Abstract
Purpose for Review
Sleep deprivation and insomnia are associated with mortality and morbidity worldwide. A pharmacological agent that improves subjective and objective measures of sleep, without significant side effects, remains nebulous. However, initial randomised controlled trials suggest Prunus cerasus (tart cherry) ingestion may be beneficial. This systematic review and meta-analysis evaluates the effect of Prunus cerasus on objective and subjective measures of sleep.
Recent Findings
We identified a total of 277 unique records, from which 8 studies of low-moderate methodological quality were included in the systematic review. Meta-analysis of subjectively recalled sleep efficiency (SE) and total sleep time (TST) were not significant. Objective SE, however, was significantly higher in the cherry cohort when compared to placebo with an effect size of 0.63 (95% CI 0.29–0.97, P < 0.01). There was low associated heterogeneity (I2 = 0%). Objective TST was significantly higher in the cherry cohorts, with a pooled effect size of 1.21 (95% CI 0.83–1.58, P < 0.01). There was high associated heterogeneity (I2 = 81.5%).
Summary
Whilst individuals may not subjectively experience a benefit, there is evidence to support significant improvements to total sleep time and sleep efficiency with the ingestion of Prunus cerasus using objective measures. Tart cherry may be the next frontier of sleep medicine and warrants further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep deprivation is a global health problem with significant implications for individuals and society. Sleep deprivation may occur in the setting of conditions such as sleep apnoea which already have established management options. However, there are other causes for sleep deprivation such as poor sleep hygiene, circadian rhythm disturbances (such as shift work) and medications [1]. Whilst guidelines for duration of sleep differ according to age group, approximately 35% of adults and 70% of high school students do not obtain the recommended amount of sleep [1, 2]. Sleep is a complex process that supports many metabolic and physiological processes and serves as a major, modifiable behaviour that is intimately related to the health of an individual [3]. Sleep deprivation is associated with an increased risk of both all-cause mortality and many leading causes of death, namely cardiovascular disease, malignancy, cerebrovascular disease, metabolic and autoimmune diseases and neurodegenerative diseases [4]. Patients with sleep deprivation also report lower quality of life compared to population norms [5]. This association with negative outcomes is more direct than that observed with sleep excess, which is likely secondary to chronic health issues [6,7,8,9]. The ingestion of Prunus cerasus (tart cherry) has been purported to assist with sleep and possibly aid in ameliorating this issue.
Sleep deprivation also poses a larger community risk. Insufficient sleep confers more immediate risks by contributing to erratic, unsafe behaviours and impulsivity, impaired judgement and daytime somnolence/microsleeps [10, 11]. This translates to an increased risk of medical errors [12], traffic accidents [13] and workplace injuries [14] and reduced academic performance. Furthermore, those with significant sleep deprivation account for a disproportionately high utilisation of health care resources [15].
Sleep deprivation may be managed with pharmacological therapies; however, these medications are not without notable side effects. Caffeine is used by many to combat daytime somnolence and fatigue but negatively impacts the quality of sleep, creating a cycle of worsening fatigue and increased dependence [16,17,18]. Many sleep-promoting agents have significant adverse effects including sedation and psychomotor impairment. They also carry the risk of addiction and abuse [19]. Many individuals may turn to other therapies to improve their sleep quality, such as cognitive behavioural therapy for insomnia (CBT-I) which has its own limitations such as a paradoxical increase in daytime somnolence which persists for 3–4 weeks, often resulting in patient dropout [20]. Other complementary therapies include nutritional supplementation; however, the evidence regarding their effect is variable [21,22,23]. One nutritional supplement that has shown early promise is Prunus cerasus (tart, sour, Montmorency cherry) [20, 24].
Tart cherries contain multiple anti-inflammatory and anti-oxidative phytonutrients including phenolic acid (polyphenols) and flavonoids [25]. This capacity for tart cherries to reduce oxidative stress has been demonstrated to reduce exercise-induced inflammation and improve muscle recovery, and it is commonly used by athletes to improve their exercise recovery and performance [26,27,28]. In these studies, some athletes report an improvement in their sleep from tart cherry supplementation; however, there is no definitive synthesis of the available evidence to prove and support the general public and clinicians in utilising Prunus cerasus for its soporific effects. Accordingly, this systematic review and meta-analysis was conducted with the aim of evaluating studies that have examined the effect of Prunus cerasus ingestion on sleep.
Methods
The methodology for this systematic review was established within a protocol prior to its conduct. This study was prospectively registered with PROSPERO (CRD42021279145) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) and Meta-analyses Of Observational Studies in Epidemiology (MOOSE) reporting guidelines [29, 30].
Search Strategy and Selection Criteria
The population, intervention, comparator group, outcome (PICO) framework was used to formulate the research question and inclusion criteria. The population was individuals, with or without prior sleep diagnosis. The intervention was Prunus cerasus (tart/sour cherry) in whole, concentrated, or supplemental form. A comparator group was not required for inclusion. The primary outcome of interest was total sleep time (both objective assessment and subjective recall). Additional outcomes of interest were other sleep parameters, including objective and subjective measures of such, melatonin levels and measures of daytime fatigue/somnolence.
Data Extraction and Analysis
Two reviewers (BS and JK) independently screened titles and abstracts, reviewed full-texts and extracted data using a standardised form. Screening of titles and abstracts was via a web application (Rayyan, Qatar Computing Research Institute, Ar-Rayyan, Qatar) [31]. Extracted data included study design and setting, population characteristics, intervention characteristics, outcomes, methodological quality information and other information relevant to the review questions. Data relevant to study outcomes was summarised to determine effect sizes across the included studies.
The determination of whether studies met inclusion criteria was undertaken in duplicate using a standardised form. To be included in the systematic review, the following criteria had to be met: (1) published in English; (2) primary research article (reviews were excluded); (3) intervention was Prunus cerasus, in any form but as a sole constituent or main active ingredient; (4) total sleep time (subjective or objective); and (5) was available in full-text. Eligibility determination was undertaken in duplicate, and instances of disagreement were resolved through discussion between the reviewers and in the event of non-agreement, a third reviewer acted as arbiter. The Joanna Briggs Institute (JBI) Critical Appraisal Checklists for Randomised Controlled Trials were used to assess the risk of bias. This risk of bias analysis was performed in duplicate. Publications not reporting primary research data were excluded. Editorials, perspectives, letters and conference abstracts were excluded. PubMed (incorporating MEDLINE), Embase and CINAHL were searched from database inception to July 31, 2022. Key search terms included sleep, insomnia, somnolence, rest, cherry, prunus and cerasus. No publication restrictions were implemented. The individual search strings employed for each database are listed in Supplementary Information. Additionally, the reference lists of included articles and grey literature sources were searched for relevant studies.
Statistical Analysis
Statistical analysis was carried out using Stata® (Version 17.0, StataCorp, Texas, USA). Where appropriate, a meta-analysis of continuous data was performed using the meta esize function. A fixed effects model was utilised as studies included in the meta-analysis largely incorporated a crossover randomised control trial design. Subjective and objective measures of sleep efficiency and total sleep time were intended for meta-analysis, a priori. Post hoc analysis of other results of interest was conducted according to data availability. Results were expressed as forest plots where appropriate, and effect size as the Hedges g statistic. P < 0.05 denoted statistical significance for intergroup comparison. Heterogeneity was assessed using the I2 test statistic. Low heterogeneity was denoted by I2 < 50%, moderate heterogeneity by I2 50–74% and high heterogeneity by I2 > 75%. Due to the limited number of studies, meta-regression, subgroup analysis and publication bias were not assessed.
Results
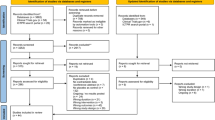
Our search identified a total of 320 records. There were 277 studies after duplicate removal (Fig. 1). After the title and abstract screening, there were 27 articles reviewed in full text. Following full text review, references lists and grey literature were searched for additional articles, and one additional text was identified for inclusion. In total, eight studies were included in this systematic review. Characteristics of the included studies are detailed in Table 1. Study publication year ranged from 2009 to 2022. Five included studies were double-blind crossover design; two were prospective cohort studies; and one study was a placebo-controlled RCT without crossover design. The five crossover studies utilised a cherry supplement, with a washout period incorporated in the crossover design. Dose and timing varied between all studies but were commonly the equivalent of ~ 100 g of fresh cherries two times per day. Six studies assessed healthy patients, whereas two assessed patients with a history of insomnia. The age range of included participants varied significantly, but all age groups were included in at least four different trials. Objective measures of sleep were the most common outcome, four utilising actigraphic recordings, one using accelerometers and one using polysomnography. Only two studies used solely subjective recordings of sleep duration.
Risk of bias analysis of the included studies demonstrated that the majority of studies were of low to moderate risk of bias (see Supplementary Information).
Subjective Sleep Measures
Four studies assessed subjective measures of sleep [32,33,34,35].
Sleep Efficiency
Three studies assessed subjective sleep efficiency in cherry vs placebo, with a pooled effect size of 0.07 (95% CI − 0.28–0.42, P = 0.94), and low associated heterogeneity (I2 = 0) [32, 33, 35] (Fig. 2).
Total Sleep Time
Three studies reported subjective total sleep time in cherry vs placebo. With a pooled effect size of 0.14 (95% CI − 0.22–0.49, P = 0.79). This result was associated with low heterogeneity (I2 = 0) [32, 33, 35] (Fig. 3). The greatest difference between cherry and placebo for total sleep time was an additional 12 min (cherry group = 421 min vs placebo = 409 min; P = 0.197), seen in healthy individuals [33]. The smallest observed difference between cherry and placebo for total sleep time was 1 min (cherry group = 475 min vs placebo = 476 min; P = > 0.05), seen in healthy individuals [32]. Losso et al. reported subjective sleep measures as a standard mean difference and demonstrated a significant improvement in habitual sleep (sleep efficiency as measured by the Pittsburgh sleep quality index) (0.5 + / − 0.5, P = 0.0331); however, a significant difference in sleep duration was not noted (0.125 + / − 0.083, P = 0.6845) [34].
Three studies assessed subjective total sleep time in pre- and post-cherry supplementation, with a pooled effect size of 0.27 (95% CI − 0.27–0.80, P = 0.11). This finding was associated with moderate heterogeneity (I2 = 0.12, 54.36%) [32, 33, 35] (Fig. 4). The greatest difference between baseline and cherry for total sleep time was an additional 29.3 min (baseline = 388.3 min vs cherry group = 417.6 min; P < 0.01), seen in individuals with insomnia [35].
Sleep Onset Latency and Naps
Not included in the meta-analysis, due to insufficient cohort data, was subjective sleep onset latency, which demonstrated variable results across the three studies. The greatest difference in sleep onset latency between placebo and cherry was a reduction of 5.3 min (cherry group = 34.2 min vs placebo = 39.5 min; P > 0.05), seen in healthy individuals [32]. In two studies, there was a trend toward reduced sleep onset latency, whilst the opposite effect was observed in the third; however, none of these observations were statistically significant (P > 0.05) [32, 35].
The only study to record subjective daytime naps demonstrated significantly less napping time in the cherry juice trial compared to baseline and the placebo trials (P = 0.031; 95% CI = 0.7–13.6 and 0.7–11.1 min, respectively) [32]. This finding was made in the absence of any significant differences in subjective sleep efficiency, sleep onset latency, wake up after sleep or total sleep times. There was, however, a significant increase in objective total sleep time in the cherry juice group vs baseline and placebo (P < 0.003; 95% CI = 15.2–39.7, 14.7–63.6, respectively).
Objective Sleep Measures
Six studies reported objective sleep measures, either utilising accelerometry, actigraphy or polysomnography [32, 34, 36,37,38,39].
Sleep Efficiency
Three studies uniformly reported sleep efficiency and, therefore, were included in a meta-analysis [32, 38, 39]. Sleep efficiency was significantly higher in the cherry cohort when compared to placebo with an effect size of 0.63 (95% CI 0.29–0.97, P < 0.01) (Fig. 5). There was low associated heterogeneity (I2 = 0%).
Total Sleep Time
Both the largest and smallest differences in placebo vs treatment (cherry) were observed in a patient cohort of similar characteristics (young, 20–30-year-old, healthy individuals). The greatest difference between cherry and placebo for total sleep time was an additional 67 min (cherry group = 425.52 min vs placebo = 358.54 min; P value not calculated) [38]. The smallest observed difference between cherry and placebo for total sleep time was an additional 22.2 min (cherry group = 408 min vs placebo = 385.8 min, P = 0.244) was reported [39]. Three studies uniformly reported total sleep time; therefore, they were included in a meta-analysis [32, 38, 39]. Total sleep time was significantly higher in the cherry cohorts, with a pooled effect size of 1.21 (95% CI 0.83–1.58, P < 0.01) (Fig. 6). This was associated with high heterogeneity (I2 = 81.5).
The greatest difference between baseline and cherry for total sleep time was an additional 47.8 min (baseline = 398 min vs cherry group = 445.8 min; P < 0.05) in healthy middle-aged volunteers [38]. There were also some notable and significant improvements in total sleep time and sleep efficiency in the study by Garrido et al. (2010); however, values are reported as a ‘fold’ increase over baseline, so actual numbers of minutes are unknown. Losso et al. were the only study to assess objective sleep measures in patients with insomnia and reported no significant difference between cherry and placebo across sleep efficiency, sleep onset latency, REM latency, wake after sleep onset and number of awakenings. However, a significant increase in total sleep time was observed, with an increase of 84 min ± 61.7 (P = 0.0182) [34]. Similarly, Garrido et al. (2009) demonstrated statistically significant improvements in actual sleep time with cherry over baseline in the young (12.3% + / − 0.5: P < 0.05), middle-aged (10% + / − 0.2: P < 0.05) and elderly (18.2% + / − 1.6: P < 0.05) [36]. Garrido et al. (2010) echo these results with improvements in actual sleep time over baseline in middle-aged and elderly volunteers, the degree of which differing based on the strain of cherry used (range from 1.15 + / − 0.05 fold increase for Pico Limón cherries to 1.45 + / − 0.07 fold increase for Pico Negro cherries (P < 0.05)) [37].
Sleep Onset Latency and Naps
The greatest difference in sleep onset latency between placebo and cherry was a reduction of 10 min (placebo = 19 min vs cherry group = 9 min; P < 0.001) in young healthy individuals [39]. No objective measures of daytime naps were undertaken.
Discussion
This systematic review is the first to synthesise the potential benefits of Prunus cerasus on sleep. Prunus cerasus use may provide an objective and clinically significant improvement in total sleep time and sleep efficiency. This objective improvement in sleep was not reflected in the participants subjective recall of total sleep time or sleep efficiency. However, the subjective meta-analysis included more participants with insomnia, a condition which is known to cause extreme deviations between subjective and objective measures of sleep [40]. Importantly, the objective meta-analysis included studies that together cover a wide age range, suggesting benefit is not restricted to a certain age group. This is also one of the rare instances where a study has performed a meta-analysis on objective measures of sleep for a supplement.
The dose of Prunus cerasus is of relevance when interpreting the present study’s findings. Generally, effective dose of cherry supplementation was derived from ~ 100 g of cherries. This amount of fresh fruit contains ~ 0.135 μg of melatonin and 9 mg of tryptophan. The clinical dosing recommendations for these compounds are actually 0.5–5 mg for melatonin and 1.2–2.4 g for tryptophan, suggesting neither of these is the direct mechanism of tart cherry’s benefits [34]. However, significant elevations in urinary melatonin metabolites [32, 37, 38] and a reduced degradation of tryptophan have been observed in the included studies [34]. Additionally, significant elevations in interleukin 1B, 8 and Tumour Necrosis Factor alpha have been demonstrated with cherry consumption which are all somnogenic cytokines [38]. The exact mechanism, however, remains unclear, and further studies may reveal a target for a new, safe and effective sleep pharmacotherapeutic agent.
Although none of the studies specifically studied Prunus cerasus supplementation in hospital inpatients, the potential for benefit from Prunus cerasus supplementation could extend beyond an outpatient, community setting and improve inpatient hospital outcomes. Currently, hospital systems pose difficulties to the achievement of healthy sleep in such a way that may potentially adversely affect patient outcomes [41, 42]. There is increased attention toward low-risk, supplementary regimens to improve patient outcomes [43]. Particularly in the absence of proven pharmacological interventions to improve sleep in hospitalised adults, Prunus cerasus supplementation may assist in patient sleep and recovery [44]. It may also be considered in hospital staff to improve sleep and, therefore, their decision-making, mood disturbances and burnout [45, 46]. However, firm evidence on its effectiveness in hospital settings is required before it’s integration into hospital care systems [47].
This review has several limitations that should be acknowledged, including the exclusion of non-English articles. The small sample size of the included studies, further confounded by the small sample sizes observed in the included studies, is another limitation. Additionally, the trials are only short-term, and there is no evidence on long-term response, so the possibility of tachyphylaxis or tolerance has not been addressed. This precludes the ability to comment on the potential for better outcomes secondary to improved sleep in long-term users.
Conclusion
This systematic review is the first to synthesise the potential benefits of Prunus cerasus (tart cherries) on sleep. Whilst individuals may subjectively not experience a benefit, objectively, there is evidence to support significant improvements to total sleep time and sleep efficiency. Notably, the benefits were observed across all age groups. This review demonstrates the therapeutic benefit of tart cherries and their potential for the reduction of sleep deprivation-related morbidity and mortality worldwide. Further research is required to ascertain whether the benefits are retained after long-term supplementation and the exact mechanism of action.
Data Availability
All data was sourced from existing literature and can be accessed by reviewing the appropriate referenced material.
References
Bandyopadhyay A, Sigua NL. What is sleep deprivation? Am J Respir Crit Care Med. 2019;199(6):P11–2.
Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Joint consensus statement of the american academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38(8):1161–83.
Kirszenblat L, van Swinderen B. The yin and yang of sleep and attention. Trends Neurosci. 2015;38(12):776–86.
Garbarino S, Lanteri P, Bragazzi NL, Magnavita N, Scoditti E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol. 2021;4(1).
Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7(4):335–49.
Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14(3):191–203.
Svensson T, Saito E, Svensson AK, Melander O, Orho-Melander M, Mimura M, et al. Association of sleep duration with all- and major-cause mortality among adults in Japan, China, Singapore, and Korea. JAMA Netw Open. 2021;4(9):e2122837.
Yin J, Jin X, Shan Z, Li S, Huang H, Li P, et al. Relationship of sleep duration with all‐cause mortality and cardiovascular events: a systematic review and dose‐response meta‐analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9).
Hou C, Lin Y, Zimmer Z, Tse LA, Fang X. Association of sleep duration with risk of all-cause mortality and poor quality of dying in oldest-old people: a community-based longitudinal study. BMC Geriatr. 2020;20(1).
Maric A, Montvai E, Werth E, Storz M, Leemann J, Weissengruber S, et al. Insufficient sleep: enhanced risk-seeking relates to low local sleep intensity. Ann Neurol. 2017;82(3):409–18.
Oginska H, Pokorski J. Fatigue and mood correlates of sleep length in three age-social groups: school children, students, and employees. Chronobiol Int. 2006;23(6):1317–28.
Kalmbach DA, Arnedt JT, Song PX, Guille C, Sen S. Sleep disturbance and short sleep as risk factors for depression and perceived medical errors in first-year residents. Sleep. 2017;40(3):zsw073.
Martiniuk ALC, Senserrick T, Lo S, Williamson A, Du W, Grunstein RR, et al. Sleep-deprived young drivers and the risk for crash. JAMA Pediatr. 2013;167(7):647.
Christian MS, Ellis APJ. Examining the effects of sleep deprivation on workplace deviance: a self-regulatory perspective. Acad Manag J. 2011;54(5):913–34.
Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA. The relationship between chronically disrupted sleep and healthcare use. Sleep. 2002;25(3):289–96.
Cook CJ, Crewther BT, Kilduff LP, Drawer S, Gaviglio CM. Skill execution and sleep deprivation: effects of acute caffeine or creatine supplementation-a randomized placebo-controlled trial. J Int Soc Sports Nutr. 2011;8(1):2.
Drapeau C, Hamel-Hebert I, Robillard R, Selmaoui B, Filipini D, Carrier J. Challenging sleep in aging: the effects of 200 mg of caffeine during the evening in young and middle-aged moderate caffeine consumers. J Sleep Res. 2006;15(2):133–41.
Moradi A, Ghahremaninejad F, Hoseini E, Talebi MN, Lohrasbi S, Farahimanesh S, et al. The effectiveness of caffeinated chewing gum in ameliorating cognitive functions affected by sleep deprivation. Sleep Sci. 2022;15(2).
Krystal AD, Prather AA, Ashbrook LH. The assessment and management of insomnia: an update. World Psychiatry. 2019;18(3):337–52.
Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13(1):40.
Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben-Shushan A, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9(1):41–50.
Chan V, Lo K. Efficacy of dietary supplements on improving sleep quality: a systematic review and meta-analysis. Postgrad Med J. 2022;98(1158):285–93.
Arab A, Rafie N, Amani R, Shirani F. The role of magnesium in sleep health: a systematic review of available literature. Biol Trace Elem Res. 2022.
Kelley D, Adkins Y, Laugero K. A Review of the Health Benefits of Cherries. Nutrients. 2018;10(3):368.
Kim DO, Heo HJ, Kim YJ, Yang HS, Lee CY. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem. 2005;53(26):9921–7.
Connolly DA, McHugh MP, Padilla-Zakour OI, Carlson L, Sayers SP. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br J Sports Med. 2006;40(8):679–83 (discussion 83).
Howatson G, McHugh MP, Hill JL, Brouner J, Jewell AP, van Someren KA, et al. Efficacy of tart cherry juice in reducing muscle damage, inflammation and oxidative stress following marathon running: 2933: Board# 80 May 30 9: 30 AM-11: 00 AM. Med Sci Sports Exerc. 2009;41(5):507–8.
Wangdi JT, Sabou V, O'Leary MF, Kelly VG, Bowtell JL. Use, Practices and attitudes of elite and sub-elite athletes towards tart cherry supplementation. Sports (Basel). 2021;9(4).
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–12.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Howatson G, Bell PG, Tallent J, Middleton B, McHugh MP, Ellis J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur J Nutr. 2012;51(8):909–16.
Kimble R, Keane KM, Lodge JK, Cheung W, Haskell-Ramsay CF, Howatson G. Polyphenol-rich tart cherries (Prunus cerasus, cv Montmorency) improve sustained attention, feelings of alertness and mental fatigue and influence the plasma metabolome in middle-aged adults: a randomised, placebo-controlled trial. Br J Nutr. 2022;1–12.
Losso JN, Finley JW, Karki N, Liu AG, Pan W, Prudente A, et al. Pilot study of tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am J Ther. 2018;25(2):e194.
Pigeon WR, Carr M, Gorman C, Perlis ML. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: a pilot study. J Med Food. 2010;13(3):579–83.
Garrido M, Espino J, González-Gómez D, Lozano M, Cubero J, Toribio-Delgado AF, et al. A nutraceutical product based on Jerte Valley cherries improves sleep and augments the antioxidant status in humans. e-SPEN Eur e-J Clin Nutr Metab. 2009;4(6):e321–3.
Garrido M, Paredes SD, Cubero J, Lozano M, Toribio-Delgado AF, Muñoz JL, et al. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J Gerontol Ser A: Biomed Sci Med Sci. 2010;65(9):909–14.
Garrido M, Gonzalez-Gomez D, Lozano M, Barriga C, Paredes S, Moratinos ABR. A Jerte valley cherry product provides beneficial effects on sleep quality. Influence on aging. J Nutr Health Aging. 2013;17(6):553–60.
Simper T, Gilmartin M, Allwood D, Taylor L, Chappell A. The effects of a sleep/recovery supplement:‘night time recharge’on sleep parameters in young adults. Nutr Health. 2019;25(4):265–74.
Zavecz Z, Nagy T, Galkó A, Nemeth D, Janacsek K. The relationship between subjective sleep quality and cognitive performance in healthy young adults: evidence from three empirical studies. Sci Rep. 2020;10(1).
Kovoor JG, Stretton B, Kerr LD, Jacobsen JHW, Hewitt JN, Ovenden CD, et al. Sleep and postoperative recovery: waking up to the evidence. ANZ J Surg. 2022;92(5):953–4.
Kovoor J, Maddern G. Seeing the light: surgical circadian rhythm. Br J Surg. 2021;108(11):1263–4.
Stretton B, Kovoor JG, Vanlint A, Maddern G, Thompson CH. Perioperative micronutrients, macroscopic benefits? J Perioper Pract. 2022;17504589221091058.
Kanji S, Mera A, Hutton B, Burry L, Rosenberg E, Macdonald E, et al. Pharmacological interventions to improve sleep in hospitalised adults: a systematic review. BMJ Open. 2016;6(7):e012108.
Kaliyaperumal D, Elango Y, Alagesan M, Santhanakrishanan I. Effects of sleep deprivation on the cognitive performance of nurses working in shift. J Clin Diagn Res. 2017;11(8):Cc01-cc3.
Rosen IM, Gimotty PA, Shea JA, Bellini LM. Evolution of sleep quantity, sleep deprivation, mood disturbances, empathy, and burnout among interns. Acad Med. 2006;81(1):82–5.
Kovoor JG, Tivey DR, Ovenden CD, Babidge WJ, Maddern GJ. Evidence, not eminence, for surgical management during COVID-19: a multifaceted systematic review and a model for rapid clinical change. BJS Open. 2021;5(4).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
Formal ethical approval was not required or sought as this study does not involve the primary testing or humans or animals. This study does not involve any human or animal testing.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stretton, B., Eranki, A., Kovoor, J. et al. Too Sour to be True? Tart Cherries (Prunus cerasus) and Sleep: a Systematic Review and Meta-analysis. Curr Sleep Medicine Rep 9, 225–233 (2023). https://doi.org/10.1007/s40675-023-00261-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-023-00261-w