Abstract
Background
The human immune system is confronted daily with a large, chemically-varied range of potentially sensitising substances. Skin sensitising substances are found, above all, in a plethora of consumer products, e. g. cosmetics, jewellery, earrings, toys, textiles, leather, other everyday commodities and, in some cases, also tattoos. These products may contain sensitisers such as fragrances, preservatives, dyes, or other additives. To provide a greater degree of consumer protection, there is a need for specific legal regulation and risk assessment, which covers each possible human exposure to a sensitising substance or mixture. This review article describes the background and pathway towards the development and implementation of an international legal framework for the classification and labelling of chemicals that contain potentially skin sensitising substances. This includes the implementation of the globally harmonized system of classification and labelling of chemicals (GHS), the classification, labelling and packaging (CLP) regulation, registration, evaluation, authorisation and restriction of chemicals (REACH), and the regulation of cosmetics, among other national laws and regulations. Assessment criteria for classification is derived from a suite of in vitro and in vivo assays, in addition to in silico approaches—validated by the organisation for economic cooperation and development (OECD)—as well as data derived from human studies.
Results
New legislation for chemical and product safety is reflected in the classification and labelling of skin sensitising substances under Category 1, Subcategory 1A or 1B, within which the threshold concentrations of several materials are regulated, e. g. p‑phenylenediamine in hair dyes, nickel in piercings, chromium VI in leather and methylisothizolinone in cosmetics. In order to minimise the risk of human contact allergy from consumer products, the scientific committee on consumer safety (SCCS) and the German federal institute for risk assessment (BfR) investigate pathways of exposure and perform risk assessments using new in vitro approaches and new (immuno-) toxicological concepts (i. e. adverse outcome pathways [AOPs], key events as well as an integrated approach to testing and assessment [IATA]). In comparison to cosmetics, substances in textiles and other consumer products are less regulated. Major efforts in research and development are necessary to decode complex substance-specific molecular mechanisms in allergic responses and to define new substance-specific thresholds. Such efforts have been continuously proposed by the BfR with regard to fragrances for over 10 years.
Conclusions
Today, skin sensitising substances can be legally regulated and labelled and, depending on the exposure, their content in consumer products can be reduced or eliminated. Furthermore, the risk assessment of potentially sensitising substances makes consumer products safer. Further improvements in research approaches are required in the area of health and consumer protection with regard to allergy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The more than 4000 known human contact allergens include numerous substances that are used in consumer products. These allergens pose a risk of triggering an allergic disease response if contact is made with the skin of individuals that have been previously sensitised to the substance in question. In addition, the risk of possible sensitisation of individuals that have not yet been sensitised to a certain allergy-inducing substance also exists. This applies to sensitising substances in foods, as well as to airborne indoor and outdoor sensitisers. In order to protect affected consumers from these health risks, it is necessary to identify, characterise and categorise substances and chemicals that may cause an allergic response. If need be, health warnings and substance labelling should be addressed and/or the use or improper use of a substance should be restricted or prohibited through appropriate regulation. At the same time, the challenge in a world made up of a global network of product exchange is to produce goods under the same harmonised safety standards and exchange them between the various trading partners under conditions that are as safe to human health as possible. This requires internationally accepted rules, such as the globally harmonized system of classification and labelling of chemicals (GHS) developed by the United Nations (UN), the related European regulation on classification, labelling and packaging (CLP) of substances and mixtures, which came into force on 20.01.2009 (EC No. 1272/2008; CLP 2008. For bibliography see electronic supplementary material.), the registration, evaluation, authorisation and restriction of chemicals regulation (REACH , in force since 01.06.2007; EC No. 1907/2006; REACH 2006. For bibliography see electronic supplementary material.), and the establishment of internationally accepted test guidelines e. g. developed by the Organisation for Economic Cooperation and Development (OECD). In addition, where necessary, some form of nationally coordinated follow-up legislation should be implemented. This will contribute jointly towards minimising the exposure risk to consumers from products containing a potentially sensitising substance. In this study, risk assessment and regulatory aspects of some contact sensitisers derived from consumer products will be discussed.
Heath protection from toxic and sensitising substances
The United Nations and GHS
The results of two UN conferences, the UN Conference on Environment and Development in Rio de Janeiro (“Rio 92” Earth Summit, 3–14 June 1992; UNCED 1992. For bibliography see electronic supplementary material.) and the World Summit on Sustainable Development in Johannesburg (26 August–4 September 2002; UN 2002. For bibliography see electronic supplementary material.), made a decisive contribution towards ensuring that all aspects of environment and human health, as well as sustainable development, attracted increased global attention, thus setting the development of the globally harmonized system of classification and labelling of chemicals (GHS) in motion. It was not only the increasing contamination of the oceans with synthetic chemicals and the dumping of toxic waste that were addressed at these meetings (Principle 17, The Rio Declaration on Environment and Development 1992; UNCED 1992), but also the development of an international regime for the safe handling of toxic chemicals. During this process, it was decided that the categorisation of toxic chemicals in the participating countries, in line with the same criteria, would be implemented in a process known as harmonisation; it was further decided to develop and apply hazard pictograms to these categories which would be valid across national boundaries (Principle 19; UNCED 1992). Corresponding mandates for action for the international community were published in the action plan known as Agenda 21. The “Environmentally Sound Management of Toxic Chemicals” is also dealt with in Chap. 19 (Agenda 21 1992. For bibliography see electronic supplementary material.), but it was to take over 10 years until the GHS, also known as the “purple book”, was published for the first time in 2003 (GHS2017, 7th edition. For bibliography see electronic supplementary material.). Substances with a potentially respiratory or cutaneous sensitising effect are also to be uniformly categorised and labelled herein as part of a global system. Generally speaking, categorisation in line with the GHS also includes the process of an evaluation of which hazards (damaging effects) may arise from a substance or mixture. A distinction is made between physical hazards, health hazards and environmental hazards. The type of hazard in question is specified by hazard classes, which are subdivided into hazard categories if necessary, which in turn express the severity of the hazard. The GHS comprises 16 hazard classes for physical/chemical hazards, 10 for health hazards and two for environmental hazards. Categorisation stages and the labelling elements to be used are prescribed for each hazard class (UBA 2014. For bibliography see electronic supplementary material.). However, since the GHS categorisations developed by the UN are not directly legally valid in each country, they have to be integrated into the legislation of individual states or communities of states.
European chemicals classification in accordance with the CLP regulation
With the goal to ensure a high level of protection for human health, the environment and trade with substances, mixtures and products, the GHS was implemented in the European Union (EU) through the adoption of the classification, labelling and packaging (CLP) regulation (EC No. 1272/2008; CLP 2008). Some regulatory deadlines, such as those for mixtures, took until 1 June 2015, roughly 23 years after Rio 92, to be agreed upon. The previously valid European classification and labelling system was also considered. The CLP regulation was and still is continuously adapted to reflect the latest findings via appropriate modifications (adaptations to the technical progress, ATPs). It is aimed in particular at manufacturers, importers, suppliers and users that are required to implement the regulation, and relates, in a complimentary fashion, to the REACH regulation, e. g. by making reference to the corresponding safety data sheets under REACH, or also by articulating the non-effectiveness of the CLP regulation, e. g. with radioactive substances or substances for scientific research and development not brought onto the market, or finished products ultimately destined for consumers, such as pharmaceuticals, foods or cosmetics (see below), which are legally regulated elsewhere. A “substance” is regarded here as defined by the CLP Reg. (Article 2) as a chemical element and its compounds “in the natural state or obtained by any manufacturing process, including any additive necessary to preserve its stability and any impurity deriving from the process used, but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition”; and “mixtures” mean “a mixture or solution composed of two or more substances” (CLP 2008. For bibliography see electronic supplementary material.). It is noteworthy from a legal point of view that a corresponding European regulation, unlike a European directive (Dir.), which has to be converted into national law to become effective, applies directly throughout the entire EU as soon as it comes into effect. This demonstrates just how important this EU legislation is, especially with regard to each affected national state, as well as to countries conducting trade with the EU. Both pieces of legislation, CLP Reg. (EC No. 1272/2008; CLP 2008. For bibliography see electronic supplementary material.) and REACH Reg. (EC No. 1907/2006; REACH 2006), jointly cover central elements of the chemicals law, currently valid in the EU, with the CLP Reg. providing information on the hazards connected with these substances and mixtures—and thereby also about potentially sensitising components—to producers and suppliers, as well as consumers and workers where necessary, through the classification and labelling of chemicals (hazard communication).
Part 3 of the CLP regulation deals explicitly with health hazards and paragraph 3.4 is concerned with “respiratory or skin sensitisation”. Where the skin is concerned, it states under 3.4.1.2 “Skin sensitiser means a substance that will lead to an allergic response following skin contact”; this is a somewhat truncated definition that can only be harmonised with the relevant immunological or allergological publications to a limited extent; however, as the central aspect of the first and second contact of a substance, and thereby the immunological memory of an adaptive immune response, it is disregarded here [1,2,3,4,5,6]. A distinction is made nevertheless a little further on under 3.4.1.3. between the induction and the elicitation phase, while it is explained under 3.4.1.5. that “[u]sually, for both skin and respiratory sensitisation, lower levels are necessary for elicitation than are required for induction”. Data on substances or mixtures are not listed here. Basically, if there is corresponding evidence, substances with a sensitising effect on the skin are classified as Hazard Category 1 or, if sufficient data is available, to sub-categories 1A (strong allergen) or 1B (other skin allergens; Table 1). Classification to a sub-category is only permitted if the corresponding data is on hand. Category 1 also means, for example, that although insufficient data are available for an according sub-category classification, sufficient human or animal data is available for Hazard Category 1.
To determine and verify information as to whether a possibly sensitising health hazard could emanate from a substance, for example, clear procedures which must be complied with are contained in article 8, paragraph 3 of the CLP Reg. It states that tests are to be conducted in accordance with the test methods mentioned under the REACH Reg. (EC No. 1907/2006; REACH 2006), or in line with “scientific principles that are internationally recognised or validated according to international procedures”. These include the OECD test guidelines (OECD 2018. For bibliography see electronic supplementary material.). “Epidemiological data and experience on the effects on humans, such as occupational and data from accident databases”, should be used to obtain more information, along with “any new scientific information” and “any other information generated under internationally recognised chemical programmes for the mixture itself or the substances contained in it”. It is also very important that the information relates to “the forms or physical states in which the substance is placed on the market and in which it can reasonably be expected to be used” (see articles 5 and 6). If new toxicological tests are conducted by manufacturers or importers for any reason, this should also be done in compliance with the REACH Reg. (see article 13).
Based on authorised data and information from experiments with animals, as well as more recently from in vitro tests, a distinction is made in CLP labelling for sensitising substances between sensitisation of the respiratory tract and of the skin. According to their classification, each substance is labelled with a different “hazard pictogram”, which is intended to convey graphically specific information about the hazard in question (Table 2). There are currently nine different pictograms. The added “signal word” is to be used to indicate the relative level of severity of hazards “to alert the reader to a potential hazard”. A distinction is made between the following two levels: “Danger” for “the more severe hazard categories” (e.g. respiratory allergy) or “warning” for “the less severe hazard categories” (e.g. skin allergy). When determining and assessing the hazard, however, it is necessary to distinguish between the categorisation of a substance (or mixture) which reflects the directly inherent hazard—which can be differentiated by its type and severity—and its risk assessment, which relates a specific hazard to the actual human (or environmental) exposure to a substance (or mixture; ECHA 2015. For bibliography see electronic supplementary material.).
Furthermore, supplementary labelling can be obligatory for certain mixtures, also with regard to sensitising substances in accordance with article 25 and annex II of the CLP Reg. (CLP 2008a. For bibliography see electronic supplementary material.). This is the case with cement and cement mixtures where the sensitising effect of chromium VI is concerned. If said labels contain the phrase “when they are hydrated, more than 0.0002% soluble chromium (VI) of the total dry weight of the cement” and if they are not yet labelled with the hazard warning (H317) “may cause an allergic skin reaction”, the following statement should be added: EUH203—“Contains chromium (VI). May produce an allergic reaction”. Mixtures that were not classified as sensitising but which contain the phrase “at least one substance classified as sensitising and present in a concentration equal to or greater than that specified in Table 3.4.6 of annex I” shall be labelled as follows: EUH208—“Contains <name of sensitising substance>. May produce an allergic reaction”.
The REACH regulation
To further simplify chemicals law, the EU enacted the regulation on the REACH; EC No. 1907/2006 (REACH 2006) on 1 June 2007. The European Chemicals Agency (ECHA) in Helsinki was founded at the same time. According to information provided by ECHA, REACH is “a regulation of the European Union, adopted to improve the protection of human health and the environment from the risks that can be posed by chemicals, while enhancing the competitiveness of the EU chemicals industry. It also promotes alternative methods for the hazard assessment of substances in order to reduce the number of tests on animals. In principle, REACH applies to all chemical substances; not only those used in industrial processes but also in our day-to-day lives, for example in cleaning products, paints as well as in articles such as clothes, furniture and electrical appliances. Therefore, the regulation has an impact on most companies across the EU”. The remarkable thing here is that REACH places the burden of proof on the companies: “To comply with the regulation, companies must identify and manage the risks linked to the substances they manufacture and market in the EU. They have to demonstrate to ECHA how the substance can be safely used, and they must communicate the risk management measures to the users. If the risks cannot be managed, authorities can restrict the use of substances in different ways. In the long run, the most hazardous substances should be substituted with less dangerous ones” (REACH 2018. For bibliography see electronic supplementary material.).
The methods for collecting and evaluating information on the properties and harmful effects of substances, e. g. those of skin-sensitising substances, are clearly regulated by REACH. This also applies to the registration process and evaluation of substances with regard to their conformity by the ECHA, or to selected substances by the member states of the EU. If individual substances pose greater risks that affect human health, such as substances that cause allergies or affect the environment, the use of hazardous substances can be restricted or even prohibited. In order to better communicate and implement REACH processes and requirements, the ECHA developed more than 20 different guideline documents on REACH and made them accessible on their website (REACH 2018a. For bibliography see electronic supplementary material.).
Alone the “guidance on information requirements and chemical safety assessment” covers two main sections, each with Concise Guidance (A–F) and related reference material (Chap. R.2–R.20; In Depth Guidance, ECHA 2018. For bibliography see electronic supplementary material.). This serves the purpose of collecting available information regarding the intrinsic properties of substances to be registered, which includes their sensitisation potential, and the assessment of this information against the requirements specified by REACH, the identification of data gaps and support to generate the additional information required to fill these data gaps. In addition to this, the guidance documents explain the basic principles of a risk assessment in accordance with REACH to the authorities concerned. A risk assessment of this kind can be necessary, for example, to justify a recommendation for the restriction or inclusion of substances in the authorisation procedure, or within the scope of a substance assessment.
In concise guidance B of the first main section of the “guidance on information requirements and chemical safety assessment” (REACH, IR & CSA, Concise Guideline B 2011. For bibliography see electronic supplementary material.), “skin and respiratory sensitisation” (B.6.2.3) is also addressed under “B.6 endpoint specific guidance”. It has been established here that the information requirements for skin sensitisation are given at production quantities in the range of 1–10 t/year. In vivo local lymph node assay (LLNA) data are observed here in standardised form under consideration of local toxicity and skin irritation, but other animal tests may also be considered if necessary. It was shown that LLNA results “correlate relatively well with the human data on skin sensitisation”, so that they can be used for hazard assessment. The pH value and other data that can be useful for classification should also be taken into account. There are no standardised information requirements for respiratory sensitisation. Human data, such as “diagnostic clinical studies, workers medical surveillance and case reports”, can be taken into consideration for assessing the sensitisation potential of substances. Relevant human data are usually given preference over animal data in line with B.6.2.3. If there is a lack of positive findings in humans, positive animal data of good quality can be used where applicable. Analysis with a (Q)SAR ([quantitative] structure activity relationship) model, which takes into account the electrophilic reactivity of a sensitising substance, may be useful for the assessment of skin sensitisation, even though QSAR models are not yet available for respiratory sensitisation.
To improve protection of consumers and the working population, however, it is also necessary to characterise a sensitising substance—or mixture—as precisely as possible, i.e. to classify it by its sensitising potency in relation to subcategories 1A and 1B. Even though in vitro data on sensitisation can now be taken into account under REACH within the scope of technical adjustments (see REACH 2017, below. For bibliography see electronic supplementary material.), no officially recognised in vitro method exists to date permitting this more precise characterisation, while officially recognised in vitro tests of respiratory sensitisation are also lacking. More detailed guidelines are given in Sect. R.7.3. In this way, qualitative risk description—linked with the corresponding risk management measures (RMM; Annex E)—forms the first approach to classification in skin sensitisation on the basis of the sensitisation potency (severe/extreme and moderate sensitisation). The derived exposure level without impairment (DNEL, derived no effect level) is determined (if possible) in order to assess the remaining probability of risks after implementation of the RMM. The determination of the DNEL can be based on data from the LLNA study and/or the validity of data using LLNA data and historic human data (REACH; IR & CSA; Concise Guidance B 2011). It can also be the case, however, that no DNEL can be derived during sensitisation. As already mentioned, more detailed notes on information requirements and the testing of chemical safety in relation to the skin—and respiratory sensitisation—are to be found in the “guidance on information requirements and chemical safety assessment, Chap. R.7a: Endpoint specific guidance Version 6.0 July 2017” (REACH 2017). Compared to previous versions, these guidelines show a very clear trend towards the increased integration of in vitro assays—even when evaluating skin sensitising substances—by means of both new EU test methods, as well as OECD test guidelines (see below). Annex VII of the REACH Reg. outlines which information requirements are necessary from a production quantity or import of 1 t/year. These also include requirements for sensitising substances (under annex VII, 8. Toxicological Information; 8.3. “Skin sensitisation”; REACH 2006). It states herein that information should be provided that allows “a conclusion whether the substance is a skin sensitiser and whether it can be presumed to have the potential to produce significant sensitisation in humans (Cat. 1A)”, and that allows for “risk assessment where required”. As outlined above, the category Cat. 1A also complies with the GHS and CLP Reg. under REACH (Fig. 1). Recently, this information requirement has also predominantly included in vitro data before use is made of data from in vivo animal tests, and preferably from LLNA data in mice. The in vitro information should be used here “from in vitro/in chemico test method(s), recognised according to article 13(3), addressing each of the following key events of skin sensitisation: a) molecular interaction with skin proteins, b) inflammatory response in keratinocytes c) activation of dendritic cells”. Where the acquisition of information on intrinsic properties of substances is concerned, article 13, paragraph 3 states that it should be conducted “in accordance with the test methods laid down in a commission regulation or in accordance with other international test methods recognised by the commission or the agency as being appropriate”; while paragraph 2 makes specific reference to another important goal under REACH of reviewing and improving test methods at regular intervals in order to reduce “testing on vertebrate animals and the number of animals involved”. This is also achieved through the regular adjustments mentioned above.
Consumer protection and product safety
Health protection and safety of consumer products constitutes an ambitious goal. In a constantly changing market this requires flexibly adapted regulatory framework in order to guarantee appropriate exposure and risk assessment. It also includes the safe or low-risk handling of (potentially) sensitising substances. These are mentioned explicitly on the one hand within the scope of legal regulations, such as the cosmetics regulation (EC 1223/2009), or are indirectly affected, as in the case of the product safety law. A regulation could still be missing here, however. The presentation made here is intended as a rough example and therefore does not in any way claim to be complete. Fig. 2 provides some information on classification and labelling of products containing sensitising substances (Fig. 2).
Generally speaking, a large number of product types regulated by various legal entities are subsumed under the term consumer products. Which laws and provisions could possibly be affected for which product is shown in a graphic provided by the German federal office of consumer protection and food safety (BVL; BVL 2018. For bibliography see electronic supplementary material.). Consumer products are regulated in Germany by the national product safety law (ProdSG 2011; BMAS 2018. For bibliography see electronic supplementary material.) unless there is an EU regulation (2001/95/EC) or a product-specific special law, as is the case with foods, feeds, medical products, plant protection products and antiques.
Cosmetic products
Cosmetics, or cosmetic products, with their “leave-on” and “rinse-off” products, are regulated separately in the EU. The regulation on cosmetic products, which also explicitly takes sensitising substances into consideration, has been in force here since 2009 (EC 1223/2009, in the new consolidated version of 01.08.2018; Cosmetics Reg. 2009, Kosmetik-VO 2009. For bibliography see electronic supplementary material.). Accordingly, “a cosmetic product made available on the market shall be safe for human health when used under normal or reasonably foreseeable conditions of use”. Cosmetic products include “creams, emulsions, lotions, gels and oils for skin care, face masks, make-up formulations (liquids, pastes powder), facial powder, body powder, foot powder, toilet soaps, deodorising soaps, perfumes, toilet water and eau de Cologne, bathing and shower additives (salt, foam, oil, gel), depilatories, deodorants and antiperspirants, hair dyes, hair waving, smoothing and styling products, hair conditioners, hair cleaners (lotions, powders, shampoos), hair care products (lotions, creams, oils), styling aids (lotions, lacquers, brilliantine), shaving products (including pre and after treatment products), make-up and make-up removal products, lip care products and cosmetics, dental and oral care products, nail care products and cosmetics, products for external intimate care, sun protection products, self-tanning products, skin bleaches, anti-wrinkle products,” to name only a few (Cosmetics Reg. 2009). The regulation therefore creates a clearly defined legal framework for the EU Single Market for cosmetics, also where health protection and product safety are concerned, as well as market monitoring. This task is assumed in Germany by the BVL. Every year, the BVL publishes a so-called federal monitoring plan (Bundesweiter Überwachungsplan, BÜP) in which cosmetics are also taken into account. Other useful tips on the legal framework for cosmetics can also be found at the BVL website (www.bvl.bund.de), such as the national German regulation on cosmetic products (D-KosmetikV) of 23 July 2014, which complements EU regulation (1223/2009) or the German Food and Feed Code (Lebensmittel‑, Bedarfsgegenstände und Futtermittelgesetzbuch, LFGB, of 1 September 2005; LFGB 2005. For bibliography see electronic supplementary material.) with bans and powers of authorisation for the protection of health, or Regulation (EU) No. 655/2013 of 11 July 2013 laying down common criteria for the justification of claims used in relation to cosmetic products. Although the product safety law (ProdSG) also applies to cosmetic products, the essential provisions are already included in cosmetics law, according to the BVL. Isothiazolones in children’s cosmetics are mentioned here as an example of BÜP results that can also have a sensitising effect [7]. Other product groups that may contain sensitising substances such as nickel are also considered [8]. Annex I of the European regulation also stipulates the minimum requirements for the safety report for each cosmetic product. The toxicological profiles of the substances used here state that particular attention should be paid to “local toxicity evaluation (skin and eye irritation), skin sensitisation, and in the case of UV absorption photo-induced toxicity”. A list of substances that are prohibited in cosmetic products is contained in annex II. The commonly occurring human contact allergen nickel is also named here (e. g. consecutive number 1093, Nickel, CAS Registry No. 7440-02-0; and consecutive number 1100, nickel sulphate, CAS Registry No. 7786-81-4). Annex III contains substances, the use of which are restricted, such as certain p‑phenylenediamines (e. g. consecutive number 8, CAS Registry No. 106-50-3), an oxidation hair dye, and stipulates the “wording of conditions of use and warnings”. In this case, it is pointed out in general use that: “a) Hair colourants can cause severe allergic reactions. Read and follow instructions. This product is not intended for use on persons under the age of 16. Temporary “black henna” tattoos may increase your risk of allergy. Do not colour your hair if:—you have a rash on your face or sensitive, irritated and damaged scalp,—you have ever experienced any reaction after colouring your hair,—you have experienced a reaction to a temporary “black henna” tattoo in the past. Contains phenylenediamines. Do not use to dye eyelashes or eyebrows”, and under b): “‘For professional use only. Hair colourants can cause severe allergic reactions. Read and follow instructions. This product is not intended for use on persons under the age of 16. Temporary “black henna” tattoos may increase your risk of allergy. Do not colour your hair if:—you have a rash on your face or sensitive, irritated and damaged scalp,—you have ever experienced any reaction after colouring your hair,—you have experienced a reaction to a temporary “black henna” tattoo in the past. Contains phenylenediamines. Wear suitable gloves’”. Annex IV provides information on colourants allowed in cosmetic products and annex V deals with preservatives, whereas annex VI addresses UV filters. According to the regulation, a panel of experts, the scientific committee on consumer safety (SCCS) advises the Commission on consumer safety. Among other things, the committee drafts opinions on the safety of the ingredients of cosmetic products, which also include substances that may trigger sensitisation. The regulation (1223/2009 version 16.04.2015) establishes that the SCCS has identified a number of substances “as likely to cause allergic reactions and it will be necessary to restrict their use and/or impose certain conditions concerning them. In order to ensure that consumers are adequately informed, the presence of these substances should be mentioned in the list of ingredients and consumers’ attention should be drawn to the presence of these ingredients. This information should improve the diagnosis of contact allergies among consumers and should enable them to avoid the use of cosmetic products which they do not tolerate. For substances which are likely to cause allergy to a significant part of the population, other restrictive measures such as a ban or a restriction of concentration should be considered”. In this regard, the SCCS also takes up a prominent position in the regulation of (potentially) sensitising substances in cosmetic products.
Food law
Everyday commodities and cosmetics are regulated by special law. As consumer products, however, they are also covered by the general ProdSG (see below) and food law. “Consumer products with not only temporary physical contact,” such as household cleaners and disinfectants, are everyday commodities, as defined by the LFGB (2005) and are therefore also regulated by the consumer goods regulation, LFGB and chemicals law where applicable (BedGgstV 1992; LFGB 2005; ChemG 1980. For bibliography see electronic supplementary material.). The chemicals law is not dealt with in more detail here (ChemG 1980).
Product safety
In order to achieve a high level of consumer protection in health protection and safety of consumers in the European community, the EU adopted directive 2001/95/EC on general product safety in 2001. The purpose of this was to ensure “that products placed on the market are safe” (EU, 2001/95/EC, version of 1.1.2010, Article 1(1); general product safety directive, GPSD; ProdS Dir 2001. For bibliography see electronic supplementary material.). In this context, a safe product “… shall mean any product which, under normal or reasonably foreseeable conditions of use … does not present any risk or only the minimum risks compatible with the product’s use, considered to be acceptable and consistent with a high level of protection for the safety and health of persons”. The above-mentioned European directive (ProdSG 2011. For bibliography see electronic supplementary material.) is reflected, among other areas in the national German product safety law (ProdSG, BGBl, [Fed. Law Gazette] I P. 2178, 2179; 2012 I P. 131; version of 1.08.2015 [BGBl. I P. 1474])—valid since 1.12.2011—and subordinate product safety ordinance based on article 8 ProdSG (1. to 14. ProdSV). This subordinate ordinance includes special regulations for electric appliances and children’s toys (ProdSV-2 2011 in the context of the toys directive 2009. For bibliography see electronic supplementary material.). There are also standards to regulate the necessary safety requirements and test bodies that are capable of advising manufacturers with regard to so-called conformity testing (BAuA 2017. For bibliography see electronic supplementary material.).
Approved in vitro/in chemico test methods for key events in skin sensitisation
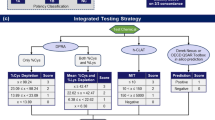
Not only since animal testing on cosmetics was banned in the EU in March 2013 has there been a trend towards more in vitro test methods in order to minimise or replace animal testing (see REACH 2006). The in vitro testing of potentially sensitising substances takes on a pioneering role here, which is impressively described in the adverse outcome pathway (AOP) concept presented by the OECD and the integrated approach to testing and assessment (IATA) concept (see below), which were transferred for the first time and applied in a very simplified form, from an immunological point of view, on the toxicological endpoint sensitisation, using the example here of four key events:
-
1.
Binding of the reactive small chemicals (haptens) to self-proteins
-
2.
Activation of keratinocytes
-
3.
Activation of dendritic cells (Langerhans cells)
-
4.
Activation of T cells
It is important here not to forget that although the immunological in vivo immune response is “reflected” in an extremely shortened manner in this way, it can be very useful for risk assessment (Fig. 3).
Chemical testing in skin sensitisation.a Testing of substances according to the adverse outcome pathway (AOP) of skin sensitisation by TG442C [17, 18], TG442D [21, 22, 69], TG442E [70, 71] and T‑cell-activation [72, 73] (no valid test yet available). b Sensitisation by animal experiments according the organisation for economic cooperation and development (OECD) test guidelines TG406 [74, 75] and TG429 [12, 16, 76]
Even though the legal regulations for “in vitro sensitisation” are definitely incomplete compared to those based on animal tests or human contact allergy, a trend can be observed towards the consideration of more alternative in vitro assays in the risk assessment of potentially sensitising substances or mixtures [9], e. g. in the sense of weight of evidence (WoE). Different concepts are currently discussed in this regard [10, 11].
OECD test methods under REACH
Unlike regulations, directive documents do not have a legal character and serve as an orientation and interpretation aid for an associated regulation. In order to guarantee more legal certainty with the test methods used under REACH, for example, currently existing or new, internationally validated OECD test methods were legally assigned to the REACH regulation by means of a new regulation (REACH Test Methods Regulation 2008; REACH Test Methods Regulation 2017; OECD 2018. For bibliography see electronic supplementary material.). In light of the complex REACH Reg., it is not surprising that the latest consolidated version of this regulation on the test methods used under REACH comprises more than 2170 pages (REACH test methods regulation 2017).
“B.6. Skin Sensitisation” (p. 287) contains a section with an important reference to the corresponding test methods, in which its states: “There is no single test method which will adequately identify all substances with a potential for sensitising human skin and which is relevant for all substances”. This is an indication of an underlying problem with the testing of (potentially) sensitising substances.
As the test for the skin sensitisation of guinea pigs with adjuvants permits more conclusions regarding a possible human reaction, the guinea pig maximisation test (GPMT) with adjuvant methods is regarded as the preferred test, even though other adjuvant tests exist. Without an adjuvant, the Bühler test is seen as the preferred test, even though its sensitivity is lower for many chemicals. Its use must therefore be justified separately. Both methods are described in detail in the regulation (B.6., 1.5.1. Guinea pig maximisation test [GPMT]; B.6., 1.5.2. Bühler Test; see OECD TG 406; OECD 2018).
The LLNA [12] (OECD TG 429; OECD 2018) is described here under B.42. Up to 40% fewer animals can be used in this test compared to the guinea pig test. “This does not necessarily imply that in all instances the LLNA should be used in place of guinea pig tests (i. e. B.6; OECD Test Guidline 406).” It should be borne in mind that by using this test method, the induction phase of skin sensitisation is examined here, and that the test method supplies quantitative data for the dose-response relationship (REACH Test Methods Regulation 2008; REACH Test Methods Regulation 2017). However, metals often provide false negative results, meaning that they would appear to take on a special role herein [6, 13,14,15] (REACH 2017). Modified LLNA test methods are also possible under certain conditions, such as a non-radioactive local lymph node assay as described under B.50., or the LLNA in combination with a bromodeoxyuridine enzyme-linked immunosorbent assay (BrdU-ELISA), as outlined in B.51 [16] (REACH Test Methods Regulation 2008).
In chemico direct peptide reactivity assay (DPRA), which has been known for many years, is also now authorised under REACH, here under B.59. For example, the DPRA uses high-performance liquid chromatography (HPLC) to measure the binding of potential skin allergens to peptides ([17,18,19]; as a molecular initiating event [MIE] of AOP skin sensitisation; OECD, TG 442C; OECD 2018, 2012. For bibliography see electronic supplementary material.). Perhaps there will shortly be a similar, fluorescent-based assay available, which should also be applicable to 96-well plates. Interlaboratory ring studies are under way.
As keratinocyte reactivity is of decisive importance for sensitisation [20], an in vitro keratinocyte test is also authorised: B.60. In vitro skin sensitisation: ARE-NRF2 Luciferase test method [21, 22] (OECD, TG 442D; OECD 2015, 2018. For bibliography see electronic supplementary material.). However, this pathway, which regulates redox enzymes, also seems to be involved in age-related diseases [23], among other responses.
New assessment criteria are required, however, if animal tests are possibly to be replaced altogether in the future. A new strategy is offered by the IATA, a “structured approach used for hazard identification (potential), hazard characterisation (potency) and/or safety assessment (potential/potency and exposure) of a chemical or group of chemicals, which strategically integrates and weights all relevant data to inform regulatory decision regarding potential hazard and/or risk and/or the need for further targeted and therefore minimal testing” (REACH Test Methods Regulation 2017; OECD IATA 2016. For bibliography see electronic supplementary material.).
EU test methods
Other test methods validated in the EU are authorised, also for sensitisation, on the basis of the OECD test guidelines, or irrespective of them, such as reduced LLNA, which may be applied under certain conditions when well-justified in individual instances (ESAC 2007; REACH 2008. For bibliography see electronic supplementary material.); or the h‑CLAT assay within the scope of the IATA concept (ECVAM 2015; OECD IATA 2016. For bibliography see electronic supplementary material.), or the DPRA (ECVAM 2013. For bibliography see electronic supplementary material.), as already mentioned.
Consumer products and skin sensitisation
Although consumer products are supposed to be safe in principal and not pose a hazard to consumer health, there is a limited risk for contact allergies in sensitive individuals that may react clinically with allergic contact dermatitis (ACD) after contact with selected products [2, 24,25,26]. A few examples are given below.
Nickel in consumer products
Nickel is still one of the most common human contact allergens and is contained in numerous consumer products, such as jewellery, watches, jeans buttons, keys and 1 euro coins, as well as several medical products, such as stents and prostheses [27,28,29]. With a rate of over 15% positive in patch test reactions, this metal also heads the hit list of contact allergens (sensitisation frequency) of the information network of German dermatological hospitals, (Informationsverbund Dermatologischer Kliniken, IVDK, Göttingen, Germany) [30].
It was assumed at the end of the 1990s that up to 20% of young women in the general population suffered from a nickel allergy and that up to 40% of positive nickel allergy sufferers were to be found proportionately among the cases of clinical contact allergies. The background here was earrings with a high nickel-content, which were even worn by small children, and an increased rate of body piercing among consumers with products containing nickel. After labelling became compulsory in Germany in 1992 and the nickel directive was adopted by the EU in 1994, a clear decline in nickel allergies was observed in women aged under 30 (from 36.7 to 25.8% in clinical cases) in Germany [31]; this observation was attributed to the EU-level nickel regulation. Similar trends were observed in Denmark [32]. After Nickel Regulation I came into effect in 2001 and Nickel Directive II in 2004, however, no further significant reduction in nickel allergy prevalence could be observed, initially among the younger population (aged 1–17 years) [33]. The possible explanations for this were: a) continued high nickel concentrations (above the established nickel migration values of 0.2 µg/cm2/week) in materials contained in earrings and body piercing, b) the permitted nickel migration limit values were possibly still too high, c) the adaptation factor for interlaboratory comparisons applied under EN 1811 (EN 1811: 1998) for determining nickel was not ideal and obscured higher nickel migration values and d) other nickel sources with skin contact could be responsible for continued positive nickel reactions among those examined. Further explanations that go beyond those outlined above are not listed in this study [33].
More data from the IVDK patch test results were subsequently evaluated (n = 74,854, 46,550 women and 28,304 men; 2005–2012) and showed a trend towards a reduction in nickel allergies in women in the age groups 1–17, 18–30 and 31–44 years over the entire period, whereas an increase was observed in 45–60 year-olds, which was explained by the possibility that these women could have become sensitised in the period before the nickel regulation came into force. In women and men aged over 60, no statistically significant trend could be observed overall [34]. Even if this trend can be partly attributed to Nickel Directive II, the positive nickel values among women still remain at a relatively high level (2011/12: 1–17 years 11.9%; 18–30 years 19.8% and 31–44 years 31.1% positive), although not as high as in southern European countries [35], but certainly comparable with the average European value of 14.5% positive nickel patch test results from five European countries [36].
To date, there is no convincing explanation for the still relatively high percentage of people with nickel allergies or positive nickel patch test results. Whether this can be explained solely by products with inadmissibly high nickel migration values or contact with other nickel-containing products is doubtful. It is worth remembering here that a positive nickel patch test does not automatically lead to a nickel allergy among those tested, not even under the conditions postulated by the ECHA (ECHA 2014. For bibliography see electronic supplementary material.), since the test would appear to be too imprecise for this [37]. At the same time, the test conditions selected for the development of the patch test are biased with regard to the expected increase in positive results, particularly when petrolatum, a vehicle and adjuvant, and 5% nickel sulphate is added. It would also appear that the observation of metals is changing with regard to the classical concept of the type IV allergy, so that from a scientific point of view, this is sometimes observed when more strictly separated, immunologically, from the covalent-binding haptens (see above). The role of cross-reactions with cobalt and palladium, for example, would also still appear to be of significance [38]. However, whether and to what extent other products such as nickel-containing/contaminated tattoos can contribute to the positive results is still open to conjecture [39,40,41,42]. Unexpected products, such as permanent make-up, can also produce an allergic nickel reaction [43]. To protect consumers from products containing nickel, the German federal institute for risk assessment (Bundesinstitut für Risikobewertung, BfR) has conducted repeated risk assessments and made reference to corresponding consumer articles, e. g. on piercing [44], toys [45] and tattooing agents [46].
P-phenylenediamine in cosmetics
With an occurrence rate of roughly 4%, contact allergy to 1,4 phenylenediamine (PPD; CAS Registry No. 106-50-3) is among the more common forms of allergy among the tested IVDK patients, whereas a prevalence of 0.8% is assumed throughout Europe [47]. PPD is a well-known oxidation hair dye that can also be hidden in henna tattoos and which is classified as a human contact allergen. Sensitisation can be induced through professional as well as private use [48]. A PPD-specific human T cell response can obviously be conveyed in a manner similar to nickel by coupling it with albumin, which also occurs in the skin and in sweat [15, 49]. A study conducted by the IVDK (2008–2013) showed that the use of a hair dye was associated with 80% of PPD-positive tested persons and that, in addition, these people frequently developed scalp dermatitis (57%), but also that age (>40), sex (woman) and profession (hairdresser) could be linked with an increased risk of PPD sensitisation [48]. Cross reactions with other aromatic p‑amino compounds are also possible.
So-called black henna tattoos, which may contain unauthorised, higher concentrations of PPD, as well as the related and severely sensitising toluene-2,5-diamine (PTD), have proven to be particularly risky in recent years [48]. The study conducted by Diepgen et al. was also able to show that black henna tattoos constitute a risk factor in developing a PPD allergy [47, 50]. Additional case studies, including studies related to critical angioedemas, underscore this risk [51,52,53,54]. It is therefore all the more surprising, and clearly inadvisable from the point of view of risk assessment and consumer protection, to recommend a self-test for PPD to consumers before hair dying. Diagnostic means belong clearly and exclusively to the area of professional expertise of a medical practitioner. In a pilot study conducted by industry with partners in various European clinics in which this approach was examined, very severe PPD immune reactions occurred in individuals among the almost 60 probands, several of whom required subsequent hospitalisation (EU, Expert Group Cosmeticovigilance). The BfR also advises against risky self-testing of this kind [55].
Fragrances in consumer products
More than 5000 different fragrances are in use today and frequent skin allergic reactions to them are among the IVDK’s so-called “hit-list” of contact allergies (sensitisation frequency). Two different fragrance mixes are used here during epicutaneous testing: Fragrance Mix I, which is to be examined more closely here, and Fragrance Mix II. Fragrance Mix I, the composition of which was developed before 1984, contains eight ingredients (seven fragrances: amyl cinnamal, cinnamyl alcohol, cinnamal, eugenol, geraniol, hydroxyl-citronellal, isoeugenol and a natural extract of Evernia prunastri [or oakmoss absolute in English], each with >1% of the emulsifier sorbitan sesquioleate [SSO]). Clinical results document an increase in sensitisation frequency in recent years from 8.4 to 9.1% compared to Fragrance Mix I [30, 56] (last IVDK data 2010–2012). This increasing trend is supported by other studies. An increased trend was also recorded in Denmark, for example, between 2011 and 2015 from 8.0% positive reactions to 10.4% among the tested women, and from 4.4 to 7.3% positive reactions in men [57]. It is worth noting here that the emulsifier can obviously also influence the reactions, which, similar to nickel, raises the question as to the role played by possible cofactors in relation to human skin reaction [58].
The lack of extensive biomonitoring for each individual substance in Fragrance Mix I—and Fragrance Mix II—is critical. There are huge information gaps here. More research must also be conducted into the stability of the individual substances and their oxidation products or interactions. Additional chemically-related structures, and possibly even products, could be hidden behind a named (volatile) individual substance, for example [59]. Questions must also be asked about fragrance allergies that are not currently covered by the fragrance mixes but which are nevertheless of significance for consumers and patients [60]. Possible immunological interactions, such as skin–lung and lung–skin must also be explored (see also linalool as a contact allergen via ambient air).
Sensitising fragrances are not only to be found in classical products such as perfume, deodorants, aftershave and other cosmetics, including toothpaste and mouthwash—they are also ubiquitous in our environment, and in other household and industrial products, as well as drugs for topical use and toys [61, 62].
To protect consumers and especially children from potentially skin-sensitising substances, 55 sensitising fragrances have been banned in toys in line with the EU toys directive (Annex II, Particular Safety Requirements, under III Chemical Properties; Toys Directive 2009, Spielzeugrichtlinie 2009. For bibliography see electronic supplementary material.). It is also stated there, however, that: “The presence of traces of these fragrances shall be allowed provided that such presence is technically unavoidable under good manufacturing practice and does not exceed 100 mg/kg.” Eleven additional fragrances must be declared if they exceed a concentration of 100 mg/kg. The BfR has also prepared an opinion on this and considers the limit value of 100 mg of banned fragrances per kilogram toy material to be too high [45]. Furthermore, fragrances in candles should also be limited [63].
A total of 26 fragrances are regulated in the cosmetics regulation (Annex III No. 67–92; Cosmetics Reg. 2009). These should be listed with their substance designations as soon as they exceed a certain concentration in a product, e. g. more than 0.01% of ingredients in “rinse-off” products (shower gel, shampoo, soap), or more than 0.001% of ingredients in “leave-on” products (cream, perfume, hair conditioner). Whether these lists should be extended or adapted to limit values is open to discussion and is usually decided by statements issued by the SCCS (see above). In light of the large variety of possible fragrances and variants thereof and (as yet) unidentified individual sensitising substances, this is bound to constitute a mere fraction of possibilities to protect consumers, but this is also understandable in view of the large data gaps and difficulties in their analytical determination. As analysis is a prerequisite for the characterisation and identification of potentially sensitising individual substances, it is of great importance to encourage the further development of analytical methods. It is possible, for example, to identify as many as 58 potentially sensitising substances in soft toys using a new mass spectrometric method [64]. The goal here is to establish a link to the allergologically relevant substances. This will in turn lead to increased demands on the testing and risk assessment authorities. It is also advisable to retain substances that are as pure as possible as reference material from the manufacturers. Patch Test Mixes I and II and individual substances could also be better classified in terms of quality using these methods.
In principle, it can be said that the cosmetics regulation and toys directive constitute a first important step towards protecting consumers from sensitising substances in the products mentioned.
Other skin-sensitising constituents
Preservatives are also an important group among skin-sensitising substances in humans. Increases in sensitisation frequency with the IVDK can also be recognised here. Accordingly, an increase in positive patch test results to 4.5% was recorded recently for the preservative mixture chloromethylisothiazolinone/methylisothiazolinone (MCI/MI) and an increase to as much as 6.8% for the individual substance MI [30]. MI is often used in cosmetics, liquid laundry detergents, wall paints and industrial processes such as paper manufacture. In the cosmetics sector, MI/MCI (highest concentration in ready-to-use preparations 0.0015% in a mixture of 5‑chloro-2-methyl-3(2Η)-isothiazolone and 2‑methyl-3(2H)-isothiazolone at a ratio of 3:1, Annex V No. 39, Cosmetics Reg.) and MI (old version: 0.01%, Annex V No. 57) are subject to regulation. In addition to this, the SCCS has dealt with MI several times and announced in 2015 (SCCS 2015. For bibliography see electronic supplementary material.) that a content of 0.0015% (15 ppm) should be regarded as safe with rinse-off products in terms of allergy induction, and that with leave-on products such as hair cosmetics, the point of view that use of articles with 100 ppm was safe was not shared in the context of allergy induction with MI (SCCS 2015; SCCS 2016. For bibliography see electronic supplementary material.). This opinion recently caused the amendment of annex V no. 57 of the cosmetics reg. (new version 2018-08-01: 0.0015% for MI).
Even though there has already been talk of an MI epidemic [65,66,67], and rinse-off cosmetics were under discussion as a possible cause, their direct causes have not been clarified in any way and will still be scrutinised intensely in the field of consumer protection in future. The BfR has also already expressed an opinion on MI and considers an influence of cosmetics on the increased sensitisation rates to be possible [68].
Other products containing sensitising substances include textiles and leather, with chromium VI and chromium III associated with leather. Remarkably, the sensitisation value with potassium dichromate has dropped from over 6% (2007) to 3% (2012) among IVDK patients [30].
Various dyes (including azo dyes) play an important role as sensitisers in textiles. Compared to preservatives, textiles to which the consumer is exposed for long periods do not appear to be particularly tightly regulated. Although the products are supposed to be safe, the data situation with regard to individual dyes (among thousands) and their potentially sensitising effect is often inadequate. There is also a lack of appropriately standardised epicutaneous tests for a corresponding mix or variety of individual substances, meaning that only a limited number of studies exist. There is still a lot of catching-up to be done here.
Outlook
Where skin sensitising substances in consumer products are concerned, new developments are constantly taking place. These affect the new—but still incomplete—regulatory classification options, with the inclusion of new in vitro tests for sensitising substances (CLP, REACH, OECD, AOP) on the one hand, and new product developments on the other, such as the increased use of fragrances and MI, which can lead, unexpectedly, to new sensitisation rates, without the mechanisms on which they are directly based being immediately recognised in a way that relates to the product. At the same time, rapid technical developments—particularly in the field of mass spectrometry (such as ICP-MS, GC-MS/MS)—also enable significantly more sensitive detection methods for sensitising substances, be they metals or organic compounds (e. g. up to 58 sensitising substances in toys with one method). There would appear to be a simultaneous data gap, however, between these very precise analytical methods, epicutaneous testing and their cofactors, and the basic understanding of different types of highly complex allergy-inducing mechanisms and threshold values, which, although desired in the regulation, often cannot be derived. A great deal more research is required is this area. Where sensitising fragrances are concerned, the BfR made the same observation 10 years ago.
Change history
15 November 2019
<Emphasis Type="Bold">Correction to:</Emphasis>
<Emphasis Type="Bold">Allergo J Int 2019 28:167–182</Emphasis>
https://doi.org/10.1007/s40629-019-0093-3
The article “Consumer protection and risk assessment: sensitising substances in consumer products”, written by Hermann-Josef Thierse and Andreas Luch, was originally published Online First without Open Access. …
Abbreviations
- ACD:
-
Allergic contact dermatitis
- AOP:
-
Adverse outcome pathway
- ATP:
-
Adaptation to the technical progress
- BfR:
-
German federal institute for risk assessment
- BÜP:
-
Federal monitoring plan
- BVL:
-
German federal office of consumer protection and food safety
- CLP:
-
Classification, labelling and packaging
- CSA:
-
Chemical safety assessment
- DNEL:
-
Derived no effect level
- DPRA:
-
Direct peptide reactivity assay
- ECHA:
-
European chemicals agency
- EU:
-
European Union
- GHS:
-
Globally harmonized system of classification and labelling of chemicals
- GPMT:
-
Guinea pig maximisation test
- GPSD:
-
General product safety directive
- HPLC:
-
High-performance liquid chromatography
- IATA:
-
Integrated approach to testing and assessment
- IVDK:
-
Information network of German dermatological hospitals
- LFGB:
-
German food, commodities and feed code (Lebensmittel‑, Bedarfsgegenstände und Futtermittelgesetzbuch)
- LLNA:
-
Local lymph node assay
- MCI:
-
Chloromethylisothiazolinone
- MI:
-
Methylisothiazolinone
- MIE:
-
Molecular initiating event
- OECD:
-
Organisation for economic cooperation and development
- PPD:
-
P-phenylenediamine
- ProdSG:
-
Product safety law
- PTD:
-
Toluene-2,5-diamine
- (Q)SAR:
-
(Quantitative) structure-activity relationship
- REACH:
-
Registration, evaluation, authorisation and restriction of chemicals
- RMM:
-
Risk management measures
- SCCS:
-
Scientific committee on consumer safety
- SSO:
-
Sorbitan sesquioleate
- UN:
-
United Nations
- UNCED:
-
United Nations conference on environment and development
- WoE:
-
Weight of evidence
References
Esser PR, Martin SF. Pathomechanisms of contact sensitization. Curr Allergy Asthma Rep. 2017;17:83.
Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D. Rook’s textbook of dermatology. 9th ed. Chichester, West Sussex, Hoboken: John Wiley & Sons; 2016.
Karlberg AT, Bergstrom MA, Borje A, Luthman K, Nilsson JL. Allergic contact dermatitis—formation, structural requirements, and reactivity of skin sensitizers. Chem Res Toxicol. 2008;21:53–69.
Martin SF, Esser PR, Schmucker S, Dietz L, Naisbitt DJ, Park BK, et al. T‑cell recognition of chemicals, protein allergens and drugs: towards the development of in vitro assays. Cell Mol Life Sci. 2010;67:4171–84.
Martin SF, Rustemeyer T, Thyssen JP. Recent advances in understanding and managing contact dermatitis. F1000Res. 2018; https://doi.org/10.12688/f1000research.13499.1.
Murphy K, Travers P, Walport M, Janeway C. Janeway’s immunobiology. 9th ed. New York: Garland Science; 2016.
Keck-Wilhelm. Isothiazolone in Kinderkosmetika. Berlin: BVL; 2016.
Vieth B. Nickelfreisetzung aus Spielzeug aus Metall (insbesondere auch Metall- und Modellbaukästen). Berlin: BVL; 2017.
Roggen EL. In vitro approaches for detection of chemical sensitization. Basic Clin Pharmacol Toxicol. 2014;115:32–40.
Hoffmann S, Kleinstreuer N, Alepee N, Allen D, Api AM, Ashikaga T, et al. Non-animal methods to predict skin sensitization (I): the Cosmetics Europe database(). Crit Rev Toxicol. 2018;48:344–58.
Kleinstreuer NC, Hoffmann S, Alepee N, Allen D, Ashikaga T, Casey W, et al. Non-animal methods to predict skin sensitization (II): an assessment of defined approaches (*). Crit Rev Toxicol. 2018;48:359–74.
Basketter DA, Gerberick GF, Kimber I, Loveless SE. The local lymph node assay: a viable alternative to currently accepted skin sensitization tests. Food Chem Toxicol. 1996;34:985–97.
Martin SF, Merfort I, Thierse HJ. Interactions of chemicals and metal ions with proteins and role for immune responses. Mini Rev Med Chem. 2006;6:247–55.
Thierse HJ, Gamerdinger K, Junkes C, Guerreiro N, Weltzien HU. T cell receptor (TCR) interaction with haptens: metal ions as non-classical haptens. Toxicology. 2005;209:101–7.
Thierse HJ, Moulon C, Allespach Y, Zimmermann B, Doetze A, Kuppig S, et al. Metal-protein complex-mediated transport and delivery of Ni2+ to TCR/MHC contact sites in nickel-specific human T cell activation. J Immunol. 2004;172:1926–34.
Williams WC, Copeland C, Boykin E, Quell SJ, Lehmann DM. Development and utilization of an ex vivo bromodeoxyuridine local lymph node assay protocol for assessing potential chemical sensitizers. J Appl Toxicol. 2015;35:29–40.
Gerberick GF, Vassallo JD, Bailey RE, Chaney JG, Morrall SW, Lepoittevin JP. Development of a peptide reactivity assay for screening contact allergens. Toxicol Sci. 2004;81:332–43.
Gerberick GF, Vassallo JD, Foertsch LM, Price BB, Chaney JG, Lepoittevin JP. Quantification of chemical peptide reactivity for screening contact allergens: a classification tree model approach. Toxicol Sci. 2007;97:417–27.
Lepoittevin J. Allergic contact dermatitis: the molecular basis. Berlin: Springer; 1998.
Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992;89:1398–402.
Natsch A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers—functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol Sci. 2010;113:284–92.
Natsch A, Bauch C, Foertsch F, Gerberick F, Normann K, Hilberer A, et al. The intra- and inter-laboratory reproducibility and predictivity of the KeratinoSens assay to predict skin sensitizers in vitro: results of a ring-study in five laboratories. Toxicol Vitro. 2011;25:733–44.
Crunkhorn S. Deal watch: abbott boosts investment in NRF2 activators for reducing oxidative stress. Nat Rev Drug Discov. 2012;11:96.
Biedermann T. Allergologie. Berlin: Springer; 2016.
Koppes SA, Engebretsen KA, Agner T, Angelova-Fischer I, Berents T, Brandner J, et al. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermatitis. 2017;77:1–16.
Rustemeyer T, van Hoogstraten IMW, von Blomberg BME, Gibbs S, Scheper RJ. Mechanisms of irritant and allergic contact dermatitis. In: Preface to the fifth edition. 2011. pp. 43–90.
Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1‑ and 2‑euro coins. Nature. 2002;419:132.
Romero-Brufau S, Best PJ, Holmes DR Jr., Mathew V, Davis MD, Sandhu GS, et al. Outcomes after coronary stent implantation in patients with metal allergy. Circ Cardiovasc Interv. 2012;5:220–6.
Thierse HJ, Luch A. Die humane Nickelallergie – Vorkommen, Mechanismen, Produktsicherheit. UMID Umwelt Mensch Informationsd. 2014;2:25–33. https://www.umweltbundesamt.de/sites/default/files/medien/378/publikationen/humane_nickelallergie_87-95.pdf. Accessed 7 Mar 2019.
Mahler V, Geier J, Schnuch A. Current trends in patch testing—new data from the German Contact Dermatitis Research Group (DKG) and the Information Network of Departments of Dermatology (IVDK). J Dtsch Dermatol Ges. 2014;12:583–92.
Schnuch A, Uter W. Decrease in nickel allergy in Germany and regulatory interventions. Contact Dermatitis. 2003;49:107–8.
Jensen CS, Lisby S, Baadsgaard O, Volund A, Menne T. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636–42.
Schnuch A, Wolter J, Geier J, Uter W. Nickel allergy is still frequent in young German females—probably because of insufficient protection from nickel-releasing objects. Contact Dermatitis. 2011;64:142–50.
Schnuch A, Schwitulla J. Decrease in nickel allergy in women after the second EU nickel directive. Contact Dermatitis. 2013;69:253–6.
Ahlstrom MG, Thyssen JP, Menne T, Johansen JD. Prevalence of nickel allergy in Europe following the EU nickel directive—a review. Contact Dermatitis. 2017;77:193–200.
Diepgen TL, Ofenloch RF, Bruze M, Bertuccio P, Cazzaniga S, Coenraads PJ, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319–29.
Johansen JD, Aalto-Korte K, Agner T, Andersen KE, Bircher A, Bruze M, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195–221.
Faurschou A, Menne T, Johansen JD, Thyssen JP. Metal allergen of the 21st century—a review on exposure, epidemiology and clinical manifestations of palladium allergy. Contact Dermatitis. 2011;64:185–95.
Aktas Sukuroglu A, Battal D, Burgaz S. Monitoring of Lawsone, p‑phenylenediamine and heavy metals in commercial temporary black henna tattoos sold in Turkey. Contact Dermatitis. 2017;76:89–95.
de Cuyper C, Lodewick E, Schreiver I, Hesse B, Seim C, Castillo-Michel H, et al. Are metals involved in tattoo-related hypersensitivity reactions? A case report. Contact Dermatitis. 2017;77:397–405.
Kang IJ, Lee MH. Quantification of para-phenylenediamine and heavy metals in henna dye. Contact Dermatitis. 2006;55:26–9.
Serup J. Medical treatment of tattoo complications. Curr Probl Dermatol. 2017;52:74–81.
Jager C, Jappe U. Contact dermatitis to permanent make up: manifestation of a pre-existing nickel allergy. J Dtsch Dermatol Ges. 2005;3:527–9.
Bf R. Piercing kann zur Sensibilisierung gegenüber Nickel führen. Berlin: BfR; 2008. Stellungnahme Nr. 046/2008 des BfR vom 10. Oktober 2008.
Bf R. Kontaktallergene in Spielzeug: Gesundheitliche Bewertung von Nickel und Duftstoffen. Berlin: BfR; 2012. Aktualisierte Stellungnahme Nr. 010/2012 des BfR vom 11. April 2012.
Bf R. Nickel in Tätowiermitteln kann Allergien auslösen. Berlin: BfR; 2013. Stellungnahme Nr. 012/2013 des BfR vom 25. Oktober 2012.
Diepgen TL, Naldi L, Bruze M, Cazzaniga S, Schuttelaar ML, Elsner P, et al. Prevalence of contact allergy to p‑phenylenediamine in the European general population. J Invest Dermatol. 2016;136:409–15.
Schubert S, Lessmann H, Schnuch A, Uter W, Geier J, IVDK. Factors associated with p‑phenylenediamine sensitization: data from the Information Network of Departments of Dermatology, 2008–2013. Contact Dermatitis. 2018;78:199–207.
Oakes T, Popple AL, Williams J, Best K, Heather JM, Ismail M, et al. The T cell response to the contact sensitizer paraphenylenediamine is characterized by a polyclonal diverse repertoire of antigen-specific receptors. Front Immunol. 2017;8:162.
Bf R. Henna-Haarfärbemittel mit p‑Phenylendiamin (PPD) stellen ein Gesundheitsrisiko dar. Berlin: BfR; 2011. Stellungnahme Nr. 024/2011 des BfR vom 19. Januar 2011.
Calogiuri G, Di Leo E, Butani L, Pizzimenti S, Incorvaia C, Macchia L, et al. Hypersensitivity reactions due to black henna tattoos and their components: are the clinical pictures related to the immune pathomechanism? Clin Mol Allergy. 2017;15:8.
Isik S, Caglayan-Sozmen S, Anal O, Karaman O, Uzuner N. Severe neck and face edema in an adolescent-delayed hypersensitivity reaction to hair dye. Pediatr Emerg Care. 2017;33:422–3.
Kind F, Scherer K, Bircher AJ. Contact dermatitis to para-phenylenediamine in hair dye following sensitization to black henna tattoos—an ongoing problem. J Dtsch Dermatol Ges. 2012;10:572–8.
Vogel TA, Coenraads PJ, Bijkersma LM, Vermeulen KM, Schuttelaar ML, Group EFS. p‑Phenylenediamine exposure in real life—a case-control study on sensitization rate, mode and elicitation reactions in the northern Netherlands. Contact Dermatitis. 2015;72:355–61.
Haarfarben BR. Selbsttest kann Allergien verursachen. Berlin: BfR; 2014. Stellungnahme Nr. 015/2014 des BfR vom 10. Februar 2014.
Schnuch A, Geier J, Lessmann H, Arnold R, Uter W. Surveillance of contact allergies: methods and results of the Information Network of Departments of Dermatology (IVDK). Arerugi. 2012;67:847–57.
Bennike NH, Zachariae C, Johansen JD. Trends in contact allergy to fragrance mix I in consecutive Danish patients with eczema from 1986 to 2015: a cross-sectional study. Br J Dermatol. 2017;176:1035–41.
Geier J, Schnuch A, Lessmann H, Uter W. Reactivity to sorbitan sesquioleate affects reactivity to fragrance mix I. Contact Dermatitis. 2015;73:296–304.
Niklasson IB, Ponting DJ, Luthman K, Karlberg AT. Bioactivation of cinnamic alcohol forms several strong skin sensitizers. Chem Res Toxicol. 2014;27:568–75.
Bennike NH, Zachariae C, Johansen JD. Non-mix fragrances are top sensitizers in consecutive dermatitis patients—a cross-sectional study of the 26 EU-labelled fragrance allergens. Contact Dermatitis. 2017;77:270–9.
Nardelli A, Carbonez A, Ottoy W, Drieghe J, Goossens A. Frequency of and trends in fragrance allergy over a 15-year period. Contact Dermatitis. 2008;58:134–41.
Nardelli A, Drieghe J, Claes L, Boey L, Goossens A. Fragrance allergens in ‘specific’ cosmetic products. Contact Dermatitis. 2011;64:212–9.
Bf R. Blei, Nickel und allergene Duftstoffe in Kerzen sollten begrenzt werden. Berlin: BfR; 2014. Stellungnahme Nr. 004/2014 des BfR vom 11. November 2013.
Wang Z, Zhang Q, Li H, Lv Q, Wang W, Bai H. Rapid and green determination of 58 fragrance allergens in plush toys. J Sep Sci. 2018;41:657–68.
Schwensen JF, Uter W, Bruze M, Svedman C, Goossens A, Wilkinson M, et al. The epidemic of methylisothiazolinone: a European prospective study. Contact Dermatitis. 2017;76:272–9.
Urwin R, Craig S, Latheef F, Wilkinson M. Methylisothiazolinone: the epidemic is declining—but not gone. Contact Dermatitis. 2017;76:301–2.
Uter W, Amario-Hita JC, Balato A, Ballmer-Weber B, Bauer A, Belloni Fortina A, et al. European Surveillance System on Contact Allergies (ESSCA): results with the European baseline series, 2013/14. J Eur Acad Dermatol Venereol. 2017;31:1516–25.
Bf R. Allergien durch Methylisothiazolinon (MI) in Kosmetika möglich. Berlin: BfR; 2013. Stellungnahme Nr. 020/2013 des BfR vom 22. Januar 2013.
Ramirez T, Mehling A, Kolle SN, Wruck CJ, Teubner W, Eltze T, et al. LuSens: a keratinocyte based ARE reporter gene assay for use in integrated testing strategies for skin sensitization hazard identification. Toxicol In Vitro. 2014;28:1482–97.
Alepee N, Piroird C, Aujoulat M, Dreyfuss S, Hoffmann S, Hohenstein A, et al. Prospective multicentre study of the U‑SENS test method for skin sensitization testing. Toxicol In Vitro. 2015;30:373–82.
Ashikaga T, Yoshida Y, Hirota M, Yoneyama K, Itagaki H, Sakaguchi H, et al. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT) I. Optimization of the h‑CLAT protocol. Toxicol In Vitro. 2006;20:767–73.
Dietz L, Esser PR, Schmucker SS, Goette I, Richter A, Schnolzer M, et al. Tracking human contact allergens: from mass spectrometric identification of peptide-bound reactive small chemicals to chemical-specific naive human T‑cell priming. Toxicol Sci. 2010;117:336–47.
Richter A, Schmucker SS, Esser PR, Traska V, Weber V, Dietz L, et al. Human T cell priming assay (hTCPA) for the identification of contact allergens based on naive T cells and DC—IFN-gamma and TNF-alpha readout. Toxicol In Vitro. 2013;27:1180–5.
Buehler EV. Delayed contact hypersensitivity in the Guinea pig. Arch Dermatol. 1965;91:171–7.
Magnusson B, Kligman AM. The identification of contact allergens by animal assay. The guinea pig maximization test. J Invest Dermatol. 1969;52:268–76.
Kimber I, Dearman RJ, Scholes EW, Basketter DA. The local lymph node assay: developments and applications. Toxicology. 1994;93:13–31.
Acknowledgements
The authors would like to thank Dr. Blair Johnston (BfR) for his critical reading of the translated manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.-J. Thierse and A. Luch declare that they have no competing interests.
Additional information
The original version of this article was revised due to a retrospective Open Access order.
Caption Electronic Supplementary Material
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Thierse, HJ., Luch, A. Consumer protection and risk assessment: sensitising substances in consumer products. Allergo J Int 28, 167–182 (2019). https://doi.org/10.1007/s40629-019-0093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-019-0093-3