Abstract
Background
A perennial house dust mite-associated allergic rhinitis has a major impact on the quality of life of patients and is associated with a high socioeconomic burden. The most common symptoms of allergic rhinitis include a runny nose and nasal congestion, sneezing, itching of nose, mouth and/or throat, and/or ocular symptoms. Affected patients often develop allergic bronchial asthma. Therapy options for allergic rhinitis include allergen avoidance, symptomatic treatment, and allergen immunotherapy. Allergen immunotherapy is the only disease-modifying treatment that can permanently alleviate the symptoms of allergic rhinitis. In July 2021, a new sublingual mite tablet was approved in Germany.
Methods
This review summarizes clinical studies on the 300 IR (index of reactivity) mite tablet in adolescents and adults with house dust mite-associated allergic rhinitis and presents the results.
Results
In the phase II and phase III studies considered here, different dosages of the mite tablet were investigated. The 300 IR mite tablet showed the best benefit–risk profile and has been approved in Europe, Japan, South Korea, Australia, and New Zealand for the treatment of house dust mite-associated allergic rhinitis.
Conclusion
Allergen immunotherapy with the 300 IR mite tablet is an effective treatment that relieves allergic symptoms, reduces the need for symptomatic medication, and improves the quality of life in both adults and adolescents with house dust mite-associated allergic rhinitis. At the same time, treatment with the 300 IR mite tablet is well tolerated. Mild to moderate reactions at the application site subside after a few days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
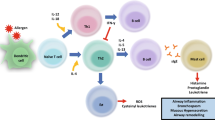
House dust mites (HDM), especially the two species Dermatophagoides pteronyssinus (D. pter.) and Dermatophagoides farinae (D. far.) are among the most common sources of indoor allergens [1,2,3]. Allergic patients react with symptoms of perennial allergic rhinitis (AR), such as nasal congestion, runny nose, sneezing, itching in the nose, mouth, or throat, or watery eyes [4]. Year-round AR has a significant impact on health-related quality of life and is associated with a high socioeconomic burden [5,6,7,8,9]. The risk of developing asthma is higher in patients with AR than in the general population, and higher in patients with HDM allergy than in patients with other inhalant allergies [10, 11].
Therapeutic options for HDM-AR include abstinence measures, symptomatic treatment, and allergen immunotherapy (AIT). We have reported on avoidance measures as a therapy elsewhere in this issue [12] and various pharmacologic therapeutic options are recommended in the literature [5,6,7, 13]. Of these, AIT is the only treatment that has a disease-modifying effect because it targets the immunologic mechanisms underlying allergic disease. It is available in the form of subcutaneous injections (SCIT) and sublingual solutions and tablets (SLIT) [5,6,7, 13,14,15].
Recently, the new sublingual mite tablet Orylmyte® (Stallergenes GmbH, 47475 Kamp-Lintfort, Deutschland; in Austria: Actair®) was approved and has been available on the market since then. This review summarizes the clinical studies of the 300 IR mite tablet in adolescents and adults with HDM-AR and presents the results of immunological investigations.
The 300 IR mite tablet
Orylmyte® was approved in 2021 as an AIT for patients 12 years of age and older with established moderate-to-severe HDM-AR [16]. The tablet, with an allergen activity of 300 IR (IR = index of reactivity), contains a standardized 1:1 mixture of freeze-dried allergen extracts obtained from bodies and feces of D. pter. and D. far.. The tablet contains a broad repertoire of major and minor allergens and thus represents the conditions of natural exposure very well [1, 17]. In particular, it contains the major allergens of group 1 (per 300 IR tablet a total of approximately 75 µg group 1 allergens: approx. 14–17 µg Der p 1 and approx. 53–68 µg Der f 1 [18]), group 2 (per 300 IR tablet a total of approx. 25 µg Der p 2 + Der f 2 [19]) and also Der p 23 [18, 20]. Der p 23 has been identified only recently as a major allergen [1, 21] which is particularly associated with the occurrence of allergic asthma [22,23,24,25].
Orylmyte® is a compressed sublingual tablet that releases allergens constantly over 2–3 min. This promotes allergen uptake by mucosal allergen-presenting cells (i.e. Langerhans cells), while reducing sudden activation of local proinflammatory cells (i.e., mast cells) [26].
Clinical development: efficacy of the 300 IR mite tablet
The 300 IR mite tablet is approved in many European countries as well as in Japan, South Korea, Australia, and New Zealand for the treatment of HDM-AR in adolescents and adults. Approval is also available in Japan, Australia, and South Korea for the treatment of children ≥ 5 years of age. The approvals are based on an extensive global clinical development program with multiple randomized, double-blind, placebo-controlled trials (RDBPC; Table 1).
Phase II study in the allergen exposure chamber
Phase I dose-escalation studies were initially conducted with doses ranging from 100 IR to 2000 IR, in which HDM tablets were well tolerated by patients with HDM-induced AR or asthma [32,33,34]. Three of these dosages were further evaluated in a phase II study in an allergen exposure chamber („environmental exposure challenge chamber“) with adults aged 18–55 years for dose finding: a total of 355 patients received daily SLIT with 100 IR, 300 IR, 500 IR, or placebo over a 6-month period, with standardized 4 h HDM allergen provocations at baseline and at 1, 2, 4, and 6 months [27]. The results showed a dose-dependent effect: while the efficacy of 100 IR was not significantly different from placebo, 300 IR and 500 IR were comparable, however with a higher dropout rate for 500 IR [27].
Phase II/III studies
Phase II/III studies in adolescents and adults were conducted for 300 IR and 500 IR doses, evaluating efficacy under natural allergen exposure in field studies.
These studies included a European phase II/III field study of 509 adults with HDM-AR aged 18–50 years [28]. 30% of patients had concomitant asthma, and 52% were polysensitized. Patients received a 300 IR or 500 IR HDM tablet or placebo once daily. Patients were treated for 1 year in seven European countries and then followed up for an additional year without treatment. The primary endpoint was the average adjusted symptom scoreFootnote 1 (AAdSS; Average Adjusted Symptom Score); secondary endpoints included symptom and medication individual scores. Treatment resulted in a comparable significant reduction in AAdSS versus placebo in patients receiving the 300 IR or 500 IR HDM tablet once daily. The effect of treatment in this study began at approximately 4 months after initiation of therapy and continued throughout the treatment period. The efficacy was maintained as a treatment-sustained effect in the treatment-free follow-up year: AAdSS continued to be significantly lower in patients treated with HDM tablet in the first year than in patients receiving placebo (Fig. 1). Efficacy results were not affected by the presence of asthma or sensitization status [28].
The 12-month treatment with the 300 IR mite tablet was effective, and the effect of the treatment was maintained in the subsequent therapy-free year [28]
In another 1‑year phase II/III field study, doses of 300 IR and 500 IR were evaluated in 968 adolescents and adults aged 12–64 years in Japan [29]. The primary endpoint here was also AAdSS. The results were comparable to those of the European study, with a significant reduction in AAdSS compared with placebo in patients treated with 300 IR and 500 IR. The difference between the two active treatment groups was not statistically significant. The onset of action in this study was evident after 8–10 weeks and persisted for the 300 IR mite tablet throughout the observation period [29].
Overall, once-daily maintenance therapy at 300 IR proved to be the therapy with the most favorable benefit–risk profile in the phase II and II/III clinical trials and was therefore chosen for further clinical development.
Global phase III study
With 1607 patients from 13 countries, the pivotal global phase III trial was the largest RDBPC study ever conducted to investigate AIT in HDM-AR [18]. The efficacy and safety of 12 months of treatment with 300 IR in adults (n = 1264) and adolescents (n = 343) with HDM-AR were investigated. Thirty-eight percent of patients had concomitant asthma, and 45% were polysensitized. The primary endpoint was the average total combined score (aTCS). Secondary efficacy endpoints included another combined symptom-medication score, symptom and medication individual scores, and quality of life.
The primary endpoint and all predefined secondary endpoints were met with statistically significant differences versus placebo. The results of important endpoints are shown in the overview (Table 2; [18]).
Regarding on-demand medication, 27% of patients treated with the 300 IR mite tablet were able to discontinue it completely, 24% were able to discontinue oral antihistamines, and 30% were able to discontinue intranasal corticosteroids [35].
AR-related quality of life, as measured by the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) score, was significantly improved overall, as well as in all 7 individual domains (activities, sleep, general discomfort, practical problems, nasal as well as ocular symptoms, and emotional well-being) in patients treated with the 300 IR mite tablet compared with placebo (Fig. 2; [18]).
A post hoc analysis of this global phase III study showed an improvement in both combined symptom-medication scores (ACSMS, average combined symptom and medication score) in the European subgroup. The ACSMS also decreased significantly by 21.7% in patients treated with 300 IR compared to placebo (p < 0.0001) [31]. Treatment effects of this magnitude are perceived by patients as a noticeable improvement in AR [31].
Clinical development: safety and tolerability of the 300 IR mite tablet
In the clinical development program studies, SLIT with the 300 IR mite tablet was well tolerated by patients with HDM-AR without or with mild asthma. In general, adverse events (AEs) were mild to moderate in intensity and occurred mostly in the first few days of treatment. Application site reactions, such as oral itching, throat irritation, or mouth swelling, were the most commonly reported reactions [36].
In the phase II study, which took place under controlled conditions in the allergen exposure chamber, AEs were reported by 90.7% of patients receiving 300 IR mite tablet and by 82.8% of patients in the placebo arm [27]. In 68.6% and 43.7% of patients, respectively, AEs were classified as adverse drug reactions (ADRs), i.e. AEs in which the association with the study medication was judged to be “possible.” Most commonly reported were application site reactions (throat irritation, oral itching, ear itching, mouth swelling). No cases of serious ADRs or anaphylaxis were reported. In addition, this study showed that AIT with 300 IR mite tablet had no adverse respiratory effects: AEs such as asthma and asthma-related symptoms (e.g., cough, dyspnea, or wheezing) occurred with comparable frequency in the 300 IR group and the placebo-treated group.
In two studies conducted in Europe [28] and Japan [29], the safety profile was comparable: the proportion of patients for whom at least one AE was documented was 88.2% in the 300 IR group and 75.5–80.0% in the placebo group. In 66.8% (300 IR) and 18.6% (placebo) of patients, AEs were potentially related to study medication [29] (in [28] corresponding rates are not given). AEs such as asthma, cough, dyspnea, and wheezing were reported with similar frequency by patients in both the active and placebo groups [28].
In the global study [18], 51.0% of patients in the 300 IR group and 14.9% in the placebo group reported at least one ADR. As expected, the most common ADRs in patients treated with 300 IR in this study were also mild to moderate local reactions at the application site, such as oral itching, throat irritation, ear itching, and mouth swelling. Adolescents and adults tolerated the treatment comparably well. The incidence and distribution of ADRs was similar in both age groups, with 49.4% of adolescents and 51.4% of adults reporting at least one ADR. Concomitant asthma did not affect the safety profile of 300 IR mite tablet.
Overall, 57% of the 1583 adults and adolescents with HDM-induced AR treated with the 300 IR mite tablet in the clinical development program reported adverse events. The safety profile in children was similar to that in adults and adolescents. Of 270 children treated with the 300 IR dosage in clinical trials, a total of 50% reported adverse events [16, 36].
Confirming the good tolerability profile observed in clinical trials are data from more than 4 years of routine post-approval use in Japan, South Korea, Australia, and New Zealand with nearly 90,000 exposed patients: The most commonly reported ADRs were local application site reactions of mild to moderate intensity [36]. This was also shown in an observational study in Japan [37].
The safety data described for the 300 IR mite tablet strengthen the existing evidence base on the beneficial safety profile of SLIT. Sublingual formulations are almost always associated with mild or moderate reactions at the application site and very rarely with severe systemic allergic reactions. This may be due to specific features associated with sublingual allergen uptake and processing that reduce the induction of proinflammatory immune responses [38, 39].
Immunomodulation by the 300 IR mite tablet
The exact mechanism of action of AIT has not been definitively determined, but it is well established that an important immunologic response to AIT is the activation of antibodies that block the allergen antibody-mediated immune response. The activated antibodies are mainly IgG and IgA antibodies, which are able to prevent the binding of IgE-allergen complexes to B cells and dendritic cells. This mechanism contributes decisively to the treatment success of AIT [40, 41].
A crucial feature in the effect of SLIT is the oral uptake of the allergen via the oral mucosa. It has already been shown that ingestion of HDM allergen extracts by SLIT has an effect on both humoral and cellular immune responses, resulting in tolerance to the allergen and subsequently improvement of allergic symptoms [2, 6, 14, 39, 42].
In the European phase II/III study [28]. HDM-specific IgG4 titers (for Der p 1, Der p 2, Der f 1, and Der f 2) increased 2‑ to 4‑fold in patients treated with the 300 IR mite tablet, an effect not observed in the placebo group [19]. Of note, treatment was not associated with a significant risk of IgE new sensitization to allergens contained in the tablet or used in the mite culture medium [19].
An up to 4‑fold increase in D. pter.- and D. far.-specific IgG4 titers was also observed in the global phase III study [18]. In a more extensive analysis, humoral responses to a large group of HDM allergens (focus: D. pter.) were specifically studied in a subgroup of patients (300 IR mite tablet versus placebo) [20]: Der p 1, Der p 2 and Der p 23 were the mite allergens with the highest sensitization rates (83, 92, and 82% of patients, respectively). In particular, after one year of therapy, an increase in Der p 1, Der p 2 and Der p 23-specific IgG and IgG4 was observed in patients treated with the 300 IR tablet. The increase in Der p 23-specific IgG antibodies by therapy demonstrates the sufficient content of biologically active Der p 23 in the tablet to elicit an immune response.
Until now, especially allergens of groups 1 and 2 (Der p 1/Der f 1 and Der p 2/Der f 2) were considered as the HDM allergens with the greatest clinical relevance. In contrast, the allergen Der p 23 has only in recent years been identified as a major allergen that is strongly associated with asthma compared to other allergens [22,23,24,25]. Parental AR and early contact with D. pter. allergens promote IgE polysensitization to various D. pter. molecules, which in turn predicts the risk for mite-induced AR and for current or future asthma [25]. Thus, the strong IgG responses to Der p 23 may be of particular benefit to appropriately sensitized or allergic patients [20].
The 300 IR mite tablet in practice
Evidence from the extensive clinical development program: In patients with confirmed HDM allergy, AIT with the 300 IR mite tablet alleviated AR symptoms, with onset of action approximately 2 months after treatment initiation [29]. The decrease in symptoms was maintained during the 1‑year AIT treatment period as well as during the remainder of the AIT-free year [28]. Although only clinical trial data for 12 months of treatment are available to date, daily, year-round therapy for 3 years is recommended according to the treatment guidelines to achieve ongoing treatment effects [16]. When treated with the 300 IR mite tablet, patients should benefit from a noticeable improvement in AR. On the ACSMS scale of 0 to 6, each of the four nasal symptoms considered scored a maximum severity of 0.75 points. A change of 0.25 points corresponds to a decrease of one symptom by one severity level, which also corresponds to the minimum clinically relevant benefit [43]. A model calculation based on an ANCOVA analysis shows what the decrease in a symptom, such as nasal congestion, by one severity level actually means [31]. This is based on the efficacy results of the global pivotal study [18]. Thus, hypothetically, a patient with average-severe HDM-AR (ACSMS = 1.45) will experience no more days of moderate or severe nasal congestion with the 300 IR mite tablet therapy over 1 year vs. 102 and 110 days, respectively, with placeboFootnote 2 (Fig. 3; [31]).
Treatment with 300 IR mite tablet is generally well tolerated, with mild to moderate reactions at the application site being the most commonly reported side effects. These occur quite predominantly in the first few days to about a week after the start of treatment and usually subside after a few days.
The first 300 IR mite tablet is taken under medical supervision, after which therapy can be safely and conveniently administered at home [16]. Thus, AIT with the 300 IR mite tablet is an effective and well-tolerated therapy that relieves allergic symptoms, reduces the need for symptomatic medication, and improves quality of life in adults and adolescents with HDM-induced AR, regardless of sensitization status or concomitant mild asthma.
Observational studies will provide further results on the efficacy and tolerability of 300 IR mite tablet therapy in clinical practice in Europe.
Notes
A symptom score adjusted for the use of on-demand medication.
Assumption: patient with ACSMS0–6 = 1.45. No change in other symptoms: itchy nose, sneezing, runny nose, and taking on-demand medication.
Abbreviations
- AAdSS:
-
average adjusted symptom score
- ADR:
-
adverse drug reaction
- AE:
-
adverse event
- AIT:
-
allergen immunotherapy
- AR:
-
allergic rhinitis
- ATCS:
-
average total combined score
- ARMS:
-
average rescue medication score
- ARCTSS:
-
average rhinoconjunctivitis rotal symptom score
- D. far.:
-
Dermatophagoides farinae
- D. pter.:
-
Dermatophagoides pteronyssinus
- EEC:
-
environmental exposure challenge chamber
- HDM:
-
house dust mite
- IR:
-
index of reactivity
- RDBPC:
-
randomized, double-blind, placebo-controlled
- RQLQ:
-
Rhinoconjunctivitis Quality of Life Questionnaire
- SCIT:
-
subcutaneous immunotherapy
- SLIT:
-
sublingual immunotherapy
References
Batard T, Baron-Bodo V, Martelet A, Le Mignon M, Lemoine P, Jain K, et al. Patterns of IgE sensitization in house dust mite-allergic patients: implications for allergen immunotherapy. Allergy. 2016;71:220–9.
Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, et al. Respiratory allergy caused by house dust mites: What do we really know? J Allergy Clin Immunol. 2015;136:38–48.
Moingeon P. Progress in the development of specific immunotherapies for house dust mite allergies. Expert Rev Vaccines. 2014;13:1463–73.
Aggarwal P, Senthilkumaran S. Dust mite allergy. Treasure Island: StatPearls; 2022.
Braido F, Arcadipane F, Marugo F, Hayashi M, Pawankar R. Allergic rhinitis: current options and future perspectives. Curr Opin Allergy Clin Immunol. 2014;14:168–76.
Brehler R, Klimek L, Kopp MV, Virchow JC. Specific immunotherapy-indications and mode of action. Dtsch Arztebl Int. 2013;110:148–58.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76.
Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106:S12–S6.
Zuberbier T, Lötvall J, Simoens S, Subramanian SV, Church MK. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69:1275–9.
Linneberg A, Henrik Nielsen N, Frølund L, Madsen F, Dirksen A, Jørgensen T. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen allergy study. Allergy. 2002;57:1048–52.
Shaaban R, Zureik M, Soussan D, Neukirch C, Heinrich J, Sunyer J, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372:1049–57.
Klimek L, Bergmann K‑C, Casper I, Klimek F, Hagemann J, Cuevas M. Karenzmaßnahmen bei Milbenallergie – ein Update. Allergo J Int. 2022.
Calderón MA, Kleine-Tebbe J, Linneberg A, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, et al. House dust mite respiratory allergy: an overview of current therapeutic strategies. J Allergy Clin Immunol Pract. 2015;3:843–55.
Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American academy of allergy, asthma & immunology/European academy of allergy and clinical immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–1296.e3.
Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6.
Stallergenes. Orylmyte-Fachinformation. 2021. https://www.stallergenesgreer.de/sites/default/files/documents/1714_Fachinfo_Orylmyte_11.2021.pdf. Accessed 13 Oct 2022.
Batard T, Hrabina A, Bi XZ, Chabre H, Lemoine P, Couret M‑N, et al. Production and proteomic characterization of pharmaceutical-grade Dermatophagoides pteronyssinus and Dermatophagoides farinae extracts for allergy vaccines. Int Arch Allergy Immunol. 2006;140:295–305.
Demoly P, Corren J, Creticos P, De Blay F, Gevaert P, Hellings P, et al. A 300 IR sublingual tablet is an effective, safe treatment for house dust mite-induced allergic rhinitis: An international, double-blind, placebo-controlled, randomized phase III clinical trial. J Allergy Clin Immunol. 2020;147:1020–1030.e10.
Baron-Bodo V, Batard T, Nguyen H, Fréreux M, Horiot S, Harwanegg C, et al. Absence of IgE neosensitization in house dust mite allergic patients following sublingual immunotherapy. Clin Exp Allergy. 2012;42:1510–8.
Potapova E, Bordas-Le Floch V, Schlederer T, Vrtala S, Huang H‑J, Canonica GW, et al. Molecular reactivity profiling upon immunotherapy with a 300 IR sublingual house dust mite tablet reveals marked humoral changes towards major allergens. Allergy. 2022;77:3084–95.
Weghofer M, Grote M, Resch Y, Casset A, Kneidinger M, Kopec J, et al. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J Immunol. 2013;190:3059–67.
Celi G, Brusca I, Scala E, Villalta D, Pastorello E, Farioli L, et al. House dust mite allergy in Italy-Diagnostic and clinical relevance of Der p 23 (and of minor allergens): A real-life, multicenter study. Allergy. 2019;74:1787–9.
Jiménez-Feijoo R, Pascal M, Moya R, Riggioni C, Domínguez O, Lózano J, et al. Molecular diagnosis in house dust mite-allergic patients suggests that der p 23 is clinically relevant in asthmatic children. J Investig Allergol Clin Immunol. 2019;30:127–32.
Posa D, Hofmaier S, Arasi S, Matricardi PM. Natural evolution of IgE responses to mite allergens and relationship to progression of allergic disease: a review. Curr Allergy Asthma Rep. 2017;17:28.
Posa D, Perna S, Resch Y, Lupinek C, Panetta V, Hofmaier S, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2016;139:541–549.e8.
Mascarell L, Batard T, Cuiné JF, Nony E. The bioavailability of allergens in allergy tablets depends on several factors. Int Arch Allergy Immunol. 2018;175:252–3.
Roux M, Devillier P, Yang WH, Montagut A, Abiteboul K, Viatte A, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts: Results of a dose-ranging study in an environmental exposure chamber. J Allergy Clin Immunol. 2016;138:451–458.e5.
Bergmann K‑C, Demoly P, Worm M, Fokkens WJ, Carrillo T, Tabar AI, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–1614.e6.
Okamoto Y, Fujieda S, Okano M, Yoshida Y, Kakudo S, Masuyama K. House dust mite sublingual tablet is effective and safe in patients with allergic rhinitis. Allergy. 2016;72:435–43.
Okamoto Y, Fujieda S, Okano M, Hida H, Kakudo S, Masuyama K. Efficacy of house dust mite sublingual tablet in the treatment of allergic rhinoconjunctivitis: A randomized trial in a pediatric population. Pediatr Allergy Immunol. 2018;30:66–73.
Pfaar O, Kleine-Tebbe J, Demoly P, Bahbah F. Clinical relevance of treatment with 300 IR house dust mite SLIT tablet. [abstract P1.10]. Allergo J Int. 2021;30:215.
Demoly P, Meziane L, LeGall M, André C, Melac M. Safety and tolerability of house dust mite tablets in sublingual immunotherapy. J Allergy Clin Immunol. 2008;121:128.
Demoly P, Le Gall M, Roux M, Zeldin RK. Safety and tolerability of high doses of sublingual tablet of house dust mite allergen extracts in subjects with house dust mite-associated respiratory allergy. Allergy. 2015;70(Suppl.101):452
Roux M, Patel P, Viatte A, Cognet-Sicé J, Zeldin RK. Safety of high doses of sub-lingual tablets of house dust mite allergen extracts in adolescents with allergic rhinitis. Allergy. 2014;69(Suppl.99):613
Pfaar O, Demoly P, Creticos P, De Blay F, Gevaert PGEU, Karagiannis E, et al. Reduction in rescue medication use in patients treated with the 300 IR house dust mite SLIT tablet. 2020. [abstract no. 1866]. Allergy 2020;75(Suppl. 109):102–3.
Worm M, Bergmann K-C, Daghildjian K, Yan K. Safety review of 300 IR house dust mite tablet from pooled data of clinical trials and post marketing experience [abstract no. 1869]. Allergy 2020;75(Suppl.109):103
Okamoto Y, Ishii K, Kato M, Hayashi H, Hata T. Safety and effectiveness of the 300 IR sublingual house dust mite allergen immunotherapy tablet: 2‑year interim analysis of a specified drug-use survey. Immunotherapy. 2021;13:1333–43.
Calderón MA, Simons FER, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67:302–11.
Moingeon P. Update on immune mechanisms associated with sublingual immunotherapy: practical implications for the clinician. J Allergy Clin Immunol Pract. 2013;1:228–41.
Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27.
Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–91.
Fujita H, Soyka MB, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. Clin Transl Allergy. 2012;2:2.
Devillier P, Brüning H, Bergmann K‑C. Determination of the minimally important difference in a nasal symptom score in house dust mite allergy. Allergy. 2019;74:2191–8.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L. Klimek reports grants and/or honoraria from Allergopharma, MEDA/Mylan, HAL Allergie, ALK Abelló, LETI Pharma, Stallergenes, Quintiles, Sanofi, grants from ASIT Biotech, Lofarma, Allergy Therapeutics, AstraZeneca, GSK, Inmunotk, Cassella med, outside the submitted work; and memberships with: AeDA, DGHNO, German Academy of Allergology and Clinical Immunology, ENT-BV, GPA and EAACI. R. Brehler reports honoraria from ALK, Allergopharma, Bencard, HAL, Leti, Stallergenes, Astra Zeneca, GSK, MedUpdate, Novertis, BiotechTools, Genentech and Circassia, outside the submitted work. M. Cuevas received personal fees and/or nonfinancial support from Novartis, Sanovi-Aventis, Allergopharma, HAL Allergie, Leti Pharma, AstraZeneca, GlaxoSmithKline, ALK Abelló, Bencard Allergie, Stallergenes, and Roxall outside of the submitted work and membership in the following organizations: AeDA, DGHNO. K.-C. Bergmann has received honoraria from Allergopharma, HAL Allergie, ALK Abelló, LETI Pharma, Stallergenes, Sanofi, Lofarma, Novartis, Bencard Allergy Therapeut., AstraZeneca and GSK in the last two years, outside the submitted work. I. Casper, F. Klimek and J. Hagemann declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klimek, L., Brehler, R., Casper, I. et al. Allergen immunotherapy in house dust mite-associated allergic rhinitis: efficacy of the 300 IR mite tablet. Allergo J Int 32, 10–17 (2023). https://doi.org/10.1007/s40629-022-00241-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-022-00241-6