Abstract
Introduction
Exposure to high ambient temperatures is associated with a risk of acute kidney injury. However, evidence comes from emergency departments or extreme weather exposures. It is unclear whether temperature-related adverse kidney outcomes can also be detected at a community level in a temperate climate zone.
Methods
In a 9.5-year retrospective cohort study we correlated estimated glomerular filtration rate (eGFR) values of Swiss adult primary care patients from the FIRE cohort (Family medicine Research using Electronic medical records) with same-day maximum local ambient temperature data. We investigated 5 temperature groups (< 15 °C, 15–19 °C, 20–24 °C, 25–29 °C and ≥ 30 °C) as well as possible interactions for patients with increased kidney vulnerability (chronic heart failure, diabetes, chronic kidney disease, therapy with renin–angiotensin–aldosterone-system (RAAS) inhibitors, diuretics or non-steroidal anti-inflammatory drugs).
Results
We included 18,000 primary care patients who altogether provided 132,176 creatinine measurements. In the unadjusted analysis, higher ambient temperatures were associated with lower eGFR across all age and vulnerability groups. In the adjusted models, we did not find a consistent association.The highest ambient temperature differences (> 25 or > 30 versus < 15 °C) were associated with marginally reduced kidney function only in patients with ≥ 3 risk factors for kidney vulnerability, with a maximum estimated glomerular filtration rate reduction of −2.9 ml/min/1.73m2 (SE 1.0), P 0.003.
Discussion
In a large primary care cohort from a temperate climate zone, we did not find an association between ambient temperatures and kidney function. A marginal inverse association in highly vulnerable patients is of unclear clinical relevance.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in heat-related kidney injury has considerably increased during recent years. Ninety-three percent of all research articles on the impact of heat on kidney health have been published within the last decade [1].
The fact that heat stress increases the risk of dehydration-related kidney damage is intuitive and beyond controversy [2]. Evidence includes meta-analytic data of increased hospitalizations with acute kidney injury (AKI) during periods of higher ambient temperatures [3] and investigations of acute and chronic kidney damage following occupational heat exposures from sunlight in agricultural and construction work or from heat processes in factories [4, 5].
However, evidence comes from emergency departments, hospital admissions or extreme scenarios like long strenuous work in very high temperatures. It is unclear whether temperature-related adverse kidney outcomes can also be detected in non-hospital-based general medicine practices within the community. This is of interrest especially in the context of global warming.
In view of the repeatedly established association between high ambient temperatures and emergency department visits with adverse kidney outcomes, we hypothesized that kidney function might be affected to some degree by temperature at the level of pre-hospital general medicine.
A Japanese study involving 102 hypertensive community-dwelling patients found reduced kidney function during summer in those with and without chronic kidney disease (CKD) [6]. Relative declines in estimated glomerular filtration rate (eGFR) were greater in CKD patients with an eGFR decrease of −13.8% (SD 9.4) during summer. However, a Chinese study of 109 community-dwelling CKD patients did not find such an association although blood pressure values were inversely correlated with outdoor temperatures [7]. Larger studies on seasonal temperature changes and kidney function in community settings do not exist. Kidney protective counseling has proven successful in very hot weather scenarios [8]. If we were to observe that kidney function is affected by ambient temperatures even within non-emergency settings, patient care could be improved by placing increased focus on patients at risk.
Taking advantage of both a large database, representative of the Swiss primary care population, and national meteorology data with highly accurate temporal and spatial resolution, we were able to match ambient temperatures at the general practitioner (GP) clinics with patients` creatinine values and medical data. We are the first to provide data on the association of ambient temperatures and kidney function in a large primary care cohort living in a temperate climate zone.
Methods
This is a retrospective cohort study: we analyzed data of primary care patients registered in the FIRE (Family medicine—Research using Electronic medical records) database. The FIRE project was established in 2009 and is an ongoing research project at the Institute of Primary Care of the University of Zurich, Switzerland. It provides structured routine medical data from Swiss primary care [10,11,12]. In brief, the database covers patient demographics, vital signs, laboratory data and prescribed medication according to Anatomical Therapeutic Chemical (ATC) coding [13, 14] as well as diagnoses based on the ICPC-2 classification (International Classification of Primary Care 2) [15]. Participating GPs extract data in an anonymized way from their electronic medical records. These data are then centrally pooled in the FIRE database and aggregated by individual consultation dates. Until August 2018, more than 400 GPs participated, representing over 5 million consultations.
Inclusion and exclusion criteria
We considered all patients in the FIRE database for whom creatinine measurements were available between 1st January, 2009 and 31st August, 2018 as potentially eligible. We excluded patients < 18 and > 100 years of age and those with < 4 creatinine measurements. We used the highest eGFR value of each patient to define his or her baseline kidney function. Therefore, we excluded these values from the analysis. A flowchart of patients and data selection process is presented in Supplemental Fig. 1.
Definitions
Kidney function
We calculated creatinine based eGFRs according to the chronic kidney disease epidemiology collaboration (CKD—EPI) formula [16] to describe the level of kidney function on a given day. For the definition of kidney disease as a vulnerability factor, we considered an individual as being affected by CKD when his/her highest eGFR value (calculated from the lowest creatinine measurement) was < 60 ml/min/1.73m2.
Temperature
To determine the temperature that an individual patient was exposed to on a given day of creatinine measurement, we synchronized the SwissMeteo-dataset (stratified by region) with geographical information of the individual patient’s GP’s practice. We used the maximum temperature of the day during which eGFR was measured. For the analyses we stratified the temperature into 5 ranges, < 15 °C, 15–19 °C, 20–24 °C, 25–29 °C and ≥ 30 °C. The MeteoSwiss-dataset contains maximum daily temperatures as gridded data with a spatial resolution of 2.2 km. These data were interpolated based on measured air temperatures at 2 m above ground level from approximately 90 stations and considering elevations [17]. The uncertainty of the gridded temperature values depends on the network density and hence varies from location to location. The uncertainty is of about 0.6 °C, but slightly higher during winter. A detailed discussion on the interpolation method and the associated uncertainties is provided by Frei (2014). For each GP, the temperature was interpolated from the neighboring cells with a weight given to the distance from the GP to the center of the grid cell.
Kidney vulnerability factors
We defined five factors that potentially increase kidney vulnerability to high ambient temperature with respect to kidney function: heart failure (HF), CKD, diabetes mellitus, RAAS inhibitors or diuretics and non-steroidal anti-inflammatory/antirheumatic drugs.
Applying these factors, we stratified our cohort into individuals with no kidney vulnerability factor (= vulnerability group 0) and those with 1, 2, 3, 4 or 5 vulnerability factors (vulnerability groups 1–5). Group 1 includes patients who exhibit any one of the above-mentioned vulnerability factors (no matter which one), while group 5 patients exhibit all 5 of the above-mentioned vulnerability factors. For the definition of the vulnerability factors and chronic diseases, we used the International Classification of Primary Care version 2 (ICPC-2) [15] adapted by O’Halloran et al. [14], the Anatomical Therapeutic Chemical (ATC) codes of medication adapted by Lamers et al. [18] as well as clinical measurements from the primary care visits. We defined HF by ICPC code K77 and diabetes mellitus using ICPC codes T89, T90, ATC code A10 as well as HbA1c values > 6.5%. We defined CKD as a creatinine-based eGFR of < 60 ml/min/1.73m2 according to CKD–EPI. We defined RAAS inhibitors by ATC code C09, diuretics by ATC codes C03 and non-steroidal anti-inflammatory/antirheumatic drugs by ATC codes M01A and M01B. We included only these medications, prescribed at least twice and at least six months apart, thus ensuring the inclusion of long-term medication.
Other patient characteristics
We used the following patient characteristics to further describe our cohort: age (stratified as ≤ 39, 40–49, 50–59, 60–69, 70–79, ≥ 80 years of age), body mass index (BMI) (stratified according to WHO) [19], arterial hypertension (presence and grading based on the mean value of all blood pressure measurements of each individual during the study, stratified according to European Society of Cardiology/European Society of Hypertension (ESC/ESH 2018) [20], CKD eGFR categories (according to KDIGO 2012) and number of chronic diseases (according to the International Classification of Primary Care version 2 (ICPC-2) [15] adapted by O’Halloran et al. [14], ATC codes of medication adapted by Lamers et al. [18] as well as clinical measurements from the primary care visits.
Statistical analysis
Descriptive data are presented as frequencies and percentages, mean and standard deviation (SD), or median and interquartile range (IQR), where appropriate. If not indicated otherwise, all percentages refer to the total number of included patients. For the univariate tables we used all available creatinine measurements of the included patients (dataset A). To visually present the effect of temperature on eGFR, a plot was created considering age group and vulnerability. Since the huge amount of single data points would not provide any information, a smoothed line was added using cubic spline and a 95% confidence region. To consider maximal eGFR of the patient as an explainable variable in the regression analysis, the maximum eGFR observation was removed from dataset A resulting in a reduction of creatinine measurements available for further analysis (dataset B). To study the effect of each variable of interest we performed a 3-level (measurment, patient and practice), bivariable and multivariable regression, also known as a mixed-model. Bivariable regression contains age and one other variable. Age was centered with a median age of 71. To consider the nonlinearity of age we used a cubic B-splines smoothing technique with 15 knots. We included interaction effect between temperature category and vulnerability level. Temperature was categorized: < 15, 15–19, 20–24, 25–29, ≥ 30 °C. To avoid data loss due to missing BMI values, patients without BMI value were categorized as not available (NA). Taken together, the multivariable regression is: GFR = B–Splines of age (centered) + sex + BMI-group + hypertension category + number of chronic disease + eGFR category according to KDIGO 2012 + temperature category x vulnerability + random effect of practice + random effect of patient within practice. Data were analyzed using R® Version 3.5.0.
Results
We included 18,000 patients from 51 GP practices who provided a total of 132,176 creatinine measurements. Mean age was 68.8 (SD 14.8) years, 51.8% were female. 31.4% suffered from chronic kidney disease, 24% had diabetes, mean BMI was 28.0 kg/m2 (SD 5.6) and 46.4% showed at least first grade mean hypertensive blood pressure values during the study period. Mean number of chronic diseases was 3.9 (SD 3.0) and patients received a cumulative average of 8.7 (SD 7.3) medications during the study period. Based on the highest eGFR value of each participant throughout the study period, patients were subdivided according to KDIGO 2012 as follows; 51.3% as G1, 38.1% as G2, 6.8 and 3% as G3a and 3b, respectively, 0.7% as G4, 0.1% as G5. Mean eGFR was 72.6 ml/min/1.73m2 (SD 25.5) in men and 75.5 ml/min/1.73m2 (SD 24.7) in women (Table 1).
eGFR, age and temperature
To highlight how age affected eGFR, all GFR estimates were plotted against age. From age 40 onward, eGFR decreased by 10 mL/min1.73m2 for each 10 year increase (Supplemental Fig. 2).
Temperature
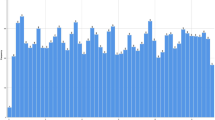
For the 132,176 creatinine measurements at the GP clinics, we had corresponding temperature data (same day, same area) for 100% of the cases. During the 9-year study period, mean maximum daily temperature was 14.6 °C (SD 9.0). Seasonal temperature changes are shown in Fig. 1b. During winter (December 22nd to March 20th), mean maximum daily temperature was 5.8 °C (SD 5.3), while it was 25.7 °C (SD 5.0) during summer (June 21st–September 23rd). Temperature ranged from a minimum of −13.4 °C to a maximum of 37.4 °C. We identified 5743 patients and 7474 measurements meeting temperature ≥ 30 °C. The average number of days per year during which the temperature exceeded 30 °C for the study period varied from zero to 33 days (Fig. 1a).
Spatial distribution and frequency of temperatures > 30 °C (a) and temperature changes (b) during the course of the study. a The map shows the average number of days per year during which the threshold temperature of 30 °C was exceeded throughout the study period. The black crosses indicate the location of the GPs. Grid cells that never exceeded the threshold are not shown. The source of the underlying relief map is the Swiss Federal Office of Topography. b Each point is the maximum of the temperature for each GP practice on each day. GP general practitioner. Dashed blue line represents mean of the daily maximum temperature over the entire observation period. Dashed red line represents temperature threshold of 30 °C
eGFR according to ambient temperature in the entire cohort
In the overall population, the unadjusted association of ambient temperature with eGFR was significant but discrete as can be appreciated in Fig. 2. Starting at ambient temperatures of around 10 °C, the higher the maximum daily temperature, the lower the associated eGFR value (Fig. 2).
Kidney vulnerability
In our population, 2088 (8.9%) participants had no vulnerability condition (group 0). Among participants, 6848 (29.2%), 8298 (35.3%), 4893 (20.8%), and 1240 (5.3%) had one, two, three and four vulnerability conditions (group 1 to 4), respectively, and 108 (0.5%) participants exhibited all five vulnerability conditions (group 5). The sum of all patients is higher than the number of included patients since any given patient could change vulnerability status during the study period. Supplemental Fig. 3 shows the distribution of the vulnerability conditions according to vulnerability group.
Unadjusted analysis of kidney vulnerability subgroups
The greater the presence of kidney vulnerability conditions, the lower the observed eGFR. This was expected since CKD was one of the vulnerability conditions and the other risk factors such as HF, diabetes mellitus or chronic NSAID use can all cause a chronic decrease in eGFR. In addition, RAAS inhibitor treatment leads to a reduction in eGFR by reducing glomerular filtration. Figure 3 displays the smoothed curve of eGFR values against temperature with 95% confidence interval considering age group and vulnerability. Confidence intervals widen at both ends of the curves due to a lower number of data points at extremes of temperatures. Interestingly, in patients without kidney risk factors there was no association between temperature and eGFR regardless of age. The presence of two or more vulnerability conditions however, seems to increase the risk of eGFR deterioration with higher temperatures, again regardless of age.
Unadjusted association between temperature and kidney function according to age and number of vulnerabilities. Smoothed curve of eGFR values against temperature with 95% confidence region considering age and vulnerability. Since age group < 60 with vulnerability = 5 only entailed 4 patients with 21 observations it was not considered in the plot. eGFR estimated glomerular filtration rate
Multivariable analysis regarding the influence of vulnerability status on temperature sensitivity of eGFR
As was seen in the unadjusted analysis, in the multivariable model we observed a significant negative correlation between the number of vulnerability factors and eGFR at T < 15 °C, (Table 2). In patients with zero kidney vulnerability factors we did not find an independent association between ambient temperature and eGFR except for the comparison between the temperature groups of 25–30 °C versus < 15 °C with an eGFR decline of −0.7 ml/min/1.73m2 (SE 0.3); P 0.03 (Table 2). With regard to the subgroups of patients exhibiting conditions that increase kidney vulnerability, significant inverse associations with eGFR were seen in vulnerability groups 3, 4 and 5 at higher temperatures. In group 3, the effect was −1.5 mL/min/1.73m2 at ≥ 30 °C (SE 0.6); P 0.015, in group 4 it was −1.9 mL/min/1.73m2 at ≥ 30 °C (SE 0.7); P 0.01, and in group 5 it was −2.9 mL/min/1.73m2 at 25–29 °C (SE 1.0); P 0.003. The effect at ≥ 30 °C was not significant; however there was only a low number of data points. In the remaining temperature and vulnerability groups, we did not detect a significant association between ambient temperature and eGFR, (Table 2). Taken together, at least > two risk factors had to be present to detect a robust association between higher outdoor temperature and lower eGFR. Except for gender, all factors chosen for adjustment were independently associated with significant eGFR changes (Supplemental Table 1).
Discussion
The main result of our study is that in a population encompassing 18,000 Swiss primary care patients, ambient temperatures > 25 °C (compared to < 15 °C) were significantly associated with a mild eGFR decline (−2.9 ml/min/1.73m2) only in patients with more than two kidney vulnerability factors.
Other studies investigating community settings were smaller or focused on special disease populations. Older hypertensive Japanese adults with and without CKD showed decreased eGFR values during summer (eGFR-7.1 ± 7.0 and −5.8 ± 5.2 ml/min/1.73m2, respectively) [6]. Italian elective cardiac surgery patients displayed elevated creatinine values during episodes of increased ambient temperatures (103 ± 61.6 vs. 98.7 ± 74.8 μmol/L, P < 0.001) [9]. However, Chinese CKD patients showed no association of kidney function with ambient temperature [7].
A recent meta-analysis suggested a relative risk (RR) of 1.012 (95% CI 1.009–1.015) for acute kidney injury (AKI) for every 1 °C increase in ambient temperature [3]. However, studies were exclusively conducted in hospital or emergency department settings. We might have missed more severe health conditions such as AKI during high ambient temperatures as these patients may have presented directly to the hospital and not to their GP.
Several factors potentially explain the absence of a robust correlation between ambient temperatures and kidney function in patients without kidney vulnerability. First, temperatures in our temperate climate zone might not be extreme enough to overwhelm the body’s compensatory mechanisms. Second, we assessed neither the participant’s behaviors and location, nor individual body temperature. Hence, it is unknown which temperatures individuals were actually exposed to. We used maximum temperature on the day of creatinine measurement, with the hypothesis of maximum volume depletion risk at the highest temperatures. Others used daytime or an average of temperatures over several days [22, 23], potentially highlighting more sustained and therefore more pronounced dehydration stressors. However, the extent of daily temperature variability is not captured by both approaches and comparative studies are needed.
With regard to kidney vulnerability, Xu et al. also found an increased risk of worsening kidney function during high ambient temperatures in patients with HF, CKD, diabetes and hypertension, which validates the conditions we chose [24]. Interestingly, our analysis of kidney vulnerability did not show age as a relevant risk factor for temperature-associated eGFR decrease.
The temperature-associated changes of eGFR we found in highly vulnerable patients may appear to be clinically negligible. However, annual eGFR changes of comparable magnitude have been associated with significantly increased mortality [25, 26]. Furthermore, as kidney autoregulation usually maintains GFR independent of systemic blood pressure [27], a mild decrease in eGFR may indicate relevant hemodynamic alterations, which potentially increase the risks for other circulatory sequelae such as stroke or myocardial ischemia.
Our study has several strengths. It is the first investigation of ambient temperature and kidney function in a large, unselected primary care cohort, providing more than 9 years of real life data for a western European population in tempered continental weather conditions. Analyzed data are of high validity as they are extracted from digital records ascertained and documented exclusively by GPs. Our study also has limitations. We could not ascertain patient behaviors or body temperatures, hence, we do not know which temperature an individual patient was exposed to on a given day. Further, a creatinine value measured during high temperatures was not necessarily from the same patient as a creatinine value measured during low temperatures in our comparative analysis. However, as our cohort comprises 132,176 creatinine measurements from 18,000 patients and as we adjusted for important confounders, we consider our results to be valid even without data on the individual’s longitudinal follow-up.
In summary, we did not find a biologically significant association between seasonal temperature variability and kidney function within a general medicine ambulatory care population. This is somewhat in contrast with data from emergency and hospital settings, where an association between ambient temperatures and kidney damage has repeatedly been observed. With regard to potential public health implications, and in light of global warming, further research is needed to understand how data from general medicine practices may contribute to identifying individual patients at risk so as to prevent temperature-related kidney damage. For future studies, prospective designs and both incorporation of behavioral data and individuals’ body temperatures might provide a considerable contribution to the topic.
Data availability
We will share the data of our investigation upon reasonable request.
References
Al-Bouwarthan M, AlMulla AA, Yaseen M (2022) The impact of heat on kidney health: a PRISMA-compliant bibliometric analysis. Medicine (Baltimore) 101(36):e30328
Chapman CL et al (2021) Kidney physiology and pathophysiology during heat stress and the modification by exercise, dehydration, heat acclimation and aging. Temperature (Austin) 8(2):108–159
Liu J et al (2021) Hot weather as a risk factor for kidney disease outcomes: a systematic review and meta-analysis of epidemiological evidence. Sci Total Environ 801:149806
Alayyannur PA, Ramdhan DH (2022) Relationship of heat stress with acute kidney disease and chronic kidney disease: a literature review. J Public Health Res 11(2):22799036221104148
Sorensen C, Garcia-Trabanino R (2019) A new Era of climate medicine-addressing heat-triggered renal disease. N Engl J Med 381(8):693–696
Masugata H et al (2011) Seasonal variation in estimated glomerular filtration rate based on serum creatinine levels in hypertensive patients. Tohoku J Exp Med 224(2):137–142
Bi SH et al (2010) Seasonal changes in blood pressure in chronic kidney disease patients. Clin Nephrol 73(3):216–220
Glaser J et al (2020) Preventing kidney injury among sugarcane workers: promising evidence from enhanced workplace interventions. Occup Environ Med 77(8):527–534
Ranucci M, Castelvecchio S, La Rovere MT (2014) Renal function changes and seasonal temperature in patients undergoing cardiac surgery. Chronobiol Int 31(2):175–181
Chmiel C et al (2011) The FIRE project: a milestone for research in primary care in Switzerland. Swiss Med Wkly 140:w13142
Schumacher LD et al (2020) Trends and between-Physician variation in laboratory testing: a retrospective longitudinal study in general practice. J Clin Med 9(6):1787
Rachamin Y et al (2022) Prescription rates, polypharmacy and prescriber variability in swiss general practice—a cross-sectional database study. Front Pharmacol. https://doi.org/10.3389/fphar.2022.832994
WHO, Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2021. Oslo, Norway, 20
O’Halloran J, Miller GC, Britt H (2004) Defining chronic conditions for primary care with ICPC-2. Fam Pract 21(4):381–386
ICPC-2, World Organization of National Colleges, Academies, and Academic Associations of General Practitioners/Family Physicians (1998) ICPC-2: international classification of primary care. Oxford University Press, Oxford
Levey AS et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Frei C (2014) Interpolation of temperature in a mountainous region using nonlinear profiles and non-Euclidean distances. Int J Climatol 34(5):1585–1605
Lamers LM, van Vliet RC (2004) The Pharmacy-based Cost Group model: validating and adjusting the classification of medications for chronic conditions to the Dutch situation. Health Policy 68(1):113–121
World Health Organization. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed 19th Jan 2023
Williams B et al (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J 39(33):3021–3104
HRA, Federal Act on Research involving Human Beings (Human Research Act, HRA) of 30 September 2011 (Status as of 26 May 2021)
Lim YH et al (2018) Ambient temperature and hospital admissions for acute kidney injury: a time-series analysis. Sci Total Environ 616–617:1134–1138
Honda T, Manjourides J, Suh H (2019) Daily ambient temperature is associated with biomarkers of kidney injury in older Americans. Environ Res 179(Pt A):108790
Xu Z et al (2020) Heat and risk of acute kidney injury: an hourly-level case-crossover study in queensland. Australia Environ Res 182:109058
Turin TC et al (2013) Change in the estimated glomerular filtration rate over time and risk of all-cause mortality. Kidney Int 83(4):684–691
Cheng TY et al (2008) Mortality risks for all causes and cardiovascular diseases and reduced GFR in a middle-aged working population in Taiwan. Am J Kidney Dis 52(6):1051–1060
Carlström M, Wilcox CS, Arendshorst WJ (2015) Renal autoregulation in health and disease. Physiol Rev 95(2):405–511
Acknowledgements
We would like to thank all GPs who participate in the FIRE project and export anonymized data from their electronic medical records.
Funding
Open access funding provided by University of Zurich. The Institute of Primary Care of the University of Zurich, Switzerland, supported our study by enabling staff members to engage in the planning, analysis and reporting of the investigation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the reporting of the work. SS, SZ, FV, MS, JS, OS and HS contributed to the management of the work. SZ, MS, PEC, JS and OS contributed to the planning of the work FV, OS and HS were responsible for the overall content as guarantors. The corresponding author, HS, attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Conflict of interest
All authors completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. The authors declare that they have no relevant or material financial interests that relate to the research described in this paper.
Ethical approval
The ethics committee of the canton of Zurich approved studies within the FIRE project. According to current Swiss law on human research, retrospective cross-sectional analysis of anonymized routine medical data requires no approval by the Zürich regional ethics committee. According to the Local Ethics Committee of the Canton of Zurich, the project did not fall under the scope of the law on human research [21] and therefore no ethical approval was necessary (BASEC No: Req-2017-00797). Patient records/information were anonymized and de-identified prior to analysis.
Human and animal rights
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Patient and public involvement
With regard to our investigation, we did not directly involve patients or the public in the planning, execution and reporting of the research. However, general practitioners participating in FIRE are informed and updated on a regular basis regarding ongoing projects and published results and can contribute to new projects. Detailed information about the FIRE database, its scientific and clinical purpose and all publications are presented on the web https://www.hausarztmedizin.uzh.ch/de/fire2.html.
Dissemination to participants and related patient and public communities
Published results will be made available on the FIRE website https://www.hausarztmedizin.uzh.ch/de/fire2.html. Participating general practitioners will be informed about the results during the subsequent rotational FIRE meeting.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schietzel, S., Zechmann, S., Valeri, F. et al. Ambient temperature and kidney function in primary care patients. J Nephrol 37, 95–105 (2024). https://doi.org/10.1007/s40620-023-01715-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-023-01715-8