorders, several questions have not been addressed so far, which include technical feasibility, patient preferences, and logistical challenges. This systematic review seeks to address these questions to support the assessment of this tool and the potential utility and disutility associated with extending its use to broader populations. A better understanding of the benefits and limitations of hPSG may inform clinicians, researchers, and healthcare policymakers about its potential future role in the provision of sleep diagnostic services.
Methods
This systematic review was conducted to identify studies assessing the technical success of hPSG for the diagnosis of sleep disorders in adults. The research followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to ensure comprehensive and transparent reporting of the review process and results [9].
Research Identification, Data Extraction, and Quality Assessment
A comprehensive literature search was performed in electronic databases including PubMed, MEDLINE, and Google Scholar from inception to August 2023, using a combination of relevant keywords and MeSH terms related to the technical feasibility of hPSG. Development of the search strategy and execution of the research was conducted with the assistance of a trained medical librarian. A detailed description of the search strategy and used keywords and MeSH terms is provided in the online supplement (Table S1).
Three independent reviewers screened the retrieved articles based on titles and abstracts for relevance to the research question. Studies that focused on technical aspects of hPSG, including the quality of recorded signals, data acquisition success rates, patient preferences, and equipment usability were eligible for assessment. The inclusion criteria for the quantitative assessment were defined as follows:
-
Research published up to August 2023 that reported original data on hPSG, acquired with standard technology
-
English language
-
Adult populations (age > 18 years)
Reviews, original articles reporting on pediatric use of hPSG, editorials, case reports, and studies not reporting relevant outcomes were excluded. Furthermore, all studies that used level III, level IV, or other types of measurement devices, that do not record direct measures of sleep as well as experimental technologies were excluded during screening at abstract level. A thorough eligibility check at full-text level was conducted to ensure that no overlapping data was included. Data from eligible studies were extracted by two reviewers using a standardized data extraction form. The extracted data included study characteristics (author, publication year, study design), participant information, sample size, type of equipment used, technical success metrics, and other relevant outcomes.
The methodological quality and risk of bias for each included study were assessed using the quality assessment tool for observational cohort and cross-sectional studies from the National Heart, Blood and Lung Institute (NHBLI) [10]. Discrepancies in quality assessment were resolved through discussion between the researchers until a consensus was reached.
Data Analysis
The technical success rate of hPSG was extracted from eligible research, and a pooled failure rate was analyzed using a random-effects nonlinear model to account for variation between studies. Heterogeneity was assessed by applying the I2 statistic, with values above 50% indicating substantial heterogeneity. Subgroup analyses were conducted based on study characteristics and study patient demographics. Statistical analyses were performed using SPSS (SPSS Statistics 29.0.1, IBM New York, USA) and Review Manager software (RevMan 5.4, The Cochrane Collaboration, 2020). A p-value of < 0.05 was considered statistically significant.
Results
Research Identified From Literature Search
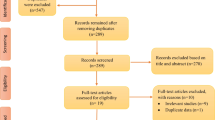
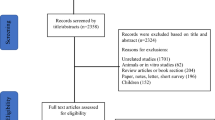
From a total of 399 studies identified through the initial literature search, 30 articles were considered eligible for inclusion in this systematic review based on their relevance to the research question and inclusion criteria (Table 2). The process of research selection is illustrated in the PRISMA flow diagram (Fig. 1).
Articles identified were published between 1998 and 2022, with increasing numbers more recently, and the majority from researchers in North America (40%) and Europe (33%). The remaining were conducted in East Asia/Pacific (20%), Latin America (3%), and the Middle East (3%). The total sample sizes amount to 14,465 participants, with individual studies ranging from 10 to 6697 subjects (mean 482 ± 1289 participants).
Data quality of eligible research, assessed with the NHBLI quality assessment tool for observational cohort and cross-sectional studies, was acceptable for all articles and none had to be excluded (Supplement Table S2).
Utilization of hPSG for Diagnosis of Sleep Disorders
The location for the set-up of hPSG systems was either the patient’s home (72%) or the hospital (28%), and the set-up was most often conducted by a trained technician (82%). Self-application by patients was used in 18% and emerged only in more recent studies, with the first article on self-applied hPSG published in 2017. The most common deployment model for hPSG was at-home application by a technician (58%), followed by technician in-hospital set-up (31%) and at-home self-application by the patient (11%). Across the studies, a wide range of PSG systems were utilized, which reflects the variety of devices available in the market. The devices used most often were the Nox A1 (Nox Medical, Reykjavik, Iceland) in 13%, and the Embletta X100 (Natus Medical, Pleasant View, USA) in 10% of studies, followed by Medatec Pamela V, Mallinkrodt Minisomno and Compumedics Safiro/S-series (7% each).
Per protocol, the majority of studies recorded one night (60.0%), followed by two nights (6.7%), three nights (3.3%), or a success-dependent approach with up to two nights (23.3%), up to three nights (3.3%) and up to five nights (3.3%). Thirty-three percent of studies (n = 10) allowed repetition of recording in case of failures, which was reported in 4.5% of studies on average (range 0–10%).
In the studies identified for this systematic review, hPSG was used to diagnose a broad range of sleep disorders, ranging from sleep-disordered breathing to general sleep complaints, insomnia, and bruxism. The populations enrolled represented a relatively typical population presenting with sleep conditions. Patients among all studies were on average 51 ± 8 years of age, predominantly male (57 ± 27%), and moderately overweight with a body mass index of 31 ± 5 kg/m2.
Technical Feasibility of hPSG
The pooled technical failure rate of home polysomnography across the eligible studies was estimated to be 7.8% (95% CI 5.5–10.1%), ranging from 0 to 23.4% (Fig. 2). Considerable heterogeneity was observed among the included studies (I2 = 97%), indicating high potential variability in technical success rates. Further analyses revealed no statistically significant correlations of hPSG study success rates with the variables age (r = 0.074, p = 0.713), body mass index (r = − 0.044, p = 0.848), male gender (r = 0.292, p = 0.157) and sample size (r = − 0.090, p = 0.635). For the three deployment models the following pooled failure rates were estimated: at-home application by technician = 5.8% (95% CI 3.7–7.9%); in-hospital application by technician = 10.0% (95% CI 3.1–16.8%) and at-home application by patient = 11.1% (95% CI 4.7–17.5%). No statistically significant correlation between the number of nights recorded and the reported technical failure rate was found (r (28) = 0.133; p = 0.483).
No adverse effects or complications from hPSG were reported by any of the studies included in this review.
Subgroup analyses were conducted for set-up location (home vs. hospital application of hPSG system) and for set-up person (technician vs. patient application of hPSG system). A difference in the technical failure rate was detected between home and hospital set-up (7.1 vs. 9.9%, Fig. 3), which was not statistically significant though (p = 0.171). A non-significant difference in technical failures was found between technician- and patient-applied hPSG (7.2 vs. 10.1%, p = 0.896, Fig. 4).
Reasons for Technical Failure
Since only a few studies used common criteria to determine the outcomes of hPSG recording, failure reasons were extracted by estimating the proportion of studies that mention the respective failure mode. Using this methodology, four major sources of hPSG failure could be identified: EEG, SpO2, airflow, and respiratory belts (Fig. 5). Twenty percent of studies did not report failure reason of home sleep studies. Differences in the occurrence and distribution of failure modes across deployment models of hPSG could not be identified.
Patient Preferences for hPSG
Though not a primary outcome in any of the studies included in this review, preferences of participants towards PSG diagnostics were assessed by 11 of 30 articles resulting in a total sample of 874 patients. The mean proportion of study participants preferring hPSG over in-lab PSG in those cohorts was 56 ± 22%, ranging from 28 to 95%. Further differentiation of preferences by subgroups could not be conducted due to limitations in the quality and quantity of available data.
Discussion
Current diagnostic approaches in sleep medicine are either limited in scalability, as in the case of in-lab PSG due to its high costs and resource intensity or offer limited diagnostic information, like with HSAT or consumer sleep trackers, which do not include direct measures of sleep such as EEG, EOG, and EMG signals. With recent developments in sleep research and their potential for improved and individualized approaches to sleep disorders, there is a need for a scalable diagnostic tool that records the signals required for advanced analyses of sleep disorders, such as phenotypization or endotypization, and supports an accurate analysis of direct sleep measures. The aim of this research was to assess the existing evidence of the technical feasibility of hPSG as a method to acquire polysomnographic signal sets outside of the clinic.
While hPSG was first mentioned in the medical literature more than three decades ago and has been used largely in clinical studies, its application in routine sleep medical practice is limited in most healthcare systems, mainly due to logistical and reimbursement-related reasons. With the rising prevalence of sleep disorders and steadily increasing demand for sleep diagnostics, hPSG may offer advantages over in-lab polysomnography as well as over simple home sleep tests [8•]. In light of the current challenges of sleep clinics across the globe to recruit and retain trained technicians for overnight monitoring in the sleep laboratory, shifting PSG towards the home could help increase the capacity of sleep programs and thus ensure access to advanced sleep diagnostics. This is even more important for the increasing populations of patients with non-OSA sleep disorders for which HSAT is not an appropriate substitute of in-lab PSG. With recent advances in the diagnostic assessment of insomnia, for example, the relevance of PSG could eventually further increase, potentially widening access issues [40•].
The results of this systematic review demonstrate that utilization of PSG at home is technically feasible and safe with low failure rates in adult populations, independent of the deployment model and whether the PSG system is applied by a technician or self-applied by the patient. Though certain variabilities of technical failure rates were found, hPSG overall allows reliable and robust signal acquisition. These findings are supported by results from an earlier review by Bruyneel and Ninane [7], in which they demonstrated a high data quality, high diagnostic accuracy, and good agreement between hPSG and in-lab PSG in six randomized cross-over trials. Though data is limited on the technical success rates with HSAT, the available literature suggests comparable outcomes with the more simplistic level III or level IV tests. For peripheral arterial tonometry, a HSAT that is increasingly used, failure rates between 0 and 19% have been reported [41,42,43,44]. The preferences elucidated in some of the studies suggest that conducting PSG at home is not only technically reliable, but also well accepted by patients, especially when the set-up is done at home [28]. In addition, a sleep recording in the comfort of the own home could also lead to a more precise picture of the natural sleep and potentially a more accurate diagnosis [45].
It is important to highlight, that though hPSG may reduce the burden on the sleep clinic and its staff, it is not free of operational challenges that need to be considered. Currently, the most common deployment model requires a technician to drive to the patient’s home to set up the system and collect the device in the morning after the recording. This approach not only has a relevant logistical complexity, but it also increases costs and the ecological footprint of the sleep test. In addition, contrary to attended in-lab PSG, electrode detachments which can happen during sleep, cannot be easily corrected with hPSG. Telemonitored at-home PSG with real-time data transmission to a data center that observes signal recording and intervenes via phone or video call, could be an opportunity to reduce signal losses or incomplete recordings [15, 23]. Recent developments towards patch-based hPSG systems, conceptionally may help reduce signal losses and improve data quality by increasing electrode adhesion and reducing the use of wires to transmit signals [46,47,48]. Those concepts need to be assessed in clinical routine and are subject of ongoing trials.
Given the shortage of trained technicians to support PSG operations in the lab and at home, current developments in the field of self-appliable PSG systems present an interesting opportunity to reduce the burden of sleep clinic staff. Though not all patients needing a sleep study will be able to apply devices themselves, early data support this concept [49]. Further miniaturization of sensors and improvements of device usability could increase the number of eligible populations.
Using hPSG for diagnosing a wide array of sleep disorders outside of the hospital may have also relevant positive economic implications. By enabling patients to conduct PSG within their own homes, this tool has the potential to meaningfully reduce the financial burden associated with clinical-grade sleep diagnostics, which traditionally involve substantial costs related to facility usage, staffing of overnight shifts, and equipment maintenance. Increased utilization of hPSG could alleviate these costs, leading to decreased healthcare expenditures and, moreover, to increased accessibility of sleep diagnostics and thus earlier identification and intervention for sleep disorders [50, 51]. In healthcare systems with limited budgets, lower costs for sleep diagnostics may also allow the allocation of greater financial resources towards treatment, treatment monitoring, and chronic care of patients with sleep disorders, and thus leading to improved overall outcomes.
Limitations
A few limitations are important to mention to the reader to reflect the results of this analysis. First of all, though extensive efforts were undertaken to identify all literature, additional studies with information relevant to the research question could be missed. Given the scope of the literature search and the results of the analysis, the potential impact should be neglectable. Within the studies identified, a variable quality was found, and only a few applied a randomized controlled design, which influences the evidence level that could be generated from the analysis. Furthermore, the technical failure rate calculated as the primary outcome of this research is an aggregated point estimate, which is statistically not precise due to the considerable heterogeneity present in the underlying data.
To estimate the value of hPSG for the diagnosis of sleep disorders comprehensively, the technical success rate and the diagnostic accuracy only reflect the input side. It is essential to dive deeper into the decision-making process to understand how clinicians use the information provided from hPSG in comparison to those derived from in-lab PSG and if downstream treatment outcomes vary, depending on which diagnostic tool was used. The authors were not able to identify any published research on this topic, so this represents an opportunity for future research.
In addition, a relevant heterogeneity in reporting outcomes of hPSG and success criteria was observed across the studies. For example, in the absence of a common definition of technical failure or sleep study success, a variety of metrics was employed in the different studies to assess outcomes, which differ as well depending on the individual study objectives and the clinical context. As such, in studies of populations with sleep-disordered breathing, oximetry signals of less than 4 h might be considered a failure, while this would be of lower relevance in a study on patients suffering from insomnia. On the other hand, a failed EEG or EOG recording might not lead to a failed study in an OSA population, as long as other relevant metrics would allow to estimate respiratory or desaturation indices.
To ensure an accurate assessment, it would be beneficial to agree on a reporting guideline with core metrics that are applied and presented in any research on sleep diagnostic tools. This is particularly important to the outcomes of this study, since a few articles included reported a study as failure only when a recording could not be obtained in the second or third attempt, which can skew the results. Other areas of medicine have adopted this approach already, which supports thorough assessment of healthcare technologies by harmonizing outcome reporting.
Conclusion
With the expected increasing demand for sleep diagnostics and limited resources for in-lab polysomnography, driven by increased awareness for sleep and greater utility of polysomnography, hPSG has the potential to secure and improve access to clinical-grade sleep diagnostics. From the data included in this systematic review, it can be concluded that hPSG has a low rate of technical failures and is safe to use in different care settings. independent of set-up location or set-up person. The most common failure reasons are related to signal acquisition during the night, which could be improved with further optimization of sensor technology. Further research is required to understand the decision-making process of physicians when using this tool in comparison with in-lab polysomnography.
Data Availability
The data that support the findings of this study are available from the corresponding author, M.B., upon reasonable request.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–98.
Chattu VK, Manzar MdD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The global problem of insufficient sleep and its serious public health implications. Healthcare. 2018;7(1):1–16.
Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14(3):191–203.
Berry RB. Chapter 13 - Polysomnography, portable monitoring, and actigraphy. In: Berry RB, editor. Fundamentals of Sleep Medicine [Internet]. Saint Louis: W.B. Saunders; 2012. p. 189–218. Available from: https://www.sciencedirect.com/science/article/pii/B9781437703269000130 [cited 2023 Aug 9]
Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.2. Am Acad Sleep Med [Internet]. 2015; Available from: www.aasmnet.org
NCA - Sleep testing for obstructive sleep apnea (OSA) (CAG-00405N) - Decision Memo [Internet]. Available from: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=227&ver [cited 2022 Nov 17]
Bruyneel M, Ninane V. Unattended home-based polysomnography for sleep disordered breathing: current concepts and perspectives. Sleep Med Rev. 2014;18(4):341–7.
• Korkalainen H, Nikkonen S, Kainulainen S, Dwivedi AK, Myllymaa S, Leppänen T, et al. Self-applied home sleep recordings: the future of sleep medicine. Sleep Med Clin. 2021;16(4):545–56. Advances in analysis techniques and wearable monitoring technologies have the potential to ease access to sleep diagnostic testing by enabling large scale at-home application.
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews | The BMJ [Internet].. Available from: https://www.bmj.com/content/372/bmj.n71 [cited 2022 Oct 20]
Study Quality Assessment Tools | NHLBI, NIH [Internet]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [cited 2023 Aug 18]
Fry JM, DiPhillipo MA, Curran K, Goldberg R, Baran AS. Full polysomnography in the home. Sleep. 1998;21(6):635–42.
Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group Sleep. 1998;21(7):759–67.
Mykytyn IJ, Sajkov D, Neill AM, Mc Evoy RD. Portable computerized polysomnography in attended and unattended settings. Chest. 1999;115(1):114–22.
Portier F, Portmann A, Czernichow P, Vascaut L, Devin E, Benhamou D, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162(3 Pt 1):814–8.
Gagnadoux F, Pelletier-Fleury N, Philippe C, Rakotonanahary D, Fleury B. Home unattended vs hospital telemonitored polysomnography in suspected obstructive sleep apnea syndrome: a randomized crossover trial. Chest. 2002;121(3):753–8.
Iber C, Redline S, Gilpin AMK, Quan SF, Zhang L, Gottlieb DJ, et al. Polysomnography performed in the unattended home versus the attended laboratory setting—sleep heart health study methodology. Sleep. 2004;27(3):536–40.
BaHammam AS. Signal failure of type 2 comprehensive unattended sleep studies in patients with suspected respiratory sleep disordered breathing. Sleep Breath. 2005;9(1):7–11.
Kurth ME, Sharkey KM, Millman RP, Corso RP, Stein MD. Insomnia among methadone-maintained individuals: the feasibility of collecting home polysomnographic recordings. J Addict Dis. 2009;28(3):219–25.
Mehra R, Stone KL, Varosy PD, Hoffman AR, Marcus GM, Blackwell T, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS Sleep) study. Arch Intern Med. 2009;169(12):1147–55.
Bruyneel M, Sanida C, Art G, Libert W, Cuvelier L, Paesmans M, et al. Sleep efficiency during sleep studies: results of a prospective study comparing home-based and in-hospital polysomnography. J Sleep Res. 2011;20(1pt2):201–6.
Campbell AJ, Neill AM. Home set-up polysomnography in the assessment of suspected obstructive sleep apnea. J Sleep Res. 2011;20(1 Pt 2):207–13.
Chung F, Liao P, Sun Y, Amirshahi B, Fazel H, Shapiro CM, et al. Perioperative practical experiences in using a level 2 portable polysomnography. Sleep Breath. 2011;15(3):367–75.
Bruyneel M, Van den Broecke S, Libert W, Ninane V. Real-time attended home-polysomnography with telematic data transmission. Int J Med Inf. 2013;82(8):696–701.
Rohling L, Blankvoort C, Mattern-Coren E, De Weerd A. Medical technology assessment of polysomnography, type 2: Full PSG at home – Difference of two unattended PSG at home systems. Sleep Med. 2013;1(14):e245.
Banhiran W, Chotinaiwattarakul W, Chongkolwatana C, Metheetrairut C. Home-based diagnosis of obstructive sleep apnea by polysomnography type 2: accuracy, reliability, and feasibility. Sleep Breath. 2014;18(4):817–23.
Crescimanno G, Greco F, Marrone O. Monitoring noninvasive ventilation in neuromuscular patients: feasibility of unattended home polysomnography and reliability of sleep diaries. Sleep Med. 2014;15(3):336–41.
Knauert MP, Yaggi HK, Redeker NS, Murphy TE, Araujo KL, Pisani MA. Feasibility study of unattended polysomnography in medical intensive care unit patients. Heart Lung. 2014;43(5):445–52.
Bruyneel M, Libert W, Ameye L, Ninane V. Comparison between home and hospital set-up for unattended home-based polysomnography: a prospective randomized study. Sleep Med. 2015;16(11):1434–8.
Hall MH, Casement MD, Troxel WM, Matthews KA, Bromberger JT, Kravitz HM, et al. Chronic stress is prospectively associated with sleep in midlife women: the SWAN sleep study. Sleep. 2015;38(10):1645–54.
Lang Carol J, Appleton Sarah L, Andrew Vakulin, Doug McEvoy R, Vincent Andrew D, Wittert Gary A, et al. Associations of undiagnosed obstructive sleep apnea and excessive daytime sleepiness with depression: an Australian population study. J Clin Sleep Med. 2017;13(4):575–82.
Levendowski D, Dawson D, Levi M, Westbrook P. Agreement between auto-scored vs. edited unattended in-home polysomnography. Sleep Med. 2017;40:e189.
Younes M, Soiferman M, Thompson W, Giannouli E. Performance of a new portable wireless sleep monitor. J Clin Sleep Med. 2017;13(2):245–58.
Andrade L, Paiva T. Ambulatory versus laboratory polysomnography in obstructive sleep apnea: comparative assessment of quality, clinical efficacy, treatment compliance, and quality of life. J Clin Sleep Med. 2018;14(8):1323–31.
Miettinen T, Myllymaa K, Westeren-Punnonen S, Ahlberg J, Hukkanen T, Toyras J, et al. Success rate and technical quality of home polysomnography with self-applicable electrode set in subjects with possible sleep bruxism. IEEE J Biomed Health Inform. 2018;22(4):1124–32.
Yoon DW, Hong IH, Baik I, Shin HW. Evaluation of the feasibility and preference of Nox-A1 type 2 ambulatory device for unattended home sleep test: a randomized crossover study. Sleep Biol Rhythms. 2019;17(3):297–304.
Cuesta R, Roebuck T, Ho S, Naughton M, McDermott E, VanBraak E, et al. P028 The Nox A1 ambulatory system is reliable when self-applied. SLEEP Adv. 2021;1(2):A30.
•• Punjabi NM, Brown T, Aurora RN, Patel SR, Stosor V, Cho JHJ, et al. Methods for home-based self-applied polysomnography: the multicenter AIDS cohort study. SLEEP Adv. 2022;3(1):zpac011. Results from this research demonstrate the feasibility of unattended home-polysomnography in a large community cohort.
Tomson H, Bender A, Lambing K, Decock D. The clinical success of 213 self-applied type 2 sleep studies. Sleep Med. 2022;1(100):S304.
Zancanella E, do Prado LF, de Carvalho LB, Machado Júnior AJ, Crespo AN, do Prado GF. Home sleep apnea testing: an accuracy study. Sleep Breath. 2022;26(1):117–23.
• Andrillon T, Solelhac G, Bouchequet P, Romano F, Le Brun MP, Brigham M, et al. Revisiting the value of polysomnographic data in insomnia: more than meets the eye. Sleep Med. 2020;66:184–200. Advanced analysis of polysomnographic data using artificial intelligence can increase the understanding of the physiological substrate of insomnia.
Onder NS, Akpinar ME, Yigit O, Gor AP. Watch peripheral arterial tonometry in the diagnosis of obstructive sleep apnea: influence of aging. Laryngoscope. 2012;122(6):1409–14.
Penzel T, Kesper K, Pinnow I, Becker HF, Vogelmeier C. Peripheral arterial tonometry, oximetry and actigraphy for ambulatory recording of sleep apnea. Physiol Meas. 2004;25(4):1025–36.
Pittman SD, Ayas NT, MacDonald MM, Malhotra A, et al. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004;27(5):923–33.
Choi JH, Kim EJ, Kim YS, Choi J, Kim TH, et al. Validation study of portable device for the diagnosis of obstructive sleep apnea according to the new AASM scoring criteria: Watch-PAT 100. Acta Otolaryngol. 2010;130(7):838–43.
Ghegan MD, Angelos PC, Stonebraker AC, Gillespie MB. Laboratory versus portable sleep studies: a meta-analysis. Laryngoscope. 2006;116(6):859–64.
Pan Q, Brulin D, Campo E. Current status and future challenges of sleep monitoring systems: systematic review. JMIR Biomed Eng. 2020;5(1):e20921.
Raschellà F, Knoops-Borm M, Sekeri M, Andries D, Oloo M, Moudab I, et al. Clinical validation of a wireless patch-based polysomnography system: a pilot study [Internet]. medRxiv; 2022. p. 2022.08.04.22278354. https://doi.org/10.1101/2022.08.04.22278354v1
Oz S, Dagay A, Katzav S, Wasserman D, Tauman R, Gerston A, et al. Monitoring sleep stages with a soft electrode array: comparison against vPSG and home-based detection of REM sleep without atonia. J Sleep Res. 2023;32(5):e13909.
Schneider H, Haisma N, Mueller S, Oloo M, Tijssen M, Stockhoff M, et al. Patient-reported experience with a wireless patch-based polysomnography system – results from a pilot study. Sleep. 2023;1(46):A240–1.
Pelletier-Fleury N, Gagnadoux F, Philippe C, Rakotonanahary D, Lanoé JL, Fleury B. A cost-minimization study of telemedicine The case of telemonitored polysomnography to diagnose obstructive sleep apnea syndrome. Int J Technol Assess Health Care. 2001;17(4):604–11.
Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep. 2011;34(6):695–709.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.B., M.T. and M.S. conceived the original idea and planned study. M.B., M.T. and M.S. carried out the literature search and quantitative analysis. M.B. and S.C. wrote the manuscript, with support from M.T., M.S., S.D. and C.S.
Corresponding author
Ethics declarations
Conflict of Interest
M.B., M.S., M.T., and S.C. received personal fees from Onera Health (The Netherlands), a manufacturer of diagnostic equipment that can be used for diagnosis of sleep disorders. C.S. received no personal fees, but institutional fees for lectures, advisory tasks, and/or scientific projects from AstraZeneca, Bayer, Bioprojet, Bristol Myers Squibb, Idorsia, Inspire Medical, Jazz, Mementor, ResMed, Sleepiz, and Zoll (Australia). S.D. declares no conflict of interest related to the topic of the article.
Human and Animal Rights and Informed Consent
Ethics committee approval was not required for this research. No studies with human participants or animals were performed by any of the authors for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Braun, M., Stockhoff, M., Tijssen, M. et al. A Systematic Review on the Technical Feasibility of Home-Polysomnography for Diagnosis of Sleep Disorders in Adults. Curr Sleep Medicine Rep 10, 276–288 (2024). https://doi.org/10.1007/s40675-024-00301-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-024-00301-z