Abstract
Background/aims
Evidence from large population-based cohorts as to the association of arterial stiffness and incident chronic kidney disease (CKD) is mixed. This large population-based study aimed to investigate whether arterial stiffness, assessed oscillometrically, was associated with incident CKD.
Methods
The study population comprised 4838 participants from the Vitamin D Assessment (ViDA) Study without known CKD (mean ± SD age = 66 ± 8). Arterial stiffness was assessed from 5 April, 2011 to 6 November, 2012 by way of aortic pulse wave velocity, estimated carotid-femoral pulse wave velocity, and aortic pulse pressure. Incident CKD was determined by linkage to national hospital discharge registers. Cox proportional hazards regression was used to assess the risk of CKD in relation to chosen arterial stiffness measures over the continuum and quartiles of values.
Results
During a mean ± SD follow-up of 10.5 ± 0.4 years, 376 participants developed incident CKD. Following adjustment for potential confounders, aortic pulse wave velocity (hazard ratio (HR) per SD increase 1.69, 95% CI 1.45–1.97), estimated carotid-femoral pulse wave velocity (HR per SD increase 1.84, 95% CI 1.54–2.19), and aortic pulse pressure (HR per SD increase 1.37, 95% CI 1.22–1.53) were associated with the incidence of CKD. The risk of incident CKD was, compared to the first quartile, higher in the fourth quartile of aortic pulse wave velocity (HR 4.72, 95% CI 2.69–8.27; Ptrend < 0.001), estimated carotid-femoral pulse wave velocity (HR 4.28, 95% CI 2.45–7.50; Ptrend < 0.001) and aortic pulse pressure (HR 2.71, 95% CI 1.88–3.91; Ptrend < 0.001).
Conclusions
Arterial stiffness, as measured by aortic pulse wave velocity, estimated carotid-femoral pulse wave velocity, and aortic pulse pressure may be utilised in clinical practice to help identify people at risk of future CKD.
Trial registration
www.anzctr.org.au identifier:ACTRN12611000402943.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) can lead to disability and is associated with premature death, particularly due to cardiovascular factors. A person is said to have CKD when their kidneys have had abnormal structure and function for > 3 months [1]. It was estimated that in 2017 11% of individuals worldwide (> 840 million people) were living with CKD [2]. Kidney function can decline by up to 90% before symptoms develop [3]. Chronic kidney disease is also largely irreversible, however, kidney function can often be preserved if the condition is properly managed. The prevalence of CKD increases with age and tends to be more common in ethnic minorities. In New Zealand, approximately 12% of the population may have CKD, with the highest prevalence estimated to be in indigenous Māori, Pacific (in New Zealand, predominantly people from the smaller Pacific nations of Samoa, Tonga and the Cook Islands) and people from South-Asia (the Indian Subcontinent)[4].
Arterial stiffness describes the rigidity of arterial walls and primarily refers to the stiffness of larger arteries and the aorta. Pulse wave velocity, a measure of the speed at which a forward pressure wave propagates in an artery is a measure of arterial stiffness, with higher measures indicating increased arterial rigidity [5]. The gold standard for evaluating aortic stiffness is the measurement of carotid-femoral pulse wave velocity [6]. Aortic pulse wave velocity can also be estimated from a blood pressure (BP) waveform obtained from a more peripheral artery, often the radial or brachial artery [6, 7]. Aortic pulse pressure is also recognised as a rough estimate of arterial stiffness [6].
Arterial stiffness may contribute to CKD incidence and progression due to increased pulsatile pressure on kidney microvasculature [5]. The evidence from large cohort studies assessing arterial stiffness and incident CKD, however, is inconclusive. Various studies, including the Framingham Heart Study [8] and the Health ABC Study [9] have found an association of arterial stiffness and incident CKD [10, 11]. Other such studies, however, have not observed a significant relationship [12, 13]. Four of these studies measured carotid-femoral pulse wave velocity by way of Doppler or tonometry [8, 9, 12, 13]. These techniques require specialized training, involve measurement from two places on the body whilst the participant lies supine, and may utilise bulky equipment. Two studies employed other measures of pulse wave velocity, namely brachial-ankle pulse wave velocity [10] and heart-ankle pulse wave velocity [11], which though easier to obtain than carotid-femoral pulse wave velocity still require the application of cuffs to all four limbs.
For arterial stiffness to be employed in clinical practice, it must be easy to measure, use a method that is acceptable to patients, and be cost-effective [14]. Technological advancements have led to the development of oscillometric devices which compute aortic pulse wave velocity using the measured brachial artery pressure waveform [6]; these devices are affordable, portable, and user-friendly as they mirror home BP devices and only require one cuff. Given that both age and mean arterial pressure influence pulse wave velocity, equations have also been formulated to estimate carotid-femoral pulse wave velocity [15].
We aimed to assess whether arterial stiffness, as assessed oscillometrically by aortic pulse wave velocity, estimated carotid-femoral pulse wave velocity, or aortic pulse pressure predicted incident CKD in a large population-based cohort, aged 50–84 years, in Auckland, New Zealand.
Materials and methods
Participants
We analysed data from the Vitamin D Assessment (ViDA) Study, a randomised controlled trial with the primary aim of evaluating the efficacy of vitamin D supplementation on reducing the incidence of cardiovascular disease (CVD)—no effect was observed [16]. Ethics approval was granted by the Ministry of Health (MoH) Multi-region Ethics committee (MEC/09/08/082). Participants provided written informed consent. Full study details have been published elsewhere [17].
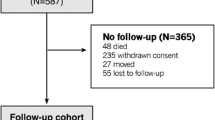
Between 5 April, 2011, and 6 November, 2012, 5250 participants primarily enlisted from 55 family practices located in Auckland, New Zealand underwent a baseline assessment at the University of Auckland, with 5108 participants included in the final study.
We excluded from the analysis 203 participants for whom arterial stiffness measures were missing or excluded (due to poor quality waveforms with a signal-to-noise ratio of < 6 dB). We further excluded 67 participants identified as having CKD for the period between 1 April 2009 and each participant’s baseline interview, resulting in 4838 participants being included in the final analysis.
CKD and follow-up
The MoH allocates New Zealand residents a National Health Index number. This number was used to track deaths and hospital discharges [utilising the International Statistical Classification of Diseases and Health Related Problems, Tenth Revision (ICD-10) coding system]. Incident CKD was identified from the first occurrence of any hospital discharge record with the relevant ICD-10 code for the period beginning with each participant’s baseline interview up to 31 August, 2022. Such data were also used to exclude participants with CKD at baseline as identified from records for the period between 1 April, 2009 and each participant’s baseline interview, together with self-reported diabetic kidney disease at the baseline interview. The relevant ICD-10 codes are reported in Supplementary Information, Table S1.
Arterial stiffness
Central BP measurements including heart rate, aortic pulse pressure and pulse waveform characteristics were obtained using a BP+ monitor (Uscom, Sydney, Australia), an oscillometric device with a correctly sized cuff placed on the upper left arm. The BP+ monitor has shown good intra-test and inter-test reliability when measuring central systolic BP [18]. The ARCSolver pulse wave algorithm (AIT Austrian Institute of Technology, GmbH, Vienna, Austria) was used to calculate aortic pulse wave velocity using the pressure waveform. This algorithm has been validated against aortic pulse wave velocity measured with an intra-aortic catheter [7]. Oscillometric devices can have inbuilt pulse wave algorithms, and hence are able to generate and save waveform parameters, including aortic pulse wave velocity, at the time of capture. Aortic mean arterial pressure was calculated as the area under the aortic pressure waveform (mmHg*seconds)/cardiac cycle duration (seconds) [19].
We implemented equations for estimated carotid-femoral pulse wave velocity based on those put forward by Greve et al. [20] and which are based on the Reference Values for Arterial Stiffness Collaboration equations [15]. These equations have predicted CVD events [20] and incident diabetes [21]. For participants with one or more CVD risk factors at baseline, estimated carotid-femoral pulse wave velocity was calculated as 4.62–0.13 × age + 0.0018 × age squared + 0.0006 × age × brachial mean arterial pressure (bMAP) + 0.0284 × bMAP. For participants with no CVD risk factors at baseline, estimated carotid-femoral pulse wave velocity was calculated as 9.587 − 0.402 × age + 0.004560 × age squared − 0.000026 × age squared × bMAP + 0.003176 × age × bMAP-0.018322 × bMAP. As defined in the development of these equations, bMAP was calculated as brachial diastolic BP + 0.4 × brachial pulse pressure. Cardiovascular disease risk factors, with criteria based on those used to develop the original equations were current tobacco smoking, hypertension (brachial systolic BP ≥ 140, or brachial diastolic BP ≥ 90, or as reported by a physician), dyslipidaemia (total cholesterol > 4.9 mmol/L, or HDL-C < 1 mmol/L, or total cholesterol/HDL-C ratio < 4, or as reported by a physician) and previous CVD events (heart attack, angina, heart failure, irregular heartbeat, transient ischaemic attack, stroke, carotid artery stenosis, or intermittent claudication as reported by a physician, or self-report of pacemaker).
Covariates
The baseline assessment included a questionnaire administered by trained interviewers adhering to a standardised protocol. Information was collected on socio-demographic factors (age, gender, self-reported ethnicity), lifestyle factors (divided into two categories: current and never/former for tobacco smoking and alcohol consumption, and > 2 h per week and ≤ 2 h per week for vigorous physical activity), medical history as reported by a physician (including diabetes, high cholesterol, hypertension and cardiovascular disease), current prescribed medications (diabetic, antihypertensives and lipid-lowering) and self-administered supplements, including vitamin D.
Participants were further identified as having diabetes or prediabetes if, between 1 April, 2009 and their baseline interview, they were identified through their National Health Index number as having an ICD-10 Code relating to diabetes or having received a prescription for diabetes medication. The relevant ICD-10 codes are reported in Supplementary Information, Table S1.
Socioeconomic status was estimated using a participant’s residential neighbourhood decile, as classified by the New Zealand Index of Deprivation 2013 (NZDep2013) (ranging from 1 to 10, with 1 representing the least deprived areas). Height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg. Body mass index (BMI) was calculated as weight (kg)/height (m2).
A non-fasting blood sample was collected and stored at a temperature of − 80 °C with samples subsequently analysed for total cholesterol, high-density lipoprotein cholesterol (HDL-C) and serum creatinine. Estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration Equation [22].
Statistical analysis
Data were analysed collectively and were also divided into aortic pulse wave velocity quartiles. The characteristics of the study population, by incident CKD status and by quartile of aortic pulse wave velocity were shown as mean ± SD or median (IQR) for continuous measures and frequency (%) for categorical measures.
The incidence rate of CKD for person-years was calculated as the number of incident cases/the total duration of follow-up (for each participant being the earlier of death date or 31 August, 2022). Comparisons of baseline characteristics, between participants who developed CKD and those who did not, were performed using t tests for continuous variables and Chi-square tests for categorical variables. We censored participants who died during the follow-up period.
Multivariable Cox regression models were used to evaluate the association between arterial stiffness as a continuous variable (measured by aortic pulse wave velocity, estimated carotid-femoral pulse wave velocity and aortic pulse pressure) and incident CKD. The analysis employed hazard ratios (HRs) with 95% CIs. We also computed HRs based on a SD increase of each arterial stiffness measure (sHR) and for quartiles of each arterial stiffness measure using the lowest level of each arterial stiffness measure as the reference group. Covariates were selected for inclusion in the models based on similar studies [9, 10, 13, 23,24,25,26].
Model 1 was adjusted for age, sex, ethnicity, BMI, eGFR, vitamin D treatment group and aortic mean arterial pressure. Model 2 comprised Model 1 plus lifestyle factors (tobacco smoking, alcohol drinking and vigorous physical activity), NZDep2013, history of CVD, diabetes/prediabetes, medications (diabetes, antihypertensives, lipid-lowering), total cholesterol, HDL-C and heart rate. Kaplan–Meier curves, stratified by quartiles of each arterial stiffness measure, were used to show the relationship between arterial stiffness and incident CKD.
SAS V9.4 (Cary, North Carolina, USA) was used for statistical analysis and all statistical tests were 2-sided.
Sensitivity analysis
To assess the influence of participants with undiagnosed CKD at baseline, we performed a sensitivity analysis where, in addition to any participants with identified CKD at baseline, we also excluded any other participants who had an eGFR < 60 ml/min per 1.73 m2, as one of the criteria for diagnosing CKD is having this reading for > 3 months [1].
Results
Baseline characteristics
Baseline characteristics of the participants (n = 4838) are presented in Table 1, overall, by incident CKD status and by quartile of baseline aortic pulse wave velocity. Mean age was 66 ± 8 years and 58% were men. The majority (84%) were European/other ethnicity, 5% identified as Māori, 6% as Pacific and 5% as South Asian. Mean ± SD arterial stiffness at baseline, as measured by aortic pulse wave velocity was 9.50 ± 1.75 m/s [median (IQR): 9.38 (2.47)], estimated carotid-femoral pulse wave velocity, 10.97 ± 1.72 m/s and aortic pulse pressure, 68 ± 16 mmHg. Of those participants, 4802 (99%) had ≥ 1 CVD risk factor.
Incident CKD
The mean ± SD length of follow up was 10.5 ± 0.4 years, during which 376 participants developed CKD, with a higher cumulative incidence for Māori (11%), Pacific (17%) and South Asian (10%), compared to European/other (7%) (Table 1).
Table 2 shows associations between measures of arterial stiffness and risk of incident CKD both overall, and by quartile of each arterial stiffness measure. The incidence of CKD overall was 7.38 per 1000 person years.
In model 1, each SD increase in aortic pulse wave velocity was associated with a 69% greater risk of incident CKD (sHR, 1.69, 95% CI 1.45–1.97), estimated carotid-femoral pulse wave velocity was associated with an 84% greater risk of incident CKD (sHR 1.84, 95% CI 1.54–2.19) and aortic pulse pressure with a 37% greater risk of incident CKD (sHR 1.37, 95% CI 1.22–1.53) after adjusting for age, sex, ethnicity, BMI, vitamin D treatment group, aortic mean arterial pressure and eGFR. The sHRs remained significant in model 2, which also adjusted for lifestyle factors, medications, diabetes/prediabetes, history of CVD, NZDep2013, heart rate, total cholesterol and HDL-C.
Figure 1 shows that the cumulative incidence of CKD increased with each increasing arterial stiffness quartile. In model 1, the risk of incident CKD was, compared to the first quartile, higher in the fourth quartile of aortic pulse wave velocity (HR 4.72, 95% CI 2.69–8.27), estimated carotid-femoral pulse wave velocity (HR 4.28, 95% CI 2.45–7.50) and aortic pulse pressure (HR 2.71, 95% CI 1.88–3.91). These associations remained significant when we adjusted for the additional covariates in model 2. There was a significant positive trend for the quartiles of all measures of arterial stiffness for both model 1 and model 2.
Applying aortic pulse wave velocity in model 1, aortic mean arterial pressure (HR 0.98, 95% CI 0.97–0.99), sex (HR for men 2.74, 95% CI 2.17–3.46), ethnicity [European/other, reference; Māori (HR 2.45, 95% CI 1.63–3.68); Pacific (HR 2.67, 95% CI 1.91–3.75); South Asian (HR 3.76, 95% CI 2.43–5.83)], BMI (HR 1.09, 95% CI 1.07–1.11) and eGFR (HR 0.92, 95% CI 0.91–0.93) were all associated with an increased risk of incident CKD (Supplementary Information, Table S2). These associations remained significant when aortic pulse wave velocity was substituted for estimated carotid-femoral pulse wave velocity or aortic pulse pressure. After adjusting for the additional covariates in model 2, all associations, for all models, apart from South Asian for aortic pulse wave velocity and aortic pulse pressure, remained significant. In addition, in model 2, tobacco smoking, vigorous physical activity, NZDep2013, CVD, diabetes and medications (antihypertensives, diabetic) were also associated with incident CKD.
Sensitivity analysis
We undertook sensitivity analysis where participants with a baseline eGFR < 60 ml/min per 1.73 m2 (n = 759) were excluded from the analysis (Supplementary Information, Table S3). For both models, when arterial stiffness was measured by aortic pulse wave velocity and estimated carotid-femoral pulse wave velocity, the risk of incident CKD was slightly higher than for when people with a baseline eGFR < 60 ml/min per 1.73 m2 were also included (for model 1: sHR 1.74, 95% CI 1.40–2.17; sHR 1.89, 95% CI 1.47–2.44, respectively) and was substantively the same for aortic pulse pressure. There was also a significant positive trend for the quartiles of all arterial stiffness measures for both models.
Discussion
In a large population-based study, with a follow-up of 10.5 years, higher arterial stiffness, as measured by aortic pulse wave velocity, estimated carotid-femoral pulse wave velocity and aortic pulse pressure, was associated with an increased risk of developing CKD. Further, our results showed that as the quartile of each arterial stiffness measure increased so did the risk of incident CKD.
To our knowledge, this is the first large population study that has investigated the association of arterial stiffness with incident CKD using oscillometrically-measured arterial stiffness. Our study findings are consistent with other large-scale population studies that have found positive associations between arterial stiffness and incident CKD. Those studies which employed carotid-femoral pulse wave velocity include the Framingham Heart Study [8] (sHR 1.17, 95% CI 1.01–1.35) and the Health ABC Study [9] (incident rate ratio per doubling of carotid-femoral pulse wave velocity 1.42, 95% CI 1.12–1.81). Positive associations were also found where arterial stiffness was measured utilising brachial-ankle pulse wave velocity [10] (odds ratio per m/s higher pulse wave velocity 1.36, 95% CI 1.09–1.70) and heart-ankle pulse wave velocity [11] (sHR 1.12, 95% CI 1.05–1.18).
In contrast, no relationship was found between carotid-femoral pulse wave velocity and incident CKD in the Rotterdam Study [13] (relative risk per SD increase in pulse wave velocity 1.05, 95% CI 0.99–1.31) or in the AGE-RS Study [12] (odds ratio per m/s higher pulse wave velocity 1.16, 95% CI 0.84–1.61). However, the Rotterdam Study [13] may have been subject to selection bias having pulse wave velocity data for only 2665 out of 3666 participants, while the AGE-RS Study [12] suffered from attrition bias after losing 310 out of 940 participants to follow-up. These studies, aside from the Health ABC Study [9], had mainly ethnically homogeneous populations and were unable to report results by ethnicity.
Māori and Pacific in this study had a higher risk of incident CKD than their European/other counterparts. In addition to having the highest prevalences of socio-economic deprivation in New Zealand, these people also have the highest prevalences of obesity, and hence diabetes and CVD [27]. This in turn is a large driver of the high CKD prevalence in this population. Māori and Pacific, however, still had higher risk of CKD in our analysis when we controlled for NZDep2013, BMI, physical activity, diabetes and CVD risk factors. Although diabetic nephropathy is the major cause of CKD in Māori and Pacific people, there is increasing evidence that there may be a familial or ethnic predisposition to CKD that is not due to diabetes or hypertension [28, 29].
The kidney is a high blood flow organ with low resistance vascular beds. Increased arterial stiffness exposes the smaller arteries of the kidney to increased pulsatile stress, possibly resulting in damage to endothelial and vascular smooth muscle cells [5]. Capillaries of the glomerulus, which are situated between afferent and efferent arterioles, may be particularly susceptible to damage, as the efferent arterioles have greater resistance than the afferent arterioles which leads to high pulsatile pressure in the glomerulus [5].
Our study has certain strengths. First, we had a long follow-up period. Second, as our study also included Māori, Pacific peoples and South Asian ethnicities, our results were generalizable to those populations and not just Europeans. Third, as outcomes were ascertained from hospital discharge registers, there was no loss to follow-up of participants.
This study has some limitations. First, as we did not clinically assess CKD, we may not have excluded all people with CKD from our initial study cohort. We did, however, control for eGFR. Further, in population studies it is widely accepted that CKD can be established with an eGFR < 60 ml/min per 1.73 m2 [30]. When we removed people at baseline who fit this definition of CKD, our conclusions remained unaltered. Second, as we had to rely on hospitalisations to identify CKD, not all people who developed CKD would have been identified. Third, as the study is observational, we cannot be completely sure of causality.
To combat the global prevalence of CKD, and as people with this condition are generally asymptomatic, new approaches to the identification and prevention of CKD are needed. We found that arterial stiffness, as measured by aortic pulse wave velocity, estimated carotid-femoral pulse wave velocity and aortic pulse pressure, was positively associated with future CKD development. Arterial stiffness, which can be easily and accurately measured, could be used as a tool in clinical practice (as part of routine BP measurement and diabetes monitoring) to help identify people at risk of CKD.
Data availability
Participants gave consent for data to be available only to the study research team
References
Levey AS, Eckardt KU, Dorman NM, Christiansen SL, Hoorn EJ, Ingelfinger JR et al (2020) Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Kidney Int 97(6):1117–1129. https://doi.org/10.1016/j.kint.2020.02.010
Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C (2019) A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Kidney Int 96(5):1048–1050. https://doi.org/10.1016/j.kint.2019.07.012
Kidney Health Australia (2020) Chronic kidney disease (CKD) management in primary care, 4th edn. Kidney Health Australia, Melbourne
Ministry of Health (2015) Managing chronic kidney disease in primary care: a national consensus statement. Ministry of Health, Wellington
Chirinos JA, Segers P, Hughes T, Townsend R (2019) Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol 74(9):1237–1263. https://doi.org/10.1016/j.jacc.2019.07.012
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D et al (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27(21):2588–2605. https://doi.org/10.1093/eurheartj/ehl254
Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T (2013) Oscillometric estimation of aortic pulse wave velocity: Comparison with intra-aortic catheter measurements. Blood Press Monit 18(3):173–176. https://doi.org/10.1097/MBP.0b013e3283614168
Vasan RS, Pan S, Xanthakis V, Beiser A, Larson MG, Seshadri S, Mitchell GF (2022) Arterial stiffness and long-term risk of health outcomes: the Framingham Heart Study. Hypertension 79(5):1045–1056. https://doi.org/10.1016/hypertensionaha.121.18776
Madero M, Peralta C, Katz R, Canada R, Fried L, Najjar S et al (2013) Association of arterial rigidity with incident kidney disease and kidney function decline: the Health ABC Study. Clin J Am Soc Nephrol 8(3):424–433. https://doi.org/10.2215/cjn.07900812
Tomiyama H, Tanaka H, Hashimoto H, Matsumoto C, Odaira M, Yamada J et al (2010) Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis 212(1):345–350. https://doi.org/10.1016/j.atherosclerosis.2010.05.033
Nagayama D, Fujishiro K, Miyoshi T, Horinaka S, Suzuki K, Shimizu K, Saiki A, Shirai K (2022) Predictive ability of arterial stiffness parameters for renal function decline: a retrospective cohort study comparing cardio-ankle vascular index, pulse wave velocity and cardio-ankle vascular index0. J Hypertens 40(7):1294–1302. https://doi.org/10.1097/hjh.0000000000003137
Huang N, Foster MC, Mitchell GF, Andresdottir MB, Eiriksdottir G, Gudmundsdottir H et al (2017) Aortic stiffness and change in glomerular filtration rate and albuminuria in older people. Nephrol Dial Transplant 32(4):677–684. https://doi.org/10.1093/ndt/gfw050
Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, Franco OH, Dehghan A (2015) Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol 10(12):2190–2197. https://doi.org/10.2215/cjn.03000315
Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS et al (2009) Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 119(17):2408–2416. https://doi.org/10.1161/circulationaha.109.192278
The Reference Values for Arterial Stiffness’ Collaboration (2010) Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values.’ Eur Heart J 31(19):2338–2350. https://doi.org/10.1093/eurheartj/ehq165
Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, Murphy J, Khaw KT, Camargo CA Jr (2017) Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study: a randomized clinical trial. JAMA Cardiol 2(6):608–616. https://doi.org/10.1001/jamacardio.2017.0175
Scragg R, Waayer D, Stewart AW, Lawes CMM, Toop L, Murphy J, Khaw KT, Camargo CA Jr (2016) The Vitamin D Assessment (ViDA) Study: design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non-vertebral fractures. J Steroid Biochem Mol Biol 164:318–325. https://doi.org/10.1016/j.jsbmb.2015.09.010
Climie RE, Schultz MG, Nikolic SB, Ahuja KD, Fell JW, Sharman JE (2012) Validity and reliability of central blood pressure estimated by upper arm oscillometric cuff pressure. Am J Hypertens 25(4):414–420. https://doi.org/10.1038/ajh.2011.238
Tien LYH, Morgan WH, Cringle SJ, Yu DY (2023) Optimal calculation of mean pressure from pulse pressure. Am J Hypertens 36(6):297–305. https://doi.org/10.1093/ajh/hpad026
Greve SV, Blicher MK, Kruger R, Sehestedt T, Gram-Kampmann E, Rasmussen S et al (2016) Estimated carotid-femoral pulse wave velocity has similar predictive value as measured carotid-femoral pulse wave velocity. J Hypertens 34(7):1279–1289. https://doi.org/10.1097/hjh.0000000000000935
Bao W, Chen C, Chen C, Zhang X, Miao H, Zhao X, Huang S, Li C (2023) Association between estimated pulse wave velocity and risk of diabetes: a large sample size cohort study. Nutr Metab Cardiovasc Dis 33(9):1716–1724. https://doi.org/10.1016/j.numecd.2023.05.032
Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T et al (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367(1):20–29. https://doi.org/10.1056/nejmoa1114248
Gottsäter M, Östling G, Persson M, Engström G, Melander O, Nilsson PM (2015) Non-hemodynamic predictors of arterial stiffness after 17 years of follow-up: the Malmö Diet and Cancer study. J Hypertens 33(5):957–965. https://doi.org/10.1097/hjh.0000000000000520
Li J, Cui L, Zhang X, Hou J, Wang A, Wu Y et al (2021) Longitudinal study of brachial-ankle pulse wave velocity and change in estimated glomerular filtration rate among Chinese adults. Kidney Blood Press Res 46(3):266–274. https://doi.org/10.1159/000510611
Liu JJ, Liu S, Lee J, Gurung RL, Yiamunaa M, Ang K et al (2020) Aortic pulse wave velocity, central pulse pressure, augmentation index and chronic kidney disease progression in individuals with type 2 diabetes: a 3 year prospective study. BMC Nephrol 21(1):359. https://doi.org/10.1186/s12882-020-02024-z
Upadhyay A, Hwang SJ, Mitchell GF, Vasan RS, Vita JA, Stantchev PI et al (2009) Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 20(9):2044–2053. https://doi.org/10.1681/asn.2009010074
Ministry of Health (2016) Annual update of key results 2015/16: New Zealand Health Survey. Ministry of Health, Wellington
Thompson CF, Simmons D, Collins JF, Cecil A (2001) Predisposition to nephropathy in Polynesians is associated with family history of renal disease, not diabetes mellitus. Diabet Med 18(1):40–46. https://doi.org/10.1046/j.1464-5491.2001.00406.x
Walker RJ, Tafunai M, Krishnan A (2019) Chronic kidney disease in New Zealand Māori and Pacific people. Semin Nephrol 39(3):297–299. https://doi.org/10.1016/j.semnephrol.2019.03.001
Bash LD, Coresh J, Köttgen A, Parekh RS, Fulop T, Wang Y, Astor BC (2009) Defining incident chronic kidney disease in the research setting: the ARIC Study. Am J Epidemiol 170(4):414–424. https://doi.org/10.1093/aje/kwp151
Acknowledgements
We thank all the research staff at the University of Auckland for help conducting the ViDA study and the New Zealand Ministry of Health for providing the hospitalisation and prescription dispensing data. We also thank the study participants and their family physicians for facilitating their participation in the ViDA Study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Angela Beros was supported by a University of Auckland Doctoral Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Ethical approval
The study was conducted in accordance with standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Human and animal rights
This study involving human participants was approved by the New Zealand Ministry of Health Multi-region Ethics committee (MEC/09/08/082).
Informed consent
Informed consent was obtained from all individual participants prior to their enrolment in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beros, A., Sluyter, J., Hughes, A. et al. Arterial stiffness and incident chronic kidney disease: a large population-based cohort study. J Nephrol 37, 1241–1250 (2024). https://doi.org/10.1007/s40620-024-01968-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-024-01968-x