Abstract
Background
When coronavirus disease 2019 (COVID-19) vaccines were introduced, they were suspected of triggering severe allergic reactions disproportionately often. This contributed to the fear of vaccination, particularly among allergy patients.
Methods
In an allergy center in eastern Bavaria, we used a skin prick test to investigate how often sensitization to COVID-19 vaccines can be detected and whether appropriate testing could significantly reduce the fear of vaccination.
Results
Comirnaty® (n = 245 tested/6.93% clearly positive reaction; Biontec/Pfizer, Mainz, Germany/New York City, NY, USA), Spikevax® (56/14.28%; Moderna, Cambridge, MA, USA), Vaxzevria® (208/4.32%; Astra Zeneca, Cambridge, England) and Jcovden® (48/4.16%; Johnson & Johnson, New Brunswick, NJ, USA) were tested by skin prick test. Most participants tested were female (83.6%) and had a history of allergies (94.8%). Depending on the result of the skin prick test, the test subjects were advised on vaccination. In a questionnaire survey approximately 1 year after testing, 75.7% of the N = 70 respondents stated that their fear of vaccination had been greatly or very greatly reduced as a result of the testing and counseling. In the follow-up survey, 88.5% of all respondents had been vaccinated at least once. No notable allergic problems occurred during the COVID-19 vaccination in study participants.
Conclusion
The study shows that simple skin prick testing could reduce fears and concerns about allergic reactions to COVID-19 vaccines, and thus significantly increase the willingness to vaccinate in the population, especially among allergy patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the coronavirus disease 2019 (COVID-19) pandemic, effective vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were developed within a very short time and approval studies [1] showed no evidence of increased rates of side effects or an increased allergenic potential of these vaccines. Shortly after approval, however, there were reports of severe anaphylactic reactions following vaccinations in the USA [2] and the UK [3], which led to great uncertainty among the population.
As a precautionary measure, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK issued an interim recommendation in December 2020 that patients with anaphylaxis to vaccines, drugs or foods should not be vaccinated with COVID-19 vaccines [4]. At the same time patients in the USA with a history of severe or immediate allergic reaction to any of the vaccine components of the messenger ribonucleic acid (mRNA) vaccines, were advised not to get vaccinated [5]. However, at the end of 2020, the Paul Ehrlich Institute saw no contraindication against COVID-19 vaccination for allergy patients or people with a history of anaphylaxis. Only known allergies against vaccine components or an allergic reaction to a prior vaccination [6] would be considered as an indication against vaccination.
In this situation, many people feared serious side effects or long-term consequences from vaccination due to the rapid development and approval of vaccines, the media outrage and overheated discussions in social media. Specifically, concerns about allergic or immunological reactions to vaccines were expressed [7,8,9]. Thus, allergy departments developed programs to test available COVID-19 vaccines to determine whether COVID-19 vaccines would actually have an increased likelihood of sensitization and subsequently allergic reactions, so that allergy patients could be properly advised on vaccination.
At the allergology department of the Wörth district hospital, allergy testing for vaccines was offered and in addition it was investigated whether allergy testing with the COVID-19 vaccines can reduce the fear of an allergic or anaphylactic reaction and whether this can also lead to an actual increase in the willingness to get vaccinated.
Materials and methods
Patient cohort
From January 2021 to February 2022, prick tests with the then available COVID-19 vaccines Comirnaty® (Biontec/Pfizer, Biontec/Pfizer, Mainz, Germany/New York City, NY, USA), Spikevax® (Moderna, Cambridge, MA, USA), Vaxzevria® (Astra Zeneca, New Brunswick, NJ, USA) and Jcovden® (Johnson & Johnson) were carried out in the allergy outpatient clinic of the district hospital in Wörth an der Donau in the greater Regensburg area. Patients of all ages presented for testing either on their own initiative or with a referral from their attending physician.
Skin prick testing
The undiluted original vaccines were used for the skin prick test (SPT). As the availability of the vaccines was still limited during that time, unused vaccine doses from that day were applied. The SPT were carried out on the skin on the volar side of the forearm. One test drop was applied to the marked skin area using a pipette or a milliliter syringe. The distance between the drops was about 4 cm. A positive and negative control was carried out to validate the reaction (histamine and saline solution). A prick lancet was then inserted into the skin so that a small amount of test solution could penetrate. After a period of 15–30 min, the skin reaction was evaluated and documented on a standardized test form. A positive test reaction referred to a pale yellow wheal (edema) with a surrounding red halo (erythema). We considered a wheal diameter of ≥ 3 mm to be a clearly positive result and no reaction was allowed to occur in the negative control with 0.9% NaCl solution (wheal diameter 0 mm). The results were evaluated according to the following classification from 0–5: 0 = no reaction; 1 = minimal reaction (+) for wheal < 3 mm; 2 = slight reaction (+) for wheal ≥ 3 to < 4 mm; 3 = medium reaction (++) for wheal ≥ 4 to < 5 mm; 4 = strong reaction (+++) for wheal ≥ 5 mm; 5 = not assessable.
Procedure after skin prick testing with vaccine
The patients received detailed information and medical advice after the test. In the event of negative test results, vaccination was recommended according to the low risk for an anaphylactic reaction, which is comparable to the general population. In the case of a positive test result, the further procedure was discussed depending on the cutaneous reaction (mild–moderate–severe). In the case of a mild reaction to a vaccine, vaccines that tested negative were generally recommended and a longer follow-up period under antihistamine administration was suggested. In the case of moderate or strong sensitization in the SPT to a vaccine, vaccination with one of the alternative vaccines that tested negative was offered or, at the patient’s request, vaccination in an inpatient setting with monitoring under antihistamine administration. In the case of several clearly positive skin reactions or a systemic, vasovagal reaction in the SPT, occurring in a few cases only, confirmation of vaccine intolerance was documented in the allergy passport and the attending physician was informed.
Patient survey
Following the allergy test, a survey was carried out using a questionnaire [10]. The main items of the questionnaire were the following: Was a vaccination given following the testing? Which vaccine was used? Where was the vaccination given? In the case of an actual vaccination, did a vaccination reaction occur after the test and if so, which one? To what extent did the test reduce the fear of vaccination (1 = not at all, 2 = slightly, 3 = moderately, 4 = strongly, 5 = very strongly)?
Initially, contact was made by telephone between February and April 2022 in order to obtain consent for the survey by means of a questionnaire. If consent to the survey was obtained by telephone, participants were contacted by post to document their consent in writing, complete the questionnaire and send it to the study center in a prepaid envelope. All study procedures were approved by the Ethics Committee of the University of Regensburg (file number 22-2810-101).
Statistical evaluation
The results of the skin prick test and the questionnaire evaluations were documented and analyzed descriptively. Metrically scaled variables were presented with mean values and standard deviation. As some of the patients were tested with several different vaccines, only those individuals who had been tested for both vaccines in the comparison could be used for the statistical evaluation of vaccine reactivity using the McNemar test. This resulted in significantly smaller sample sizes for the comparisons of the vaccines than can be seen in the descriptive presentation. The frequency of positive allergic reactions to vaccines in the SPT was compared with the frequency of positive reactions to Comirnaty® as the standard.
Results
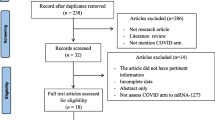
Between January 2021 and February 2022, 250 people were tested for allergic sensitization to COVID-19 vaccines by skin prick test. Of these, n = 70 were successfully included in the follow-up survey on anxiety reduction and willingness to vaccinate, as shown in the flowchart in the supplementary material. The demographic data for both the entire study population and the subgroup that participated in the survey are shown in Table 1. The patients came mainly from the greater Regensburg area (92%), were between 16 and 88 years old, 6 times more often female, and almost 95% had a history of allergy. The subpopulation of n = 70 who ultimately took part in the questionnaire survey did not differ significantly from the overall test population in terms of the parameters analyzed (Table 1, column 2).
At the beginning of testing, only the vaccines Comirnaty® and Vaxzevria® were approved and available for testing. Later, the vaccines Spikevax® and Jcovden® were also approved and were therefore available for testing. The number of tests per patient depended on the availability of the vaccines, but also on individual patient wishes: n = 31 (12.4%) people were tested for only one of the available vaccines, 160 subjects (64%) were tested for two different vaccines, a further n = 30 people (12%) were tested for 3, and n = 29 (11.6%) were tested for all 4 vaccines. 198 people did not react to any vaccine, n = 24 reacted to one, n = 18 to 2, n = 7 to 3 and n = 2 to all 4 vaccines tested. In one person, the SPT could not be assessed due to a strong histaminergic reaction and swelling of the entire forearm to the positive control. The number of all positive skin test reactions (regardless of their severity) in relation to the tests carried out for each vaccine was n = 37 (17.8%) for Vaxzevria®, n = 33 (13.5%) for Comirnaty®, n = 12 (21.4%) for Spikevax® and n = 9 (18.8%) for Jcovden®. The minimal reactions n = 45 (8.1%) were in most cases due to urticaria factitia. Late reactions also occurred in a total of 2 test subjects 12–24 h after testing. Clinically clearly positive reactions (mild, moderate, severe) occurred in 4–14% of the respective tests as shown in Table 2. Of the patients who had a history of polyvalent allergy (n = 127), 8 (6.29%) had a clearly positive reaction in the SPT to a COVID-19 vaccine, in patients who had a history of polyethylene glycol (PEG) allergy (n = 7), this was the case in 1 patient, and of the 2 patients with a history of anaphylactic reaction grade II or higher, none reacted to any of the COVID-19 vaccines tested.
With Comirnaty®, n = 17 out of 241 (6.9%) had clearly positive skin reactions, of which 3 patients (1.2%) had severe skin reactions. The vaccine Spikevax® was only tested in n = 56 subjects, but showed clearly positive skin reactions in 8 subjects (14.3%) and a severe test reaction in 2 (3.6%) of these. Vaxzevria® led to a clearly positive reaction in 9 (4.32%) of a total of 208 skin tests and to a strong test reaction in 1 person (1%). Finally, Jcovden® was tested in 48 patients, showed a clearly positive reaction in 2 (4.16%) and a strong reaction in 1 person (2.1%) in the skin test.
To statistically compare the SPT reactivity between vaccines in our study setting, it was not possible to use complete groups but only individuals, as some probands had been tested with several different vaccines. This resulted in significantly smaller comparison groups. Comirnaty® was used as the “standard” for all comparison because this vaccine had been used and tested most frequently from the beginning (Fig. 1). This subevaluation showed no significant (p < 0.05) differences in terms of allergic potential between the vaccines when compared to Comirnaty®. Of note, all subjects in the subevaluation who were tested for Comirnaty® and Spikevax® reacted to both vaccines.
Venn diagram (a) and pairwise statistical comparison using McNemar test (b) of sensitizations by skin prick test (SPT) against COVID‑19 vaccines using Comirnaty as reference (Note: Comirnaty® was used as the standard for comparison because this vaccine had been used and tested most frequently from the outset). A Comirnaty® (Biontec/Pfizer), B Spikevax® (Moderna), C Vaxzevria® (Astra Zeneca), D Jcovden® (Johnson & Johnson), neg. negative, pos. positive
In the questionnaire survey, n = 53 of 70 test subjects stated that SPT testing and counseling had led to a very strong or strong reduction in anxiety (75.7%) regarding the vaccination. In 13 people (18.5%) there was a moderate or slight reduction and in 4 patients (5.7%) there was no reduction in anxiety about the vaccination as a result of the testing and counseling (Fig. 2). Of the total of n = 70 patients surveyed, n = 62 people (88.5%) were vaccinated with one of the COVID-19 vaccines available at the time of follow-up, 11.5% were not vaccinated, half of whom had received a vaccination recommendation (Fig. 3). Overall, none of the SPT-recommended vaccinations (n = 62) resulted in a severe allergic reaction, 4 patients reported local cutaneous vaccination reactions and 2 reported short-term mild dizziness after vaccination. The gender-related evaluation shows a very similar picture in terms of both anxiety reduction and the decision to vaccinate.
Discussion
Depending on the vaccine, a clearly positive skin test reaction occurred in 4–15% of the vaccine tests in the test subjects, almost all of whom had a history of allergy. In a pairwise comparison with Comirnaty, no significant differences in sensitization to the vaccines were found in the statistically analyzable subpopulation. An SPT for COVID-19 vaccines and appropriate counseling led to a reduction in vaccination anxiety in many patients and to vaccination in an overwhelming majority of patients.
The vaccine testing was carried out due to a clinical need after media reports at the end of 2020 had caused considerable uncertainty, especially among allergy patients, regarding the tolerability of COVID-19 vaccines. Most of the 250 participants in the SPT were therefore tested at their own request, but also according to German Society for Applied Allergology (AeDA) recommendations at the time. Although we were able to reach n = 156 people by telephone who verbally consented to the survey, we ultimately only received written consent and a completed questionnaire from n = 70 participants. Presumably, a postal response with additional completion of the questionnaire was too complicated or too time-consuming for most people. In our study, as in comparable studies, a very high proportion of female participants was found [11,12,13]. One of the reasons for this may be that women are more willing to critically review health issues [14]. The reporting rate of allergic anaphylactic reactions is also higher among women [15, 16]. This study did not only include patients who were willing/ready to be vaccinated; some participants were quite critical of the COVID-19 vaccination and had not necessarily completed the test with the aim of being vaccinated.
The test solutions were unused vaccine doses and not standardized test solutions, as these were not yet available at the time of testing. Undiluted vaccine solutions were used for the SPT, but it was not formally checked whether the necessary concentration of the individual substances was reliably sufficient to detect immunological sensitization (sensitivity). On the other hand, there is still no data on the influence of storage (cooling) on the skin reaction in the SPT. It has also not yet been conclusively clarified to what extent a skin reaction is triggered in people who previously tolerated the vaccine during vaccination (specificity) [11]. However, as negative SPT and positive results were measurable and correlate very well with tolerance in the subsequent vaccination, it is unlikely that the SPT did not produce relevant results.
After the test, 88.5% of all respondents had been vaccinated at least once, and some had already been vaccinated twice or three times at the time of the survey approximately 1 year after testing. Furthermore, 75.6% reported a strong to very strong reduction in anxiety after the test. However, other factors in addition to testing and counseling could have influenced the willingness to be vaccinated and the reduction in anxiety: A growing acceptance or mandatory vaccination in some professions, further studies on the safety of COVID-19 vaccines, as well as a more balanced reporting in the media.
A more structured patient history using the criteria of the Paul Ehrlich Institute (PEI), as later published in a German study [13], might also have been sufficient to make an allergic vaccination very unlikely. However, skin prick testing is an inexpensive and simple diagnostic procedure from an economic point of view [17]. Therefore, in the pandemic situation with so many individual concerns, we thought it to be advantageous to use SPT to demonstrate with little effort that a vaccination was harmless to a specific patient. No study ever compared the two approaches (medical history according to PEI and counseling versus medical history, skin prick test and counseling as in our study) in terms of anxiety reduction and willingness to vaccinate.
Consulting alone could also have had an anxiety-reducing effect. Interestingly, a study from Hong Kong came to the conclusion that a consultation in an allergy center increased the willingness to vaccinate [18]. However, several studies conclude that allergology testing contributes significantly to anxiety reduction and increased willingness to vaccinate [12, 13].
The starting point for our analysis of the effect of allergological testing on vaccination fear and willingness to vaccinate was the testing of a relatively large number of allergy patients. Of note, there were quite different sensitization rates for vaccines by SPT. Spikevax® with 14.3% and Jcovden® with 4.2% were the extremes in our testing. However, not all vaccines were tested in all subjects and not in the same number of subjects. Therefore, these figures should definitely be viewed with caution. In the statistical comparison with Comirnaty®, no statistically significant difference in sensitization to the vaccines used could be demonstrated due to variable overlaps when individual vaccines were compared in pairwise comparisons in subjects who all had received vaccines against Comirnaty® as a reference. This comparison between vaccines was not the primary objective of this study and therefore, the study was not designed for this purpose.
In the meantime, the rate of confirmed cases of anaphylaxis for Comirnaty® has been reported at 0.2 per 100,000 vaccine doses administered, for Spikevax® at 0.08/100,000, for Vaxzevria® at approximately 0.35/100,000 and for Jcovden® at approximately 0.1/100,000 doses [9, 19]. Fortunately, anaphylaxis resulting in death in connection with COVID-19 vaccines has not yet been reported or described despite millions of vaccinations [20]. The COVID-19 vaccines therefore do not appear to have an increased risk of clinically relevant allergic reactions. The procedure described here helped to reduce the fear of vaccination among allergy patients and increase willingness to vaccinate with little effort. Programs of this kind may also be useful in future pandemics and vaccination campaigns to reduce preconceived concerns regarding negative health effects.
Abbreviations
- AeDA:
-
German Society for Applied Allergology
- MHRA:
-
Medicines and Healthcare products Regulatory Agency
- PEG:
-
Polyethylene glycol
- PEI:
-
Paul-Ehrlich-Institute
- SPT:
-
Skin prick test
References
www.stiftung-gesundheitswissen.de/wissen/covid-19-impfung/wirksamkeit-und-sicherheit. Accessed 23 Apr 2024.
Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. Am J Transplant. 2021;21:1332–7.
Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan PW. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51:861–3.
www.gov.uk/government/news/confirmation-of-guidance-to-vaccination-centres-on-managing-allergic-reactions-following-covid-19-vaccination-with-the-pfizer-biontech-vaccine. Accessed 23 Apr 2024.
Banerji A, Wickner PG, Saff R, Stone CA Jr, Robinson LB, Long AA, et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J Allergy Clin Immunol Pract. 2021;9:1423–37.
www.pei.de/DE/newsroom/positionen/covid-19-impfstoffe/stellungnahme-allergiker.html. Accessed 23 Apr 2024.
Klimek L, Jakob T. COVID-19-Impfung: Welche Rolle spielt die Angst vor allergischen Reaktionen? Allergo J. 2021;30(6):3.
Koock U. Sorgen (ernst) nehmen: Die Covid-Impfung. Heilberufe. 2021;73(2):40–1.
Mahler V, Junker AC. Anaphylaxis to additives in vaccines. Allergo J Int. 2022;31:123–36.
www.we-care.de/media/pages/home/8870682dbb-1709714375/fragebogen-zur-allergietestung.pdf. Accessed 23 Apr 2024.
Alexiou A, Irmer ML, Bauer A, Treudler R, Wurpts G. Dickel H et al. SARS-CoV‑2 und Allergie – was haben wir gelernt? AL. 2023;46:382–91.
Brockow K, Wang R, Mathes S, Bent R, Faihs V, Eberlein B, Darsow U, Biedermann T. SARS-CoV‑2 and allergy—what have we learned after two and a half years? Allergol Sel. 2023;7:101–12.
Bent R, Faihs V, Darsow U, Biedermann T, Brockow K. Organisation und Erkenntnisse einer Pilotstudie zur allergologischen Abklärung einer COVID-19-Impfstoffallergie in der Weihnachtswoche 2021. AL. 2022;45:812–22.
Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26:100495.
Barth I, Weißer K, Gaston-Tischberger D, Mahler V, Keller-Stanislawski B. Anaphylactic reactions after COVID-19 vaccination in Germany. Allergol Sel. 2023;7:90–100.
www.pei.de/DE/newsroom/hp-meldungen/2021/210409-was-bei-positiver-allergieanamnese-impfung-covid-19-beachten.html. Accessed 23 Apr 2024.
Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, et al. The skin prick test—European standards. Clin Transl Allergy. 2013;3:3.
Chiang V, Saha C, Yim J, Au EYL, Kan AKC, Hui KSH, et al. The Role of the Allergist in Coronavirus Disease 2019 Vaccine Allergy Safety: A Pilot Study on a “Hub-and-Spoke” Model for Population-Wide Allergy Service. Ann Allergy Asthma Immunol. 2022;129:308–12:e1.
www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html. Accessed 23 Apr 2024.
Acknowledgements
The authors would like to thank all study participants and the staff of the Allergy Center at the Kreisklinik Wörth an der Donau.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Study design: MK, WS, TB, Data collection: TB, WS, Statistical analysis: SB, Manuscript presentation: MK, TP.
Corresponding author
Ethics declarations
Conflict of interest
T. Pindel, S. Brandstetter, W. Sieber and M. Kabesch declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Approval was granted by the Ethics Committee of the University of Regensburg (reference 22-2810-101). Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors W. Sieber and M. Kabesch contributed equally to the manuscript.
Supplementary Information
Supplement Fig. 1:
Flowchart for patient selection
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pindel, T., Brandstetter, S., Sieber, W. et al. Allergy skin prick tests with COVID-19 vaccines and their contribution to improve vaccination readiness and reduce anxiety. Allergo J Int (2024). https://doi.org/10.1007/s40629-024-00296-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40629-024-00296-7