Summary
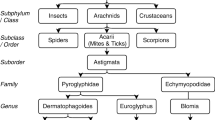
House dust mites are among the most important allergy triggers worldwide. While mites of the genus Dermatophagoides occur almost worldwide, the tropical mite Blomia tropicalis and storage mites are only of importance for certain areas or groups of people. The most important allergens of Dermatophagoides pteronyssinus are Der p 1, Der p 2, and Der p 23 with immunoglobulin E (IgE)-binding frequencies of more than 70% and high allergenic activity. Also of importance are Der p 5, Der p 7, and Der p 21, which have IgE-binding frequencies of about 30%. According to the current state of knowledge, these six allergens are the allergens of clinical relevance which are also required for diagnosis and immunotherapy with individual components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
House dust mites (HDM) are one of the most important allergen sources in the home and are widespread worldwide except in very cold and dry regions [1]. In HDM-populated areas, more than 50% of allergic patients are sensitized to HDM and it is estimated that approximately 65–130 million people worldwide suffer from HDM allergy [2, 3]. HDM allergic patients show symptoms such as allergic rhinitis or atopic dermatitis, and untreated HDM allergy is also a major risk factor for the development of asthma [4]. The HDM species Dermatophagoides pteronyssinus and D. farinae are considered the most important triggers of HDM allergy worldwide and are highly homologous and cross-reactive [5, 6]. In tropical and subtropical countries, the tropical mite Blomia tropicalis is predominant, but the two Dermatophagoides species are also found there [7]. Although Blomia tropicalis and Dermatophagoides contain homologous allergens, little IgE cross-reactivity is shown between the two species [8, 9]. Therefore, in regions colonized by Blomia tropicalis, allergic patients often suffer from two different mite allergies, which are difficult to differentiate diagnostically and need to be treated differently. In addition, in very humid housing conditions, storage mites of the species Lepidoglyphus destructor, Tyrophagus putrescentiae, and Glycyphagus domesticus are often found in house dust, but storage mites are predominantly considered a hazard of some occupational groups (e.g., farmers, bakers), where they represent an important risk factor for the development of occupational asthma [10,11,12]. While storage mites are cross-reactive with each other, IgE cross-reactivity between storage mites and HDM is very low [13]. Therefore, an accurate diagnosis is required to identify the allergenic mite species and to target therapy to patients. Mite extracts are often not suitable for making an accurate diagnosis because they cannot distinguish between co-sensitization and cross-sensitization. In addition, mite extracts are difficult to standardize, have different allergen contents, and sometimes important allergens are missing from the extracts, which means that not all mite allergic patients can be diagnosed with the available extracts [14]. Therefore, it is important to produce the single allergens of the mites, either in the form of natural allergens purified from the mites or in the form of recombinant allergens produced in prokaryotic or eukaryotic expression systems. With the help of these single allergens, it is possible to determine the exact IgE sensitization pattern of a patient and to distinguish between co-sensitizations and cross-sensitizations. This also allows the success of an immunotherapy with mite extracts to be estimated in advance.
The allergens of HDM and their significance
Currently, 39 allergens of the house dust mite genus Dermatophagoides have already been recognized by the Allergen Nomenclature Subcommittee of the World Health Organization (WHO) and the International Union of Immunological Societies (IUIS) [15]. Depending on the mite species (Dermatophagoides pteronyssinus or Dermatophagoides farinae), allergens are designated Der p or Der f. Allergens are numbered (1–39) according to their chronological discovery or homology to previously identified allergens in other mite species (Table 1).
Allergens with high relevance
Group 1, 2 and 23 allergens of HDM are called major allergens because they have high IgE binding frequency and high allergenic activity [16, 17]. Der p 1 is a cysteine protease with a molecular weight of 24 kDa, which is produced as a proform and becomes biologically active by posttranslational cleavage of the propeptide [18]. The protease activity of Der p 1 can cut the transmembrane molecules occludin and claudin, destroying the barrier of the bronchial epithelium [19]. Group 1 allergens are found in large amounts in house dust and about 80–90% of all mite allergy sufferers are sensitized to group 1 allergens [16, 20]. Group 2 allergens contain an MD-2-related lipid recognition domain that can bind lipopolysaccharides (LPS), allowing them to activate Toll-like receptor4 (TLR4) [21]. Der p 2 has a molecular weight of 14 kDa, is localized in the intestine and feces of mites, and is recognized by 80–100% of mite allergic individuals [16, 22]. Der p 23 is a peritrophin-like protein that contains a chitin-binding domain in the sequence but does not bind chitin [17, 23]. Approximately 70–80% of HDM-allergic patients are sensitized to Der p 23, which has a molecular weight of 8 kDa [17]. All three major allergens (Der p 1, Der p 2, Der p 23) cause severe allergic symptoms and are, thus, of great clinical significance [24]. Moreover, it has been shown that childhood sensitization to HDM starts with the development of IgE antibodies to these three allergens [25].
Allergens with medium relevance
The group 5, 7 and 21 allergens of HDM are referred to as allergens of medium importance. All three have an IgE-binding frequency of about 30% and are thus not major allergens, but they have high allergenic activity [24]. Der p 5 and Der p 21 are proteins with a molecular weight of 15 kDa whose biological function is still largely unknown. Sequence homologies exist between the two allergens but no cross-reactivity; thus, they are two independent allergens [26]. Der p 7 has a molecular weight of 22 kDa and shows structural similarities with LPS-binding proteins [27]. Divergent data exist for Der p 4, which is recognized by 20–30% of HDM-allergic patients in some studies, but weakly binds IgE in most populations [25, 28]. The divergent data for Der p 4 may be due to cross-reactivity, but this has only been partially explored [29].
Allergens with low relevance
According to the current state of knowledge, the other allergens of HDM are only of minor importance or are only relevant for certain patient groups. Der p 11, the paramyosin of the mite, is of minor relevance for patients with respiratory symptoms, whereas for patients with atopic dermatitis, it is a major allergen with an IgE-binding frequency of more than 50% [30]. It is suggested that in patients with atopic dermatitis this allergen, as well as others present only in the mite body but not in the mite feces, may lead to sensitization via the skin [30]. For two other allergens (Der p 20, Der p 37), it has been shown that patients who react to these allergens suffer more frequently from asthma [31, 32]. Der p 10, mite tropomyosin, is recognized by only about 10% of mite allergic patients, but shows high sequence homology to tropomyosins of other invertebrates (e.g., shellfish, cockroaches, parasites, and mosquitoes) [33]. Therefore, a high cross-reactivity between tropomyosins from invertebrates can be found. Tropomyosin is the major allergen in crustaceans, where it can cause severe symptoms in allergic individuals [34].
The allergens of the storage mites and the tropical mite Blomia tropicalis
Far less well studied than HDM of the genus Dermatophagoides are the allergens of storage mites and Blomia tropicalis. Although a number of allergens have already been described in these species, little is known about their clinical significance (Tables 1 and 3). The described allergens show homologies to the corresponding groups of HDM allergens, but little cross-reactivity [8]. As with HDM, group 2 allergen is a major allergen in storage mites [35]. Interestingly, Blo t 5 and Blo t 21 have been described as the major allergens in Blomia tropicalis, but little is known about the importance of Blo t 1 and Blo t 2, and a group 23 allergen of Blomia tropicalis has not yet been described [36].
Abbreviations
- HDM:
-
House dust mites
- IgE:
-
Immunoglobulin E
- IUIS:
-
International Union of Immunological Societies
- LPS:
-
Lipopolysaccharides
- MW:
-
Molecular weight
- TLR4:
-
Toll-like receptor 4
- WHO:
-
World Health Organization
References
Arlian LG. Water balance and humidity requirements of house dust mites. Exp Appl Acarol. 1992;16:15–35.
Sánchez-Borges M, Fernandez-Caldas E, Thomas WR, Chapman MD, Lee BW, Caraballo L, et al. International consensus (ICON) on: clinical consequences of mite hypersensitivity, a global problem. World Allergy Organ J. 2017;10:14.
González-Pérez R, Pineda F, Poza-Guedes P, Castillo M, Matheu V, Sánchez-Machín I. Molecular allergen profiling of dual mite sensitization in severe allergic rhinitis. J Investig Allergol Clin Immunol. 2020;30(6):421–9.
Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22.
Yasueda H, Mita H, Yui Y, Shida T. Comparative analysis of physicochemical and immunochemical properties of the two major allergens from Dermatophagoides pteronyssinus and the corresponding allergens from Dermatophagoides farinae. Int Arch Allergy Appl Immunol. 1989;88:402–7.
Heymann PW, Chapman MD, Aalberse RC, Fox JW, Platts-Mills TA. Antigenic and structural analysis of group II allergens (Der f II and Der p II) from house dust mites (Dermatophagoides spp). J Allergy Clin Immunol. 1989;83:1055–67.
Chew FT, Zhang L, Ho TM, Lee BW. House dust mite fauna of tropical Singapore. Clin Exp Allergy. 1999;29:201–6.
Cheong N, Soon SC, Ramos JD, Kuo IC, Kolatkar PR, Lee BW, et al. Lack of human IgE cross-reactivity between mite allergens Blo t 1 and Der p 1. Allergy. 2003;58:912–20.
Meno KH, Kastrup JS, Kuo IC, Chua KY, Gajhede M. The structure of the mite allergen Blo t 1 explains the limited antibody cross-reactivity to Der p 1. Allergy. 2017;72:665–70.
Iversen M, Dahl R. Allergy to storage mites in asthmatic patients and its relation to damp housing conditions. Allergy. 1990;45:81–5.
Iversen M, Korsgaard J, Hallas T, Dahl R. Mite allergy and exposure to storage mites and house dust mites in farmers. Clin Exp Allergy. 1990;20:211–9.
van Hage-Hamsten M, Johansson E. Clinical and immunologic aspects of storage mite allergy. Allergy. 1998;53(48 Suppl):49–53.
Zhang C, Li J, Lai X, Zheng Y, Gjesing B, Spangfort MD, Zhong N. House dust mite and storage mite IgE reactivity in allergic patients from Guangzhou, China. Asian Pac J Allergy Immunol. 2012;30:294–300.
Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, Thomas WR, Alessandri C, Chen KW, de Blay F, Valenta R, Vrtala S. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol. 2012;159:253–62.
Chapman MD, Pomés A, Breiteneder H, Ferreira F. Nomenclature and structural biology of allergens. J Allergy Clin Immunol. 2007;119:414–20.
Thomas WR, Smith WA, Hales BJ, Mills KL, O’Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002;129:1–18.
Weghofer M, Grote M, Resch Y, Casset A, Kneidinger M, Kopec J, Thomas WR, Fernández-Caldas E, Kabesch M, Ferrara R, Mari A, Purohit A, Pauli G, Horak F, Keller W, Valent P, Valenta R, Vrtala S. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J Immunol. 2013;190:3059–67.
Lienard D, Dinh OT, van Oort E, Van Overtvelt L, Bonneau C, Wambre E, Bardor M, Cosette P, Didier-Laurent A, de Borne FD, Delon R, van Ree R, Moingeon P, Faye L, Gomord V. Suspension-cultured BY‑2 tobacco cells produce and mature immunologically active house dust mite allergens. Plant Biotechnol J. 2007;5:93–108.
Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–33.
Wahn U, Lau S, Bergmann R, Kulig M, Forster J, Bergmann K, Bauer CP, Guggenmoos-Holzmann I. Indoor allergen exposure is a risk factor for sensitization during the first three years of life. J Allergy Clin Immunol. 1997;99:763–9.
Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–8.
Park GM, Lee SM, Lee IY, Ree HI, Kim KS, Hong CS, et al. Localization of a major allergen, Der p 2, in the gut and faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2000;30(9):1293–7.
Soh WT, Le Mignon M, Suratannon N, Satitsuksanoa P, Chatchatee P, Wongpiyaboron J, et al. The house dust mite major allergen der p 23 displays O‑glycan-independent IgE reactivities but no chitin-binding activity. Int Arch Allergy Immunol. 2015;168(3):150–60.
Huang HJ, Resch-Marat Y, Rodriguez-Dominguez A, Chen KW, Kiss R, Zieglmayer P, et al. Underestimation of house dust mite-specific IgE with extract-based ImmunoCAPs compared with molecular ImmunoCAPs. J Allergy Clin Immunol. 2018;142(5):1656–1659.e9.
Posa D, Perna S, Resch Y, Lupinek C, Panetta V, Hofmaier S, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139(2):541–549.e8.
Weghofer M, Dall’Antonia Y, Grote M, Stocklinger A, Kneidinger M, Balic N, et al. Characterization of Der p 21, a new important allergen derived from the gut of house dust mites. Allergy. 2008;63(6):758–67.
Mueller GA, Edwards LL, Aloor JJ, Fessler MB, Glesner J, Pomes A, et al. The structure of the dust mite allergen Der p 7 reveals similarities to innate immune proteins. J Allergy Clin Immunol. 2010;125(4):909–991.e4.
Chen KW, Zieglmayer P, Zieglmayer R, Lemell P, Horak F, Bunu CP, et al. Selection of house dust mite-allergic patients by molecular diagnosis may enhance success of specific immunotherapy. J Allergy Clin Immunol. 2019;143(3):1248–1252.e12.
Walton SF, Slender A, Pizutto S, Mounsey KE, Opresecu F, Thomas WR, et al. Analysis of IgE binding patterns to house dust mite allergens in scabies-endemic communities: insights for both diseases. Clin Exp Allergy. 2015;45(12):1868–72.
Banerjee S, Resch Y, Chen KW, Swoboda I, Focke-Tejkl M, Blatt K, et al. Der p 11 is a major allergen for house dust mite-allergic patients suffering from atopic dermatitis. J Invest Dermatol. 2015;135(1):102–9.
Sarzsinszky E, Lupinek C, Vrtala S, Huang HJ, Hofer G, Keller W, et al. Expression in Escherichia coli and purification of folded Dder p 20, the arginine kinase from Dermatophagoides pteronyssinus: a possible biomarker for allergic asthma. Allergy Asthma Immunol Res. 2021;13(1):154–63.
Huang HJ, Resch-Marat Y, Casset A, Weghofer M, Zieglmayer P, Zieglmayer R, et al. IgE recognition of the house dust mite allergen Der p 37 is associated with asthma. J Allergy Clin Immunol. 2021;149(3):1031–43.
Papia F, Bellia C, Uasuf CG. Tropomyosin: a panallergen that causes a worldwide allergic problem. Allergy Asthma Proc. 2021;42(5):e145–e51.
Reese G, Ayuso R, Lehrer SB. Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol. 1999;119(4):247–58.
Olsson S, van Hage-Hamsten M. Allergens from house dust and storage mites: similarities and differences, with emphasis on the storage mite Lepidoglyphus destructor. Clin Exp Allergy. 2000;30(7):912–9.
dos Anjos Carvalho K, de Melo-Neto OP, Magalhães FB, Ponte JC, Felipe FA, dos Santos MC, et al. Blomia tropicalis Blo t 5 and Blo t 21 recombinant allergens might confer higher specificity to serodiagnostic assays than whole mite extract. BMC Immunol. 2013;14:11.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Vrtala declares that she has no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vrtala, S. Allergens from house dust and storage mites. Allergo J Int 31, 267–271 (2022). https://doi.org/10.1007/s40629-022-00226-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-022-00226-5