Abstract
Purpose of Review
Insomnia and sleep apnoea are the two most prevalent sleep disorders and frequently co-exist. Co-morbid insomnia and sleep apnoea (COMISA) is increasingly recognised as a highly prevalent condition that is associated with worse sleep, daytime function, physical and mental health compared to either disorder alone. Compared to people with sleep apnoea alone, those with COMISA are less likely to accept and use positive airway pressure therapy, the most effective treatment for sleep apnoea. Given the high prevalence, morbidity and complexities in effectively managing COMISA, it is critical to develop a better understanding of the aetiology, consequences and effective treatments for this condition. This report aims to provide an overview of recent COMISA research.
Recent Findings
This report presents an overview of emerging areas of COMISA research over the past 5 years, including (1) mental and physical health associations of COMISA, (2) bi-directional relationships between insomnia and sleep apnoea, (3) positive airway pressure therapy for COMISA and (4) cognitive behavioural therapy for COMISA. Future research directions are discussed, including tailored treatment approaches and implementation programs to improve recognition and management of COMISA.
Summary
COMISA is a highly prevalent and debilitating condition in sleep clinic and population-based settings. Emerging research aims to develop and implement more effective and tailored treatment approaches for COMISA, to improve sleep, mental health, physical health and quality of life in people with COMISA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insomnia and obstructive sleep apnoea (OSA) are the two most prevalent sleep disorders and frequently co-exist [1•]. Insomnia is characterised by self-reported difficulties initiating sleep and/or maintaining sleep, with associated daytime impairment, and is defined as a chronic condition when persisting for at least 3 months [2]. OSA is characterised by repetitive brief narrowing (hypopnoea) and/or closure (apnoea) of the upper airway during sleep, resulting in reduced oxygen saturation, cortical arousals, reduced sleep quality and daytime impairment [2]. Chronic insomnia and OSA each occur in approximately 5–20% of the general adult population, with prevalence estimates varying by severity threshold, assessment tools and sample characteristics [3, 4]. Both insomnia and OSA are associated with increased risk of physical and mental health problems, and incur high economic costs through healthcare utilisation, reduced quality of life and reduced workplace productivity [5,6,7,8].
Although the co-morbidity of insomnia and OSA was first described in 1973 [9•], there were very few studies focusing on this area for the next three decades. In 1999 and 2001, Lichstein [10•] and Krakow [11•] reported on the high prevalence of co-occurring insomnia and OSA and ignited an increase in research attention to this field. In 2017, we coined the term co-morbid insomnia and sleep apnoea (COMISA), with the aim of generating more research and clinical interest in this prevalent yet under-recognised condition [1•]. Over the following 6 years, the COMISA field gradually received an increasing amount of research and clinical attention. It has since become apparent that COMISA is a prevalent condition that is associated with significant mental and physical morbidity, and is more difficult to treat compared to either condition alone [12, 13]. Approximately 30–50% of patients with OSA report clinically significant insomnia symptoms, while 30–40% of patients with insomnia have co-morbid OSA [14•] (Fig. 1). The high co-morbidity of insomnia and OSA has been reported consistently in population-based samples, sleep clinics, hospital clinics and specialist insomnia treatment services [14•, 15, 16•].
Co-morbid insomnia and sleep apnoea (COMISA) is prevalent in 30–40% of patients with insomnia, and 30–50% of patients with obstructive sleep apnea (OSA). Figure adapted with permission from Sweetman et al. [14•]
This report aims to present an overview of recent and emerging research in the field of COMISA and highlight future research and translation opportunities.
Mental and Physical Health Associations of COMISA
COMISA is generally associated with worse overall mental health, physical health, daytime function, quality of life and sleep, compared to people with neither disorder, and often compared to those with insomnia alone and OSA alone [1•, 17].
Sleep and mental health share strong bi-directional relationships [18]. Disturbed sleep is a frequent symptom of depression, and people with sleep disorders are at increased risk of experiencing depression [19]. Given the associations of both insomnia and depression [5], and OSA and depression [6], it is unsurprising that COMISA is also associated with a large impact on depression presence and severity. For example, Lang and colleagues reported that among 700 community-dwelling males, those with COMISA had a significantly higher prevalence of depression (42.6%), compared to those with insomnia (21.6%), or OSA alone (8.4%) [20]. A recent review of 15 studies by Jeon and colleagues [21] found that COMISA was associated with elevated depression symptoms, with the insomnia component of the disorder playing the predominant role. The exact mechanisms linking COMISA and depression are not well understood. A simple additive effect of nocturnal and daytime symptoms of each disorder may contribute to a reduced quality of life, poor mood and depression. Alternatively, more complex interactions between the mechanisms and manifestations of the two disorders may play a unique role in reducing mental health in people with COMISA [14•]. Night-to-night variability in sleep (insomnia symptoms, OSA severity, perceptions of sleep quality) and daytime symptoms (sleepiness, fatigue, depression) have not been studied in the context of COMISA [22].
Three recent population-based studies have reported that COMISA is associated with an increased risk of all-cause mortality, compared to people with neither insomnia nor OSA [23, 24, 25•]. Lechat and colleagues [25•] used the Sleep Heart Health Study data to investigate the association of co-morbid insomnia (difficulties initiating and/or maintaining sleep and daytime impairment on at least 15 nights/month) and OSA (apnoea-hypopnoea index ≥ 15) and all-cause mortality in 5236 participants. After controlling for socio-demographics, behavioural factors, chronic conditions and potential mediators/moderators, COMISA was associated with a 47% increased risk of all-cause mortality compared to people with neither insomnia nor OSA over 15 years of follow-up. In the NHANES data (N = 6877) [23], self-reported insomnia symptoms and a high-risk of OSA (according to the STOP-Bang tool [26]) were similarly associated with a 56% increased risk of all-cause mortality compared to people with neither insomnia nor OSA in the fully adjusted model (Fig. 2). Results were robust across multiple sensitivity definitions of high-risk OSA. Finally, in the Wisconsin Sleep Cohort (N = 1115) Lechat and colleagues [24] reported that the combination of infrequent insomnia symptoms and at least mild OSA was associated with a 71% increased risk of mortality compared to neither insomnia nor sleep apnoea in adjusted models.
Unadjusted Kaplan-Meier’s curve showing association of sleep disorder group and mortality in the National Health and Nutrition Examination Survey data. Co-morbid insomnia and self-reported high-risk OSA (COMISA) are associated with an increased risk of all-cause mortality, compared to neither insomnia nor sleep apnoea. Figure copied from Sweetman et al. [23], via CC-BY licence.
The association between COMISA and a 50–70% increased risk of all-cause mortality may be mediated by several possible mental health, physical health and treatment access/acceptance pathways. For example, the association of COMISA and depression may contribute to increased overall mortality risk in those with COMISA [20, 21]. Potential associations between COMISA, depression, suicide risk and the impact of treatment/s on mitigating these risks require further research [27]. Physical health consequences of COMISA may also mediate the association with mortality. Lechat and colleagues [28] also recently reported that COMISA is uniquely associated with a 75% increased prevalence of cardiovascular disease (95% confidence interval = 1.14–2.67), in the Sleep Heart Health Study. We also observed an unadjusted association between COMISA and risk of incident cardiovascular events (hazard ratio: 2.00, 95% CI = 1.33–2.99; after excluding participants with prevalent cardiovascular disease at baseline) which was no longer significant in fully controlled models [28]. Previous research has investigated cross-sectional associations between COMISA and cardiovascular disease/health; however, more longitudinal studies investigating COMISA and incident cardiovascular outcomes are required [29,30,31,32]. Third, COMISA may also be associated with mortality risk due to the reduced access, acceptance and use of evidence-based treatments for OSA and insomnia, compared to treatment access/use in people with either disorder alone. Relationships between COMISA and treatment access and use are described in more detail below; however, there is a consistent body of literature reporting that patients with COMISA are less likely to accept and use positive airway pressure therapy, compared to patients with OSA alone [12]. Furthermore, insomnia is frequently managed with sedative and hypnotic medicines and sub-optimal ‘sleep hygiene’ advice, rather than the recommended treatment—cognitive behavioural therapy for insomnia (CBT-I) [33]. Hypnotics have been investigated as a potential treatment approach in specific sub-samples of patients with OSA, but are not appropriate for all patients [34]. For example, sedative-hypnotics may exacerbate sleep apnoea severity in individuals with severe hypoxemia and should be avoided due to depressant effects on respiratory control mechanics. Furthermore, hypnotics may be associated with next-day sedation and sleepiness, especially in older adults [35] or those with pre-existing daytime sleepiness. Among people with insomnia symptoms and undiagnosed OSA, sedative-hypnotics prescriptions for insomnia may exacerbate daytime sleepiness and increase risk of falls/fractures, or motor-vehicle accidents, exacerbating the daytime/health consequences of the condition. Indeed, hypnotics are not recommended for the management of insomnia in older adults or as a long-term management approach, as the therapeutic benefit is outweighed by patterns of dependence, abuse and adverse event risks [35]. Similarly, although CBT-I is an effective treatment for insomnia in the presence of OSA [36•], it is associated with a small increase in daytime sleepiness during the first 1–2 weeks of sleep restriction therapy [37]. There is at least one documented case of a sleepiness-related motor-vehicle accident during sleep restriction therapy in a patient with undiagnosed OSA [38]. This highlights the importance of identifying COMISA in those presenting with insomnia symptoms and close monitoring of daytime sleepiness/alertness during sleep restriction protocols in such patients.
The three recent population-based studies reporting a 50–70% increase in mortality risk among people with COMISA compared to those with neither condition is of obvious concern [23, 24, 25•]. These potential mental health, physical health and treatment access/use mediators between COMISA and mortality are entirely speculative. More research is needed to understand the specific features of COMISA that are associated with mortality risk, the factors that mediate this relationship, and the potential role of different interventions and treatment approaches in reducing this risk.
Bi-directional Relationships Between Insomnia and OSA
There is evidence that insomnia and OSA are bi-directionally related [14•]. Interactions between the mechanisms and manifestations of each condition may contribute to the development and exacerbation of the other disorder, resulting in greater morbidity and reduced response to treatments that are effective for the management of each disorder presenting alone. A hypothesis that is generally well accepted in the sleep medicine community is that OSA can directly contribute to insomnia symptoms. For example, respiratory events and post-apneic arousals may cause more frequent awakenings from sleep with increased sympathetic activity, and consequently, insomnia symptoms. These insomnia symptoms may be dependent on the presence of OSA, or compensatory (perpetuating) behaviours and cognitive pathways could emerge over time, which would result in the insomnia becoming a functionally independent co-morbid condition [39, 40]. This hypothesis is supported by cohort studies reporting that OSA may increase the risk of incident insomnia symptoms [41], and clinical trials reporting a moderate improvement in insomnia symptoms among some patients with COMISA following PAP therapy [14•, 42].
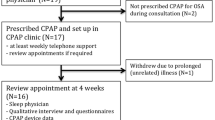
The reverse of this pathway has received comparatively less attention. It is also possible that insomnia symptoms contribute to the exacerbation of OSA through increased cognitive and physiological activity (also referred to as ‘hyperarousal’) delaying transition to stable sleep, and modifying the respiratory arousal threshold. This may exacerbate sleep apnea severity in those with a pre-existing anatomical pre-disposition to airway collapse [14•]. This hypothesis is supported by studies reporting that experimentally induced sleep deprivation/restriction may exacerbate some features of OSA [14•], and a randomised controlled trial reporting that CBT-I reduces OSA severity in 145 patients with co-morbid insomnia and moderate/severe untreated OSA (Fig. 3, also described below). Studies investigating longitudinal trajectories of COMISA development from neither condition, insomnia alone and sleep apnoea alone are needed [43].
Cognitive behavioural therapy for insomnia (CBT-I) is associated with a greater reduction in the apnoea-hypopnoea index (AHI) from pre-treatment to 6-week follow-up than no-treatment control. Figure adapted with permission from Sweetman et al. [14•]
Several emerging studies aim to investigate the respiratory arousal threshold in people with COMISA [44,45,46]. The respiratory arousal threshold is a non-anatomical trait that contributes to OSA pathophysiology in approximately one-third of patients with OSA. Premature arousal/awakening to relatively minor respiratory stimuli and termination of respiratory events may contribute to cyclic periods of respiratory instability, and overall exacerbation of sleep apnea severity [47]. We hypothesised that insomnia may reduce the threshold to arouse to both external stimuli (e.g. light, sound, temperature) and internal stimuli (including respiratory stimuli) throughout the night [14•]. Hence, in people with an anatomical compromise to airway collapse (e.g. narrow upper airway), a state of heightened conditioned cognitive and physiological arousal in the evening that is frequently present in people with insomnia may moderate the respiratory arousal threshold to exacerbate OSA severity [14•]. This emerging area of COMISA research may be important to refine existing treatments and develop new treatment approaches tailored to the underlying features of the condition [44, 45].
Positive Airway Pressure Therapy for COMISA
Positive airway pressure (PAP) therapy combined with weight management advice is the recommended ‘first-line’ treatment for moderate and severe OSA [48]. Although PAP therapy improves many nocturnal and resulting daytime symptoms of OSA, many patients find it difficult to wear nasal/oro-nasal masks that deliver positive air pressure for the duration of the sleep period. Consequently, many patients reject PAP therapy or experience patterns of sub-optimal use over time [49].
Unsurprisingly, COMISA is associated with reduced adherence to PAP therapy, compared to OSA alone [12]. For example, it can be difficult for a patient with chronic insomnia to embrace a treatment that requires strapping a mask and tubing their head, which administers air pressure to the nose/throat, despite their pre-existing difficulties falling asleep, long awakenings throughout the night and a pre-disposition to avoid activities that may ‘harm’ their sleep. There are several ways that insomnia symptoms may reduce PAP adherence. In patients with COMISA, PAP therapy may exacerbate pre-existing insomnia symptoms [50], may be viewed as a ‘threat’ to sleep (which patients with insomnia are generally more protective of), or may provide less therapeutic benefit if insomnia symptoms persist despite adequate PAP use. Consequently, people with COMISA are ~ 30% less likely to accept PAP compared to those with sleep apnoea alone [51], and among patients that do start PAP therapy, nightly use is ~ 2 times lower in patients with COMISA compared to sleep apnoea alone [1•, 12, 51, 52].
Despite this overall effect of co-morbid insomnia on reducing PAP use, a minority of people with COMISA do accept PAP therapy, show adequate PAP use and experience improvement of both insomnia symptoms and OSA with PAP [53]. A recent review identified that PAP therapy is associated with a 20–50% improvement in insomnia severity among patients with COMISA (potentially driven by improvements in daytime symptoms of insomnia which are shared by both insomnia and OSA) [14•]. An emerging area of research aims to identify this PAP-responsive sub-group of people with COMISA, to refine tailored treatment approaches.
Until this PAP-responsive group of patients with COMISA can be reliably identified, PAP therapy appears to be a sub-optimal ‘fist line’ treatment for most patients with COMISA. Early negative experiences with PAP therapy may reduce the likelihood of future PAP acceptance and use. Consequently, several research groups have suggested that patients with COMISA should receive targeted behavioural insomnia treatment to improve insomnia symptoms, reduce this barrier to PAP use and facilitate more positive/successful initial experiences with PAP therapy [1•, 13, 15].
Managing COMISA with Cognitive Behavioural Therapy for Insomnia
Cognitive behavioural therapy for insomnia (CBT-I) is the recommended ‘first-line’ treatment for insomnia [48, 54, 55]. CBT-I is a multi-component therapy that aims to identify and target the underlying cognitive and behavioural mechanisms that maintain insomnia, to gradually improve sleep and daytime function. We recently reported in a systematic review and meta-analysis that CBT-I is associated with a large reduction in insomnia severity among patients with treated and untreated co-morbid OSA (Hedge’s g = − 0.89, 95% confidence interval = − 1.35 to − 0.43) [36•]. We identified 14 unique studies including 1040 patients investigating the effect of CBT-I in patients with COMISA, of which 9 studies were appropriate for meta-analysis (Fig. 4). Sub-group meta-analyses also indicated that CBT-I improves insomnia symptoms among patients with treated and untreated OSA, highlighting the importance of future research to investigate different sequences of CBT-I and PAP therapy commencement [36•].
Forest plot of effect of CBT-I on the Insomnia Severity Index in patients with COMISA (Hedge’s g, 95% confidence interval). Of interest, the two studies with 95% confidence intervals crossing 0 had the smallest COMISA sample sizes. Reproduced from Sweetman et al. [36•] through CC-BY license
CBT-I may also improve manifestations and subsequent management of OSA in patients with untreated OSA. For example, we used randomised controlled trial data to investigate the effect of a 4-session psychologist-delivered CBT-I program, versus no-treatment control, on changes in the apnoea-hypopnoea index from pre-treatment to 6-week follow-up in 145 patients with co-morbid insomnia and untreated moderate/severe OSA. Home-based polysomnography studies indicated that the CBT-I group experienced a significantly greater reduction in the apnoea-hypopnoea index from pre-treatment to 6 weeks (5.5-point reduction) than the control group (2-point increase) adjusting for body posture and sleep stage (Fig. 3). This small improvement in OSA severity may have been due to reduced physiological and cognitive ‘hyperarousal’, or consolidating sleep periods to reduce time spent in ‘light’ transitional sleep stages following CBT-I. CBT-I was also associated with a reduction in objective awakening frequency and duration. Future studies should investigate the effect of CBT-I on OSA severity, and mechanisms underpinning this relationship. For example, it may be possible to use wearable/nearable sleep monitoring equipment to track indices of OSA severity from night-to-night of CBT-I, to understand the treatment components and timing of CBT-I that is associated with the largest improvement of OSA severity. Non-anatomical traits of OSA, including the respiratory arousal threshold, may be extracted from polysomnography studies before and after CBT-I to also understand the effect of insomnia treatment on moderating these underlying traits of sleep apnoea.
Several research groups have also hypothesised that treating insomnia may improve subsequent PAP adherence [56, 57•, 58, 59]. Four recent randomised controlled trials have investigated the effect of CBT-I versus education/no-treatment control on improving insomnia symptoms and increasing subsequent use of PAP therapy in patients with COMISA. Sweetman [57•] and Alessi [58] reported increased adherence to PAP therapy following CBT-I versus control, while Ong [59] and Bjorvatn [56] reported no effect of CBT-I on PAP use (see Sweetman et al. [36•] for a review of methodological differences between studies). Future studies are required to understand the specific COMISA sub-groups, settings, CBT-I components and duration, and comparator conditions, that are associated with the greatest effects of CBT-I on subsequent PAP use.
Although CBT-I is the recommended ‘first-line’ treatment for insomnia, and is effective in patients with COMISA, very few patients with insomnia presently access this treatment [60]. Regarding the management of COMISA, it may be possible to implement insomnia screening, diagnostic and CBT-I pathways in sleep clinic settings that currently specialise in the diagnosis and management of OSA alone. This would provide an evidence-based assessment and treatment pathway for the 30–50% of OSA patients with clinically significant co-morbid insomnia. For example, brief insomnia screening questionnaires such as the Insomnia Severity Index [61] may be administered alongside other routine intake questionnaires (sleepiness, chronic conditions, etc.). Patients reporting clinically significant nocturnal and daytime insomnia symptoms [62] could be considered for further diagnostic assessment (e.g. with a 1-week sleep diary, daytime function questionnaires, and a follow-up consultation with a behavioural sleep medicine specialist), and referral for CBT-I. Given some evidence suggesting that CBT-I can improve subsequent acceptance and use of PAP therapy, it may be appropriate to administer CBT-I prior to commencing PAP therapy [57•]. Many patients experience a > 6-week waiting period between diagnostic and PAP titration/commencement appointments, which may allow for CBT-I to be administered without delaying PAP therapy commencement. CBT-I has been translated to several modalities including delivery by psychologists, psychology students, nurses, and other health professionals, tele-health and group delivery, brief behavioural therapy for insomnia and self-guided or hybrid online/clinician guided CBT-I through interactive online programs [36•]. Moderate to large effects have been reported for several of these different modalities [63]; however, most studies of CBT-I in COMISA populations have focused on psychologist/clinician-delivered treatment. Consequently, future research is required to understand the effectiveness of other CBT-I modalities in patients with untreated co-morbid OSA (for efficacy, safety/side effects, and acceptability, and optimal timing with PAP commencement). Larger mixed-methods implementation programs to understand the feasibility, barriers and facilitators to implementing different CBT-I modalities in sleep clinic settings are needed, to improve the current management of patients with COMISA.
Conclusion
COMISA is a highly prevalent condition that is associated with greater morbidity and more complex treatment decisions compared to either insomnia or OSA alone. Although first described in 1973, the field of COMISA has only recently received an increase in research and clinical attention. Given the high prevalence of COMISA in sleep clinic and population-based settings, it is critical to undertake more research to understand the aetiology of COMISA, develop more effective and personalised treatments, and to implement these COMISA management pathways throughout the health system.
Abbreviations
- CBT-I:
-
Cognitive behavioural therapy for insomnia
- COMISA:
-
Co-morbid insomnia and sleep apnoea
- OSA:
-
Obstructive sleep apnoea
- PAP:
-
Positive airway pressure
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Sweetman A, Lack L, Catcheside P, Antic NA, Chai-Coetzer C, Smith S, et al. Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Med Rev. 2017;33:28–38. This review article coined the COMISA phrase.
The American Academy of Sleep Medicine. 3rd ed. International Classification of Sleep Disorders (ICSD-3), Diagnostic and coding manual: Westchester; 2014.
Reynolds A, Appleton S, Gill T, Adams R. Chronic insomnia disorder in Australia: a report to the Sleep Health Foundation. Sleep Health Foundation Special Report; 2019.
Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81.
Sweetman A, Van Ryswyk E, Vakulin A, Lack L, Reed R, Battersby M, et al. Co-occurring depression and insomnia in Australian primary care: a narrative review of recent scientific evidence. Med J Aust. 2021;215(5):230–6.
Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166(16):1709–15.
Natsky A, Vakulin A, Chai-Coetzer C, Lack L, McEvoy R, Lovato N, et al. Economic evaluation of cognitive behavioural therapy for insomnia (CBT-I) for improving health outcomes in adult populations: a systematic review. Sleep Med Rev. 2020;54. https://doi.org/10.1016/j.smrv.2020.101351.
Li L, Gan Y, Zhou X, Jiang H, Zhao Y, Tian Q, et al. Insomnia and the risk of hypertension: a meta-analysis of prospective cohort studies. Sleep Med Rev. 2020;101403. https://doi.org/10.1016/j.smrv.2020.101403.
Guilleminault C, Eldridge FL, Dement WC. Insomnia with sleep apnea: a new syndrome. Science. 1973;181(4102):856–8. This was the first peer-reviewed article to identify the co-existence of insomnia and sleep apnea.
Lichstein KL, Riedel BW, Lester KW, Aguillard RN. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67(3):405–10. This article reported on the high prevalence of OSA in a sample of people with insomnia.
Krakow B, Melendrez D, Ferreira E, Clark J, Warner TD, Sisley B, Sklar D. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest. 2001;120(6):1923–9. This article reported on the high prevalence of insomnia in a sample of people with sleep-disordered breathing.
Sweetman A, Lack L, Crawford M, Wallace DM. Co-morbid insomnia and sleep apnea (COMISA): Assessment and management approaches. Sleep Medicine Clinics. 2021;in press(A review of PAP therapy for the treatment of OSA).
Ong JC, Crawford MR, Wallace DM. Sleep apnea and insomnia: emerging evidence for effective clinical management. Chest. 2020;159(5):2020–8.
Sweetman A, Lack L, McEvoy RD, Smith S, Eckert DJ, Osman A, et al. Bi-directional relationships between co-morbid insomnia and sleep apnea (COMISA). Sleep Med Rev. 2021;101519. https://doi.org/10.1016/j.smrv.2021.101519. Outlines potential bi-directional relationships between insomnia and OSA.
Luyster FS, Buysse DJ, Strollo PJ. Comorbid insomnia and obstructive sleep apnea: Challenges for clinical practice and research. J ClinSleep Med. 2010;6(2):196–204.
Zhang Y, Ren R, Lei F, Zhou J, Zhang J, Wing Y-K, et al. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2019;45:1–17. A systematic review and meta-analysis of COMISA prevalence.
Sweetman A, Lack L, Bastien C. Co-morbid insomnia and sleep apnea (COMISA): prevalence, consequences, methodological considerations, and recent randomized controlled trials. Brain Sci. 2019;9(12):371.
Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14(1):35–46.
Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–9.
Lang CJ, Appleton SL, Vakulin A, McEvoy RD, Wittert GA, Martin SA, et al. Co-morbid OSA and insomnia increases depression prevalence and severity in men. Respirology. 2017;22:1407–15.
Jeon B, Luyster FS, Callan JA, Chasens ER. Depressive symptoms in comorbid obstructive sleep apnea and insomnia: an integrative review. West J Nurs Res. 2021;0193945921989656. https://doi.org/10.1177/0193945921989656.
Lechat B, Naik G, Reynolds A, Aishah A, Scott H, Loffler KA, et al. Multi-night prevalence, variability, and diagnostic misclassification of obstructive sleep apnea. Am J Respir Crit Care Med. 2022;205(5):563–569. https://doi.org/10.1164/rccm.202107-1761OC.1519.
Sweetman A, Lechat B, Appleton S, Reynolds A, Adams R, Melaku YA. Association of co-morbid insomnia and sleep apnoea symptoms with all cause mortality: analysis of the NHANES 2005–2008 data. Sleep Epidemiol. 2022;(2):100043. https://doi.org/10.1016/j.sleepe.2022.100043.
Lechat B, Loffler KA , Wallace DM, Reynolds A, Appleton SL , Scott H, et al. All-cause mortality in people with co-occurring insomnia symptoms and sleep apnea: analysis of the Wisconsin Sleep Cohort. Nat Sci Sleep. 2022;14:14:1817-1828. https://doi.org/10.2147/NSS.S379252.
Lechat B, Appleton S, Melaku Y, Hansen K, McEvoy RD, Adams R, et al. Co-morbid insomnia and obstructive sleep apnoea is associated with all-cause mortality. Euro Resp J. 2021;in press. https://doi.org/10.1183/13993003.01958-2021. First report of the association between COMISA and all-cause mortality.
Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631–8.
Sweetman A, Adams R. Co-morbid insomnia and sleep apnea (COMISA) as a potential predictor of suicide and self-harm. Commentary on Udholm et al. Obstructive sleep apnea and risk of suicide and self-harm: a Danish Nationwide Cohort Study. Sleep. 2022;45(6):zsac043. https://doi.org/10.1093/sleep/zsac043.
Lechat B, Appleton S, Melaku Y, Hansen K, McEvoy R, Adams R, et al. The association of co-morbid insomnia and obstructive sleep apnoea with prevalent cardiovascular disease and incident cardiovascular events. J Sleep Res. 2022;(5):e13563. https://doi.org/10.1111/jsr.13563.
Sivertsen B, Björnsdóttir E, Øverland S, Bjorvatn B, Salo P. The joint contribution of insomnia and obstructive sleep apnoea on sickness absence. J Sleep Res. 2013;22(2):223–30.
Vozoris NT. Sleep apnea-plus: Prevalence, risk factors, and association with cardiovascular diseases using United States population-level data. Sleep Med. 2012;13(6):637–44.
Luyster FS, Kip KE, Buysse DJ, Aiyer AN, Reis SE, Strollo PJ. Traditional and nontraditional cardiovascular risk factors in comorbid insomnia and sleep apnea. Sleep. 2014;37(3):593–600.
Gupta MA, Knapp K. Cardiovascular and psychiatric morbidity in obstructive sleep apnea (OSA) with insomnia (sleep apnea plus) versus obstructive sleep apnea without insomnia: a case-control study from a nationally representative US sample. Public Libr Sci One. 2014;9(3). https://doi.org/10.1371/journal.pone.0090021.
Haycock J, Grivell N, Redman A, Saini B, Vakulin A, Lack L, et al. Primary care management of chronic insomnia: a qualitative analysis of the attitudes and experiences of Australian general practitioners. BMC Fam Pract. 2021;22(158):1–11.
Carter SG, Eckert DJ. Effects of hypnotics on obstructive sleep apnea endotypes and severity: novel insights into pathophysiology and treatment. Sleep Med Rev. 2021;101492. https://doi.org/10.1016/j.smrv.2021.101492.
Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169.
Sweetman A, Farrell S, Wallace D, Crawford M. The effect of cognitive behavioural therapy for insomnia in people with co-morbid insomnia and sleep apnoea: a systematic review and meta-analysis. J Sleep Res. 2023;e13847. https://doi.org/10.1111/jsr.13847. Systematic review and meta-analysis of the effect of CBT-I on insomnia severity in people with COMISA.
Sweetman A, McEvoy R, Smith S, Catcheside P, Antic N, Chai-Coetzer C, et al. The effect of cognitive and behavioral therapy for insomnia on week-to-week changes in sleepiness and sleep parameters in insomnia patients with co-morbid moderate and severe sleep apnea: A randomized controlled trial. Sleep. 2020;43(7):zsaa002. https://doi.org/10.1093/sleep/zsaa002.
Lack L, Gradisar, Harris, Tietzel, Jayaram, McEvoy. Treating a co-morbid insomnia/obstructive sleep apnea case with bedtime restriction therapy. Bedtime restriction and motor-vehicle accident risk Sleep Down Under. 2003;Conference Abstract.
Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93.
Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56.
Fernandez-Mendoza J, Vgontzas AN, Bixler EO, Singareddy R, Shaffer ML, Calhoun SL, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35(5):689–97.
Lundetræ RS, Saxvig IW, Aurlien H, Lehmann S, Bjorvatn B. Effect of continuous positive airway pressure on symptoms and prevalence of insomnia in patients with obstructive sleep apnea: a longitudinal study. Front Psychol. 2021;12:691495. https://doi.org/10.3389/fpsyg.2021.691495.
Sweetman A, Lechat B. Incidence, persistence and remission of co-morbid insomnia and sleep apnea (COMISA) over time in the Wisconsin Sleep Cohort data. Sleep Adv. 2022;in press.
Zheng JN, Tong B, Sweetman A, Eckert DJ, Osman A. The insomnia severity index is related to the respiratory arousal thershold in people with co-morbid insomnia and sleep apnoea (COMISA). Sleep Adv. 2022;in press. https://doi.org/10.1093/sleepadvances/zpac029.156.
Brooker E, Thomson L, Landry S, Edwards B, Drummond S. O013 The pathogenesis of obstructive sleep apnea in individuals with comorbid insomnia and obstructive sleep apnoea (COMISA). Sleep Adv. 2021;2(1):A6–A7. https://doi.org/10.1093/sleepadvances/zpab014.012.
Tsai C-Y, Kuan Y-C, Hsu W-H, Lin Y-T, Hsu C-R, Lo K, et al. Differentiation model for insomnia disorder and the respiratory arousal threshold phenotype in obstructive sleep apnea in the Taiwanese population based on oximetry and anthropometric features. Diagnostics. 2022;12(1):50.
Osman AM, Carter SG, Carberry JC, Eckert DJ. Obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:21.
Australasian Sleep Association. Sleep heatlh primary care resource: evidence-based resources and information to assess and manage adult patients with Obstructive Sleep Apnoea and Insomnia, 2021. Available from: https://www.sleepprimarycareresources.org.au/. Accessed 20 June 2023
Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–8.
Caetano Mota P, Morais Cardoso S, Drummond M, Santos AC, Almeida J, Winck JC. Prevalence of new-onset insomnia in patients with obstructive sleep apnoea syndrome treated with nocturnal ventilatory support. Port J Pulmonol. 2012;18(1):15–21.
Tan D, Appleton A, Chai-Coetzer CL, Lovato N, Vakulin A, Adams R, et al. Health and treatment correlates of obstructive sleep apnoea (OSA) alone and comorbid with insomnia (COMISA) in a community-based sample. Effect of COMISA on CPAP acceptance and use ASA Adelaide Meeting. 2022.
Smith S, Dunn N, Douglas J, Jorgensen G. Sleep onset insomnia is associated with reduced adherence to CPAP therapy. Sleep Biol Rhythm. 2009;7:A74.
Björnsdóttir E, Janson C, Sigurdsson JF, Gehrman P, Perlis M, Juliusson S, Arnardottir ES, Kuna ST, Pack AI, Gislason T, Benediktsdóttir B. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep. 2013;36(12):1901–9.
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–33.
Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700.
Bjorvatn B, Berge T, Lehmann S, Pallesen S, Saxvig I. No effect of a self-help book for insomnia in patients with obstructive sleep apnea and comorbid chronic insomnia–a randomized controlled trial. Front Psychol. 2018;9:2413.
Sweetman A, Lack L, Catcheside P, Antic N, Smith S, Chai-Coetzer C, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with co-morbid insomnia: a randomized clinical trial. Sleep. 2019;42(12). https://doi.org/10.1093/sleep/zsz178. First RCT to report that CBT-I improves acceptance and use of PAP therapy in patients with COMISA.
Alessi CA, Fung CH, Dzierzewski JM, Fiorentino L, Stepnowsky C, Rodriguez Tapia JC, et al. Randomized controlled trial of an integrated approach to treating insomnia and improving use of positive airway pressure therapy in veterans with comorbid insomnia disorder and obstructive sleep apnea. Sleep. 2021;44(4). https://doi.org/10.1093/sleep/zsaa235.
Ong JC, Crawford MR, Dawson SC, Fogg LF, Turner AD, Wyatt JK, et al. A randomized controlled trial of CBT-I and PAP for obstructive sleep apnea and comorbid insomnia: main outcomes from the MATRICS study. Sleep. 2020;43(9):zsaa041. https://doi.org/10.1093/sleep/zsaa041.
Miller CB, Valenti L, Harrison CM, Bartlett DJ, Glozier N, Cross NE, et al. Time trends in the family physician management of insomnia: the Australian experience (2000–2015). J Clin Sleep Med. 2017;13(06):785–90.
Bastien C, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307.
Sweetman A, Lack L, McEvoy D, et al. Predictors of Insomnia Severity Index Profiles in US veterans with obstructive sleep apnea (JC-19–00217). J Clin Sleep Med. 2019;15(12):1717–9.
Simon L, Steinmetz L, Feige B, Benz F, Spiegelhalder K, Baumeister H. Comparative efficacy of onsite, digital, and other settings for cognitive behavioral therapy for insomnia: a systematic review and network meta-analysis. Sci Rep. 2023;13(1):1929.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
AS reports research equipment and/or funding support from the National Health and Medical Research Council, Flinders University, Hospital Research Foundation, Big Health, Philips Respironics, Compumedics, ResMed and commissioned/consultancy work for Australian Doctor, Sleep Review Mag and Cerebra.
Human and Animal Rights and Informed Consent
No ethics approvals were required for this review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sweetman, A. Co-morbid Insomnia and Sleep Apnoea (COMISA): Latest Research from an Emerging Field. Curr Sleep Medicine Rep 9, 180–189 (2023). https://doi.org/10.1007/s40675-023-00262-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-023-00262-9