Abstract
COVID-19, a disease caused by a novel coronavirus (SARS-CoV-2), is a major global threat that has turned into a pandemic. Despite the emergence of multiple vaccination alternatives and developing therapeutic options, dramatic short- and long-term clinical outcomes have been recorded with more than 250 million infected people and over 5 million deaths as of November 2021. COVID-19 presents various respiratory, cardiovascular, neuropsychiatric, musculoskeletal and kidney features during the acute phase; nevertheless, renal involvement in the post-infection period has recently been emphasized. The present review aims to evaluate the growing literature on kidney involvement in the SARS-CoV-2 infection along with clinical features reported both in the acute phase of the infection and in the post-acute COVID-19 period by assessing potential pathophysiological frameworks explaining such conditions. Chronic kidney disease and development of acute kidney injury (AKI) in the course of initial hospitalization are associated with high mortality and morbidity rates. Moreover, growing evidence suggests a decline in renal function in the 6-to-12-month follow-up period even in patients without any signs of AKI during the acute phase. Despite such concerns there are no guidelines regulating the follow-up period or therapeutic alternatives for such patient population. In conclusion, the burden of COVID-19 on the kidney is yet to be determined. Future prospective large scale studies are needed with long follow-up periods assessing kidney involvement via multiple parameters such as biopsy studies, urinalysis, measurement of serum creatinine and cystatin C, directly measured glomerular filtration rate, and assessment of tubular function via urinary β2-microglobulin measurements.
Graphical abstract

Similar content being viewed by others
Introduction
COVID-19, a disease caused by a novel coronavirus (SARS-CoV-2), is a major global human threat that has turned into a pandemic [1, 2]. It is the second one among the latest outbreaks caused by a coronavirus family member, following the severe SARS-CoV and the Middle East respiratory syndrome (MERS)-CoV. COVID-19 presents various respiratory, cardiovascular, neuropsychiatric, musculoskeletal and kidney features during the acute infection phase; nevertheless, renal involvement in the post-infection period has recently been highlighted. Despite the emergence of multiple vaccination alternatives and developing therapeutic options, dramatic short- and long-term clinical outcomes have been recorded with more than 250 million infected people and over 5 million deaths as of November 2021 [3, 4]. The most common presenting symptoms include shortness of breath, fatigue, fever and cough, all of which are commonly observed between days 4 and 5 from exposure [5]. The most common causes of mortality and morbidity among infected patients are respiratory complications followed by cardiovascular events; nevertheless, pathological investigations and autopsy studies demonstrated the involvement of almost all systems, including: central and peripheral nervous, gastrointestinal, musculoskeletal and renal systems [6]. In addition to cardiovascular and respiratory comorbidities, chronic kidney disease (CKD), diabetes mellitus and immunocompromising disorders are associated with higher hospitalization and mortality rates [7].
Although various pharmaco-therapeutic alternatives including chloroquine/hydroxychloroquine, favipravir, azithromycin, lopinavir-ritonavir, molnupravir, and ivermectin have been proposed for the treatment of COVID-19, few antiviral agents such as remdesivir and nirmatrelvir-ritonavir have been approved by the US Food and Drug Administration to date [8]. Data on their safety are limited: studies so far have shown significant adverse reactions with remdesivir (high serum liver enzymes and serum creatinine, respiratory failure), chloroquine (retinal toxicity), ivermectin (neurotoxicity and psychosis), and favipravir (high serum liver enzymes) [9, 10].
The present narrative review aims to evaluate the growing literature on kidney involvement in COVID-19 along with clinical features reported both in the acute phase of infection and in the post-acute COVID-19 period by assessing potential pathophysiological frameworks explaining such conditions: in January 2022 we started our literature search in three databases, PubMed/Medline, Web of Science and Google Scholar. The titles and abstracts of all studies found were individually assessed with regard to their suitability for this narrative review. After deleting unrelated articles and studies the full texts of all remaining studies were individually assessed.
Post-acute COVID-19 syndrome
Presence and/or persistence of symptoms, not attributable to any other disease, 8–12 weeks after the onset of COVID-19, define the post-acute COVID-19 syndrome [11]. The most common symptoms include fatigue, joint and muscle pain, fever, dyspnea and cough; furthermore, multiple system involvement has been reported [11]. An observational study conducted on 1250 US patients showed that over 32% of them experienced persisting or new-onset symptoms; more than 15% required re-hospitalization with more than 6% of mortality [12]. Similar rates of mortality, re-hospitalization and persistent symptoms have also been reported in some European studies [13, 14]. In a large scale study including more than 1300 hospitalized patients, only 40% were independent in their activities within 30 days of discharge [15]. The most common cardiovascular symptoms were chest pain, myocarditis and palpitations, while the most common neuropsychiatric symptoms included anxiety-depression, sleep disturbances and headache [16, 17]. The main pathophysiological mechanisms underlying the post-acute COVID-19 syndrome are immunological and inflammatory alterations, virus-specific pathophysiological changes and other common post-infectious sequelae depending on the organ system involved.
Direct kidney involvement in COVID-19
The genome of SARS-CoV-2 is a positive‐sense single‐stranded RNA of less than 30 kb that encodes 14 open reading frames including envelope, spike, nucleocapsid, matrix and accessory proteins [18]. The spike protein is involved in the attachment of the viral envelope to the angiotensin converting enzyme 2 (ACE2) receptors via an N-terminal S1 subunit and fusion of the viral envelope to the host cell membrane via a C-terminal S2 subunit [19]. A step referred as priming occurs after the attachment to the ACE2 receptors. Through this route the spike protein is cleaved by endosomal proteases (19) [19]. At this route of the viral entry SARS-CoV-2 requires a transmembrane protease serine 2 (TMPRSS2), similarly to the other members of the coronavirus family. The ACE receptors and the TMPRSS2 are co-localized in the kidney mostly in the epithelial cells of the proximal tubules and collecting ducts, with additional presence in the podocytes and mesangial cells, though to a lesser degree (Fig. 1) [20]. Despite not being the most common entry route for SARS-CoV-2, another potential route that depends on the receptor CD147 commonly expressed on the proximal tubular epithelial cells has been identified. These findings are supported by the inhibition of the viral entry and amplification in certain cell lines incubated with meplazumab, an anti-CD147 antibody [21].
Although direct viral entry into the renal tissue has been shown in multiple studies and is supported by the presence of viral inclusion bodies on biopsy specimens, it is unclear whether the clinical kidney outcomes are caused by direct cytopathic effects, indirect mechanisms, or both [22, 23]. A detectable viral load has been observed by utilizing the RT-PCR method in certain autopsy specimens which may indicate direct cytopathic effects; nevertheless, it is important not to overlook the possibility of those viral inclusions being remnants of renal cell endocytosis or clathrin-coated vesicles, since not all inclusions include a viral RNA: such an example is observed in cases of COVID-19-associated collapsing glomerulopathy (Fig. 1) [24,25,26,27]. Therefore, further studies are required to determine the underlying pathophysiology of kidney involvement in COVID-19.
Kidney involvement in the acute phase of COVID-19
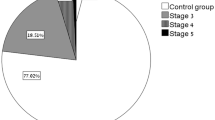
High rates of acute kidney injury (AKI), elevated serum creatinine levels, electrolyte abnormalities and elevated serum urea levels have been reported in the acute phase of COVID-19, even though kidney involvement is not the most common cause of mortality or morbidity in COVID-19 patients [28,29,30]. Rates of AKI vary between 5 and 43% among infected patients through different pathophysiological mechanisms including acute tubular injury, endothelial damage, thrombotic microangiopathy, pre-renal azotemia, hypoxic injury and collapsing glomerulopathy (Fig. 2a) [6, 30]. A recent meta-analysis including 30,839 patients estimated AKI prevalence in 28% of them and the need for dialysis in 9% of the hospitalized patients; however, the rates of both AKI and need for dialysis were considerably higher in patients admitted to the intensive care units (Fig. 2b) [31]. Additionally, rates of AKI and need for dialysis were much greater during the course of COVID-19 compared to influenza respiratory infections, as demonstrated by large scale comparison studies [32, 33]. Many mechanisms of kidney involvement in the acute phase of COVID-19 have been proposed: systemic factors including hemodynamic instability in response to sepsis or multi-organ failure (most commonly found in the so-called pre-renal AKI), cytokine-mediated injury and direct viral invasion, as described in the previous section. The main causes of pre-renal AKI in the acute phase of COVID-19 include hypotension, multi-organ failure, poor renal perfusion due to the effects of non-invasive or invasive mechanical ventilation, rhabdomyolysis, hypovolemia due to poor oral intake or increased losses through various mechanisms including insensible perspiration or vomiting [34, 35].
High rates of proteinuria and loss of electrolytes due to proximal tubular damage have been shown in a Belgian study conducted on 49 hospitalized patients [36]. Some studies have shown proteinuria [36, 37]. In most cases proteinuria was low in degree, whereas cases of collapsing glomerulopathy showed severe proteinuria. Additionally, the degree of proteinuria was found to be associated with the severity of COVID-19 in a study conducted on 45 subjects [38]. Studies analyzing the presence of hematuria are limited: hematuria was present in 26.7% of the 701 subjects enrolled in a study [39]. Both hematuria and proteinuria were positively correlated with in-hospital deaths in COVID-19 patients [39].
In addition to AKI or acute-on-chronic kidney disease operating through various mechanisms, patients with poor baseline kidney function, as shown by a low estimated glomerular filtration rate (eGFR) or elevated serum creatinine or blood urea nitrogen levels, had a poor prognosis in terms of hospitalization and mortality during COVID-19 [30, 39, 40]. Therefore, poor renal function is a bad prognostic factor in addition to being a complication of COVID-19 (Fig. 2).
Kidney involvement in the post-acute COVID-19 period
AKI and requirement for kidney replacement therapy (KRT) were relatively common features of severe COVID-19, especially in patients admitted to the intensive care unit, with up to 25% incidence; furthermore, both were important predictors of survival [41, 42]. Approximately 28% of hospitalized COVID-19 patients were also affected by AKI and 9% of them required KRT [31]. Nevertheless, studies investigating renal function during the post-acute COVID-19 period are limited in number and in terms of follow-up duration. A study conducted on 56 hemodialysis patients infected with SARS-CoV-2 with a follow-up period of 12 months demonstrated a higher mortality rate compared to 154 hemodialysis patients without COVID-19 (hazard ratio 3.0, P < 0.01), with 30% of mortality occurring during the initial hospitalization [43]. Another important finding of that study was the rapid decline in anti-SARS-CoV-2 IgG levels in the hemodialysis patients at the 12-month follow-up [43]. A Chinese study showed that 35% of the 1733 subjects recovering from COVID-19 and with a baseline eGFR > 90 ml/min/1.73 m2 had a statistically significant decline in eGFR at the six-month follow-up, including 13% who did not demonstrate any signs of AKI during the initial hospitalization period [44]. Similar findings were observed in a study conducted on US veterans, in which the risk of a decline in renal function and urinary tract infections were correlated with the severity of the initial COVID-19 infection [45]. Most of the patients demonstrated a variable degree of recovery of renal function and a decline in the need for KRT in a study conducted on 74 hospitalized COVID-19 patients with AKI requiring KRT during the post-acute COVID-19 period (median follow-up period of 151 days) [46]. Similar findings were reported in many other retrospective cohort or observational studies, in which only the presence of CKD at baseline appeared to be a risk factor for higher rates of KRT requirement [47, 48]. Additionally, higher rates of AKI, significant decline in eGFR, major adverse kidney events and end-stage kidney disease were observed in the 30-day survivors of COVID-19 compared to a non-infected control group in a study including 89,216 infected patients and 1,637,457 controls [49].
Kidney transplant patients are a population that is highly vulnerable to COVID-19 due to the use of immunosuppressive medications, low levels and shortened persistence of antibody titers following either vaccination or active infection, a pro-coagulant state caused by the CKD status and medications, and greater need for hospitalization and medical assistance. Kidney transplant patients are more prone to develop signs of kidney injury during the initial hospitalization period, in addition to higher rates of morbidity and mortality, compared to the general population [50]. Furthermore, almost 90% of the kidney transplant patients who survived COVID-19 developed general symptoms of post-acute COVID-19 syndrome, such as fatigue, malaise and joint pain. The most common laboratory findings included shortened activated partial thromboplastin time, elevated fibrinogen and D-dimer levels, all of which are consistent with a pro-coagulant state [51]. Rates of symptoms and laboratory abnormalities associated with the post-acute COVID-19 syndrome were statistically significantly higher in kidney transplant patients [51, 52]. Despite such findings, high rates of graft survival were reported in relatively large scale studies during the post-acute COVID-19 period [53]. However, some of the major limitations of these studies were the short follow-up period, the lack of biopsy-proven histopathological changes, and the lack of a clear cause-effect relationship. Additionally, there is no consensus on the therapeutic alternatives for patients with renal involvement in the post-acute period, despite the presence of certain recommendations regarding other organ systems, such as the cardiovascular, respiratory and neuropsychiatric ones.
Pathophysiological mechanisms of kidney involvement in the COVID-19 period
COVID-19 might affect the kidney in multiple ways and the contribution of these factors can vary over time. Many of the direct and indirect effects of SARS-CoV-2 may persist during recovery after hospital discharge and could lead to repeat episodes of sepsis, recurrent AKI and increased risk of CKD. Furthermore, the relationship between COVID-19 and CKD is likely to be bidirectional. Mild CKD might increase the risk of COVID-19 and associated AKI, whereas increased severity of AKI might be associated with persistent kidney dysfunction, delayed recovery and/or need for long-term dialysis.
Various pathophysiological mechanisms including tubular injury, endothelial damage, inflammatory mediators, complement activation, micro- or macrovascular injury, and podocyte injury have been proposed to explain the renal features reported during the post-acute COVID-19 period (Fig. 2c).
Tubular injury
In addition to potential direct viral toxicity, local inflammatory processes including release of cytokines and activation of complement, medication-induced tubular injury, rhabdomyolysis, hypovolemia caused by fluid losses due to fever or diarrhea, hypotension or septic shock, pro-coagulant status, and activation of the renin–angiotensin–aldosterone system are all potential contributors to tubular injury (Fig. 2c) [6, 54, 55]. This hypothesis has been supported by numerous autopsy studies revealing high rates of acute tubular necrosis; one biopsy study conducted on 240 subjects demonstrated myoglobin cast nephropathy in 3.3% of patients [52]; additionally, two cases of biopsy-proven vitamin C-related oxalate nephropathy have been reported [56]. Lack of long-term follow-up periods, dependence on a single parameter, mostly serum creatinine to assess renal function, absence of urinalysis and relatively small study populations are major limitations of these studies. Nevertheless, we cannot underestimate the possibility of unresolved tubular injury that is not reflected in clinical or laboratory features.
Endothelial activation and microvascular injury
Commonly encountered during COVID-19, elevated lactate dehydrogenase levels, prolonged prothrombin and partial thromboplastin times, thrombocytopenia and occurrence of deep vein thrombosis or pulmonary embolism are features of a pro-coagulant state and/or disseminated intravascular coagulation (DIC) (Fig. 2c) [57]. Thrombocyte activation is thought to be the main mechanism for DIC in COVID-19, since SARS-CoV-2 can bind to thrombocytes via ACE2 receptors and activate certain signaling pathways [58]. Additionally, activation of inflammatory pathways and complement via the release of pathogen-associated molecular pattern (PAMP) and damage-associated molecular pattern (DAMP) molecules may lead to the release of pro-coagulant substances and tissue factors involved in the extrinsic pathway of coagulation [59]. Recent studies have demonstrated a role of an excessive formation of neutrophil extracellular traps in a phenomenon referred to as immunothrombosis in severe cases of COVID-19 [60]. Additionally, features of thrombotic microangiopathy have been demonstrated in 3.3% of 240 patients [52]. Supporting evidence for thrombotic complications includes rare cases of renal artery or renal vein thrombosis leading to renal infarction [61].
Podocyte injury
Collapsing glomerulopathy is the most commonly reported glomerular disease in COVID-19 patients: it is associated with APOL1 gene polymorphisms especially in patients of African ancestry (Fig. 2c) [62]. Upregulation of the APOL1 gene via a viral infection leads to activation of interferon and toll-like receptors, thus leading to dysregulation of podocytes and glomeruli [63, 64]. Many other viral infections associated with interferon release, such as human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), cytomegalovirus and Parvovirus B19, have been linked to collapsing glomerulopathy [65,66,67]. Furthermore, rare cases of minimal change disease and focal segmental glomerulosclerosis have been reported in COVID-19 patients.
Other glomerulopathies
Rare cases of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, anti-glomerular basement membrane (GBM) disease and immunoglobulin A vasculitis without nephropathy have been reported in COVID-19 patients [68, 69].
Future perspectives
The burden of COVID-19 on the kidney is yet to be determined. The COVID-19 pandemic is likely to have a long-term impact even on patients who have not been infected by the virus, owing to delays in care for chronic diseases, such as CKD, diabetes and hypertension. This area is understudied but might have the largest impact at the population level and couls disproportionately affect vulnerable individuals, particularly those with low socioeconomic status and poor access to health care. Studies are urgently needed to investigate this area and confirm the importance of measures to catch up on preventive care for at-risk individuals [67]. To gain a better understanding of the effects of COVID-19 on the kidney, large integrated health-care systems and collaborations that enable rapid collection and analysis of clinical, laboratory and diagnostic data should continue to provide access to their databases for researchers who are investigating the long-term sequelae of COVID-19. Translational studies in which patients with COVID-19 undergo data and biospecimen collection during hospitalization and recovery are key to understanding clinical and biological outcomes and for investigating mechanisms [67]. Future prospective large scale studies are needed with long follow-up periods assessing renal function via multiple parameters such as biopsy studies, urinalysis, measurement of serum creatinine and cystatin C, directly measured GFR, and assessment of tubular function via urinary β2-microglobulin measurements. Several ongoing clinical trials are investigating this issue (Table 1). In the meantime, careful follow-up of kidney function should be performed in COVID-19 patients with prioritization of those who had AKI or are affected by CKD.
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069
Sousa G, Garces T, Cestari V, Florêncio R, Moreira T, Pereira M (2020) Mortality and survival of COVID-19. Epidemiol Infect 148:e123
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC et al (2020) Remdesivir for the treatment of Covid-19. N Engl J Med 383(19):1813–1826
Chams N, Chams S, Badran R, Shams A, Araji A, Raad M et al (2020) COVID-19: a multidisciplinary review. Front Public Health 8:383
Copur S, Kanbay A, Afsar B, Elsurer Afsar R, Kanbay M (2020) Pathological features of COVID-19 infection from biopsy and autopsy series. Tuberk Toraks 68(2):160–167
Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J et al (2020) Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 81(2):e16–e25
Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Fotiou D, Migkou M, Tzanninis IG et al (2021) Emerging treatment strategies for COVID-19 infection. Clin Exp Med 21(2):167–179
Charan J, Kaur RJ, Bhardwaj P, Haque M, Sharma P, Misra S et al (2021) Rapid review of suspected adverse drug events due to remdesivir in the WHO database; findings and implications. Expert Rev Clin Pharmacol 14(1):95–103
Majumder J, Minko T (2021) Recent developments on therapeutic and diagnostic approaches for COVID-19. Aaps j 23(1):14
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS et al (2021) Post-acute COVID-19 syndrome. Nat Med 27(4):601–615
Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC (2021) Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med 174(4):576–578
Arnold DT, Hamilton FW, Milne A, Morley AJ, Viner J, Attwood M et al (2021) Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 76(4):399–401
Carfì A, Bernabei R, Landi F (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324(6):603–605
Bowles KH, McDonald M, Barrón Y, Kennedy E, O’Connor M, Mikkelsen M (2021) Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med 174(3):316–325
Taquet M, Luciano S, Geddes JR, Harrison PJ (2021) Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 8(2):130–140
Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H et al (2020) Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open 3(7):e2014780
Abduljalil JM, Abduljalil BM (2020) Epidemiology, genome, and clinical features of the pandemic SARS-CoV-2: a recent view. New Microbes New Infect 35:100672
Khailany RA, Safdar M, Ozaslan M (2020) Genomic characterization of a novel SARS-CoV-2. Gene Rep 19:100682
Pramod S, Kheetan M, Ogu I, Alsanani A, Khitan Z (2021) Viral nephropathies, adding SARS-CoV-2 to the list. Int J Nephrol Renovasc Dis 14:157–164
Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P et al (2020) CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther 5(1):283
Fehr AR, Perlman S (2015) Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 1282:1–23
Parmar MS (2021) Acute kidney injury associated with COVID-19-Cumulative evidence and rationale supporting against direct kidney injury (infection). Nephrology (Carlton) 26(3):239–247
Bosch BJ, van der Zee R, de Haan CA, Rottier PJ (2003) The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol 77(16):8801–8811
Nasr SH, Kopp JB (2020) COVID-19-associated collapsing glomerulopathy: an emerging entity. Kidney Int Rep 5(6):759–761
Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L et al (2020) Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383(6):590–592
Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT et al (2020) SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396(10251):597–598
Apetrii M, Enache S, Siriopol D, Burlacu A, Kanbay A, Kanbay M et al (2020) A brand-new cardiorenal syndrome in the COVID-19 setting. Clin Kidney J 13(3):291–296
Carriazo S, Kanbay M, Ortiz A (2020) Kidney disease and electrolytes in COVID-19: more than meets the eye. Clin Kidney J 13(3):274–280
Kanbay M, Medetalibeyoglu A, Kanbay A, Cevik E, Tanriover C, Baygul A et al (2021) Acute kidney injury in hospitalized COVID-19 patients. Int Urol Nephrol, pp. 1–8. https://doi.org/10.1007/s11255-021-02972-x
Silver SA, Beaubien-Souligny W, Shah PS, Harel S, Blum D, Kishibe T et al (2021) The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: a systematic review and meta-analysis. Kidney Med 3(1):83-98.e1
Birkelo BC, Parr SK, Perkins AM, Greevy RA Jr, Hung AM, Shah SC et al (2021) Comparison of COVID-19 versus influenza on the incidence, features, and recovery from acute kidney injury in hospitalized United States Veterans. Kidney Int 100:894
Xie Y, Bowe B, Maddukuri G, Al-Aly Z (2020) Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ 371:m4677
Abdelsalam M, Abd El Wahab AM, Nassar MK, Samaan E, Eldeep A, Abdalbary M et al (2022) Kidneys in SARS-CoV-2 Era; a challenge of multiple faces. Ther Apher Dial. https://doi.org/10.1111/1744-9987.13792
Chong WH, Saha BK (2021) Relationship between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the etiology of acute kidney injury (AKI). Am J Med Sci 361(3):287–296
Werion A, Belkhir L, Perrot M, Schmit G, Aydin S, Chen Z et al (2020) SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int 98(5):1296–1307
Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C et al (2020) Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31(6):1157–1165
Ouahmi H, Courjon J, Morand L, François J, Bruckert V, Lombardi R et al (2021) Proteinuria as a biomarker for COVID-19 severity. Front Physiol 12:611772
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L et al (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97(5):829–838
Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T et al (2020) Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest 158(1):97–105
Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L (2020) Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med 46(7):1339–1348
Ertuğlu LA, Kanbay A, Afşar B, Elsürer Afşar R, Kanbay M (2020) COVID-19 and acute kidney injury. Tuberk Toraks 68(4):407–418
Carriazo SM-FSSC, Cano J, Goma E, Avello A, Ortiz A, Gonzalez-Parra E (2021) Increased one-year mortality in hemodialysis patients with COVID-19: a prospective, observational study. Clin Kidney J 15:432
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X et al (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397(10270):220–232
Al-Aly Z, Xie Y, Bowe B (2021) High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594(7862):259–264
Stockmann H, Hardenberg J-HB, Aigner A, Hinze C, Gotthardt I, Stier B et al (2021) High rates of long-term renal recovery in survivors of coronavirus disease 2019-associated acute kidney injury requiring kidney replacement therapy. Kidney Int 99(4):1021–1022
Nugent J, Aklilu A, Yamamoto Y, Simonov M, Li F, Biswas A et al (2021) Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open 4(3):e211095-e
Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S et al (2021) AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 32(1):151–160
Bowe B, Xie Y, Xu E, Al-Aly Z (2021) Kidney outcomes in long COVID. J Am Soc Nephrol 32(11):2851–2862
Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ et al (2020) COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transpl 20(11):3140–3148
Basic-Jukic N, Juric I, Furic-Cunko V, Katalinic L, Radic J, Bosnjak Z et al (2021) Follow-up of renal transplant recipients after acute COVID-19—a prospective cohort single-center study. Immun Inflamm Dis 9(4):1563–1572
Och A, Tylicki P, Polewska K, Puchalska-Reglińska E, Parczewska A, Szabat K et al (2021) Persistent post-COVID-19 syndrome in hemodialyzed patients—a longitudinal cohort study from the north of Poland. J Clin Med 10(19):4451
Kute VB, Ray DS, Yadav DK, Pathak V, Bhalla AK, Godara S et al (2021) A multicenter cohort study from india of 75 kidney transplants in recipients recovered after COVID-19. Transplantation 105(7):1423–1432
Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M et al (2020) COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol 16(12):747–764
Long JD, Strohbehn I, Sawtell R, Bhattacharyya R, Sise ME (2021) COVID-19 survival and its impact on chronic kidney disease. Transl Res
Fontana F, Cazzato S, Giovanella S, Ballestri M, Leonelli M, Mori G et al (2020) Oxalate Nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int Rep 5(10):1815–1822
Asakura H, Ogawa H (2021) COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol 113(1):45–57
Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y et al (2020) SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol 13(1):120
Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD (2020) Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis 27(5):365–376
Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ (2015) Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 74(7):1417–1424
Mui LW, Lau JF, Lee HK (2021) Thromboembolic complications of COVID-19. Emerg Radiol 28(2):423–429
Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M et al (2020) AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J Am Soc Nephrol 31(8):1688–1695
Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V et al (2015) Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87(2):332–342
Friedman DJ, Pollak MR (2016) Apolipoprotein L1 and kidney disease in African Americans. Trends Endocrinol Metab 27(4):204–215
Moudgil A, Nast CC, Bagga A, Wei L, Nurmamet A, Cohen AH et al (2001) Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int 59(6):2126–2133
Wyatt CM, Klotman PE, D’Agati VD (2008) HIV-associated nephropathy: clinical presentation, pathology, and epidemiology in the era of antiretroviral therapy. Semin Nephrol 28(6):513–522
Chandra P, Kopp JB (2013) Viruses and collapsing glomerulopathy: a brief critical review. Clin Kidney J 6(1):1–5
Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R et al (2020) COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol 31(9):1948–1958
Uppal NN, Kello N, Shah HH, Khanin Y, De Oleo IR, Epstein E et al (2020) De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep 5(11):2079–2083
Acknowledgements
Mehmet Kanbay gratefully acknowledges the use of the services and facilities of the Koc University Research Center for Translational Medicine (KUTTAM), funded by the Presidency of Turkey, Presidency of Strategy and Budget. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Presidency of Strategy and Budget.
Funding
No funding agency granted the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No verbal and written informed consent was necessary for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Copur, S., Berkkan, M., Basile, C. et al. Post-acute COVID-19 syndrome and kidney diseases: what do we know?. J Nephrol 35, 795–805 (2022). https://doi.org/10.1007/s40620-022-01296-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01296-y