Abstract
Background
Hypertensive disorders of pregnancy are associated with chronic kidney disease. Early detection of renal dysfunction enables implementation of strategies to prevent progression. International guidelines recommend review at 6–8 weeks postpartum to identify persistent hypertension and abnormal renal function, but evidence for the efficacy of this review is limited.
Methods
All women attending a specialist fetal-maternal medicine clinic for hypertensive disorders of pregnancy (pre-eclampsia, chronic hypertension, gestational hypertension) were invited for a 6–8 weeks postpartum review of their blood pressure and renal function in order to establish the prevalence and independent predictors of renal dysfunction. Renal dysfunction was defined as low estimated Glomerular Filtration Rate (eGFR < 60 ml/min/1.73 m2) or proteinuria (24-h protein excretion > 150 mg or urinary albumin-to-creatinine ratio > 3 mg/mmol). All women attending a specialist clinic for hypertensive disorders were invited for a 6–8 weeks postpartum review of their blood pressure and renal function. Demographics, pregnancy and renal outcomes were prospectively collected.
Results
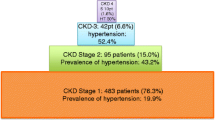
Between 2013 and 2019, 740 of 1050 (70.4%) women who had a pregnancy complicated by a hypertensive disorder attended their 6–8 weeks postpartum visit. Renal dysfunction was present in 32% of the total cohort and in 46% and 22% of women with and without pre-eclampsia, respectively. Multivariate logistic regression demonstrated that independent predictors were pre-eclampsia, chronic hypertension, highest measured antenatal serum creatinine, highest measured antenatal 24-h urinary protein, and blood pressure ≥ 140/90 mmHg at the postnatal visit.
Conclusions
Renal dysfunction was present in one in three women with hypertensive disorders of pregnancy at 6–8 weeks postpartum. This includes women with gestational hypertension and chronic hypertension without superimposed pre-eclampsia, and thus these women should also be offered postnatal review.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertensive disorders of pregnancy affect 8–12% of pregnancies worldwide and are one of the leading causes of maternal and perinatal mortality [1]. In addition to immediate risks to maternal health, hypertensive disorders of pregnancy also negatively affect future health, including cardiovascular disease with risk related to the severity of the disorder [2]. Large epidemiological studies have also reported that women with pre-eclampsia [3, 4] or any of the hypertensive disorders of pregnancy [5, 6], have up to 10-times higher risk of developing end stage kidney disease (ESKD), compared to women with uncomplicated pregnancies.
The association between the hypertensive disorders of pregnancy and future chronic kidney disease (CKD) is less clear. A meta-analysis of seven cohort studies reported that at a weighted mean of 7 years postpartum, women with previous preeclampsia have a four-fold increased risk of microalbuminuria and women with severe pre-eclampsia had an eight-fold increased risk. However, there was no difference in estimated glomerular filtration rate (eGFR) in women with previous pre-eclampsia compared to women with uncomplicated pregnancies [7]. More recently, the risk of albuminuria and CKD after pre-eclampsia was not reported to be significantly higher in a large meta-analysis but data were heterogeneous with incomplete follow-up [4]. On the contrary, data from another meta-analysis suggested that both previous pre-eclampsia and gestational hypertension were associated with increased risk of future CKD [6].
In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guidelines recommend a medical review 6–8 weeks postpartum for all women with hypertensive disorders of pregnancy in order to assess future risk for cardiovascular disease and plan lifestyle modifications and appropriate follow-up, but renal assessment is advised only in women with pre-eclampsia [8]. However, due to variations in pre-eclampsia definition [9, 10], lack (of ascertainment) of confirmed diagnosis of pre-eclampsia at term and potential risk of future CKD across the spectrum of hypertensive disorders of pregnancy [6], it is unclear whether postnatal renal risk assessment is needed for all women with hypertensive disorders of pregnancy or if risk factors can be used to target those at greatest risk.
We sought to (1) establish the prevalence of reduced eGFR or proteinuria in women with different hypertensive disorders of pregnancy attending their routine postnatal visit and (2) determine which of these disorders and other risk factors are associated with low eGFR or proteinuria postpartum.
Materials and methods
All women with hypertensive disorders of pregnancy that were managed by a fetal-maternal medicine clinic at a University maternity unit in London, UK, were invited to attend a 6–8 weeks postnatal visit in the clinic between January 2013 and March 2019. Women with known kidney disease or with proteinuria before 20 weeks’ gestation were excluded. Blood pressure was measured twice with a validated automatic device [11] and the average of the two readings was reported. Serum creatinine concentration was quantified by a hospital laboratory IDMS traceable assay [12], and eGFR was calculated using the 2009 CKD-EPI equation [13]. Postpartum assessment of urinary protein excretion was performed according to national guidelines: 24-h urine protein until 2014 [14] and subsequently by spot albumin to creatinine ratio (ACR) [15]. Urinary protein was measured by the pyrogallol red molybdate dye-binding assay, albumin with the immunonephelometric method [16] and urinary creatinine with modified Jaffe’s reaction [12].
Maternal demographic characteristics at the booking visit, as well as antenatal and postnatal laboratory and clinical parameters and pregnancy outcomes were extracted from the hospital databases. We used the maternal weight at the booking visit and not the pre-pregnancy weight because the former is objectively measured by clinicians whilst the latter may suffer reporting bias by a significant number of patients. Antenatal urinary protein excretion was assessed with 24-h urine protein. The highest measured antenatal values for 24-h urine protein, serum creatinine, aspartate aminotransferase, systolic and diastolic blood pressure were reported. Women with persistent hypertension or renal dysfunction were referred to their general practitioner or a nephrology physician (KB) for ongoing follow-up.
Definitions
Hypertensive disorders of pregnancy were defined according to the 2018 International Society for Hypertension in Pregnancy (ISSHP-2018) recommendations [17]. Birthweight percentiles for gestational age were defined according to the Fetal Medicine Foundation charts [18].
For the purposes of analysis, 24-h urine protein excretion > 150 mg and > 500 mg were estimated to be > 3 mg/mmol and > 30 mg/mmol, respectively [14, 15]. Renal outcomes were categorised according KDIGO 2012 guidelines [19] with renal dysfunction defined as the presence of proteinuria or eGFR below 60 ml/min/1.73 m2, in the absence of confirmation of CKD status after 90 days. Maternal characteristics and pregnancy outcomes in women with and without renal dysfunction were compared.
Caesarean sections were categorised as emergency and elective [20]. An unplanned emergency caesarean section is when there is threat to the life of the woman or fetus and needs be performed within 75 min [20]. A planned emergency caesarean section is when there is a need for early delivery, without immediate compromise of mother or baby [20]. An elective caesarean section is when there are no maternal or fetal concerns and is planned at the end of pregnancy.
Statistical analysis
Kolmogorov–Smirnov test was used to test for normality and data presented according to distribution. Comparison between the hypertensive disorders of pregnancy was performed with the Kruskall-Wallis or Mann–Whitney-U test for numerical data and chi-square test for categorical data. The Bonferroni correction was used to correct for multiple comparisons.
Univariate binary logistic regression was used to assess associations between antenatal and postnatal variables and renal outcomes at the 6–8 weeks postnatal visit. Multivariate logistic regression was performed to assess the independent contribution of each of these variables in the prediction of renal outcomes at the 6–8 weeks postnatal visit. Maternal parameters assessed in the logistic regression models included demographic characteristics (maternal race, chronic hypertension, use of antihypertensive medications at booking), antenatal factors (the highest recorded values for total protein excretion in 24-h urine collection, serum creatinine and aspartate transaminase, systolic and diastolic blood pressure) and postnatal factors (blood pressure ≥ 140/90 mmHg at the postnatal visit). In addition, variables related to pregnancy outcome assessed in the logistic regression models were gestational age at delivery, mode of delivery, the development of pre-eclampsia and birthweight percentile.
The area under the receiver operating characteristic curve (ROC curve) was used to graphically compare the performance of the multivariate model vs pre-eclampsia.
The study was registered as a service evaluation at King’s College Hospital NHS Foundation Trust (KCH -approval M005).
Results
Study population
Between 2013 and 2019, 740 of 1050 (70.4%) women with hypertensive disorders of pregnancy that were managed by a fetal-maternal medicine clinic at King’s College Hospital, London, UK attended their 6–8 weeks postpartum visit at a median (IQR) of 6.5 (5.9–7.2) weeks after delivery; 53 women were excluded because they had evidence of kidney disease or proteinuria before 20 weeks’ gestation. Of the remaining 687, 240 (35%) had received care in the specialist clinic due to chronic hypertension and 447 (65%) developed new onset hypertension during their pregnancies (Table 1, Supplementary Table 1). Pre-eclampsia complicated 284 (41%) pregnancies from the total cohort including 66 (27%) women with chronic hypertension and 218 (49%) women with new onset hypertension.
Features of renal dysfunction
Overall, 244 (40%) women had ACR > 3 mg/mmol (24-h protein > 150 mg) and/or eGFR ≤ 90 ml/min/1.73 m2 at postnatal visit (Table 1) including 217 (32%) with features of renal dysfunction: 187 (27%) A2 and 30 (4%) A3. 86 (13%) women had eGFR between 60 and 89 ml/min/1.73 m2 (G2) but only 26 (30%) had concurrent proteinuria (G2A2 or G2A3) (Table 1).
Risk factors for renal dysfunction
Women with pre-eclampsia (including pre-eclampsia superimposed on chronic hypertension) were more likely to have features of renal dysfunction at the postpartum visit than women with gestational hypertension or uncomplicated chronic hypertension. However, 37 (16%) and 50 (29%) women with gestational hypertension or uncomplicated chronic hypertension, respectively, also had features of renal dysfunction at the postpartum visit (Table 1).
Comparison of maternal characteristics, pregnancy outcomes and antenatal and postnatal visit parameters between women with and without features of renal dysfunction at the postnatal visit are presented in Table 2. Women with features of renal dysfunction were more likely to be of black ethnicity and to have been prescribed antihypertensive medications at booking (Table 2) than women without features of renal dysfunction. Women with features of renal dysfunction also had earlier deliveries and smaller babies, were less likely to have had a vaginal delivery or an elective caesarean section and more likely to have had a planned emergency caesarean section.
The highest antenatal 24-h urine protein, serum creatinine, aspartate aminotransferase and systolic and diastolic blood pressure levels were observed in women with features of renal dysfunction, and a higher proportion of these women also had blood pressure > 140/90 mmHg at their postpartum visit compared to those without renal dysfunction.
There were no differences between maternal demographics, pregnancy outcomes and antenatal kidney function between women with eGFR > 90 and 60–89 ml/min/1.73 m2 without ACR > 3 mg/mmol (24-h protein > 150 mg) (Supplementary Table 2).
Logistic regression analysis
Significant associations with features of renal dysfunction at postnatal visit identified by univariate binary logistic regression are shown in Table 3. Multivariate logistic regression demonstrated that pre-eclampsia was associated with a two-fold increased risk of features of renal dysfunction at postnatal visit compared to women with other gestational hypertension and uncomplicated chronic hypertension (Table 3). Chronic hypertension during pregnancy or having blood pressure > 140/90 mmHg at postpartum visit were also associated with increased risk of features of renal dysfunction. An increase in highest antenatal serum creatinine concentration by 1 µmol/l, and in highest antenatal protein excretion in 24-h urine collection by 100 mg increased the risk of postpartum features of renal dysfunction by 2% and 4%, respectively (Table 3). The area under the operating characteristic curve for the final multivariate model was 0.73 (95% CI 0.68–0.77), which was superior to that of a model with pre-eclampsia as the only predictor, following the NICE recommendation, 0.64 (95% CI 0.59–0.68), p < 0.001.
Discussion
Approximately one in three women with hypertensive disorders of pregnancy had features of renal dysfunction at 6–8 weeks postnatal visit. Nearly half of the women with pre-eclampsia or superimposed pre-eclampsia had features of renal dysfunction, but also one in five women with gestational hypertension of chronic hypertension without superimposed pre-eclampsia had features of renal dysfunction and would not previously have been investigated according to NICE recommendations. The majority of women had features of mild renal dysfunction (G1A2 or G1A3); however, one in eight women with hypertensive disorders of pregnancy had features of G2A2 or G2A3 with eGFR 60–89 ml/min/1.73 m2 postpartum. Maternal black ethnicity, antihypertensive medication at booking, mode and gestational age at delivery, low birthweight centile, pre-eclampsia, chronic hypertension, highest antenatal serum creatinine and 24-h protein excretion and postnatal hypertension were associated with abnormal postpartum kidney function, but only the latter five variables were independent predictors.
Estimates of features of renal dysfunction prevalence in women with hypertensive disorders of pregnancy are few and report variable thresholds of proteinuria at different time points postpartum, and to our knowledge there are no other studies describing features of renal dysfunction according to KDIGO criteria at the 6–8 weeks postpartum visit. One study (N = 121) reported persistent proteinuria (> 300 mg per 24 h) in 21% of women with pre-eclampsia at six weeks postpartum, that decreased to 14% and 2% at three months and two years postpartum, respectively [21]. Another study of 775 primiparous women with pre-eclampsia reported that overall, 14% had ACR > 3 mg/mmol at 4–24 months postpartum, but of those reviewed at 16–20 weeks postpartum (33% of women) had ACR > 3 mg/mmol [22]. A third study examining women with hypertensive disorders of pregnancy at six weeks postpartum, reported that 14%, 10% and 4% of women with pre-eclampsia (N = 288), superimposed pre-eclampsia (N = 30) and chronic hypertension (N = 51) had protein:creatinine ratio (PCR) > 30 mg/mmol [23]. Whilst challenging to compare with the findings from our cohort due to different proteinuria thresholds and time of assessment, together they support that pre-eclampsia and severity of antenatal proteinuria are associated with persistent postpartum proteinuria [21, 22]. However, in the latter study, none of the women with gestational hypertension (N = 94) had PCR > 30 mg/mmol at six weeks postpartum [23], whereas 16% of our cohort had ACR > 3 mg/mmol which likely reflects the lower threshold reported and the higher prevalence of women with chronic hypertension and of black ethnicity in our cohort.
The high proportion of women with gestational hypertension or chronic hypertension without superimposed pre-eclampsia with features of renal dysfunction postpartum suggests that antenatal, intrapartum or peripartum kidney injury or disease may have been under-recognised. Routine antenatal proteinuria assessment is done by urine dipstick, which has a sensitivity to diagnose antenatal proteinuria of only 40–60% [24]. It is therefore plausible that some women diagnosed with gestational or chronic hypertension did not have a formal quantification to confirm proteinuria over diagnostic threshold for pre-eclampsia as a result of false negative dipstick results. Accurate assessment of urine ACR throughout pregnancy in women with gestational hypertension and chronic hypertension could identify the proportion of women with pre-existing antenatal glomerular proteinuria which is persistent postpartum. In addition, other obstetric factors may contribute to peripartum acute kidney injury (AKI), which could have led to ensuing proteinuria after the antenatal period. Risk factors for AKI including maternal sepsis and haemorrhage frequently occur peri- or post-partum. It is likely that all women with hypertensive disorders of pregnancy are vulnerable to further peripartum renal insults which may lead to longer term kidney pathology.
It is now recognised that AKI in non-pregnant populations is associated with progression to CKD even in children and young adults, and subclinical CKD has been proposed to contribute to future pregnancy complications in women of child-bearing age [25, 26]. In our data, the highest antenatal creatinine was associated with features of renal dysfunction at the postpartum visit which suggests that AKI was present in the antenatal period. It is likely that antenatal AKI is associated with severe pre-eclampsia, as evidenced by a doubling in the risk for features of renal dysfunction by the presence of pre-eclampsia in the multivariate model.
We also report that approximately one in 12 women had eGFR < 90 ml/min/1.73m2 at postnatal visit, and there were no differences according to type of hypertensive disorder of pregnancy. The proportion of women with reduced eGFR was similar to that reported at more than 4 months postpartum in a study of women with new-onset pre-eclampsia[22] but it was higher than that reported by others at six weeks [23]. This may be related to higher numbers of women with chronic hypertension and of black ethnicity in our cohort, which are established risk factors for CKD [27, 28]. Reduced GFR is a recognised risk factor for the development of hypertensive disorders of pregnancy [29], and it is unknown whether reduced GFR was pre-existing prior to pregnancy and masked by gestational changes in creatinine concentration during pregnancy or previously undetected as many women were referred at time of onset of the hypertensive disorder of pregnancy.
Alternatively, renal dysfunction at the postpartum visit could be a consequence of inadequate blood pressure control after pregnancy. In keeping with others [21, 22], higher antenatal systolic and diastolic blood pressures were present in women with postpartum features of renal dysfunction, which may also reflect the severity of hypertensive disorder of pregnancy, and one in four women had blood pressure > 140/90 mmHg at postnatal assessment which may reflect inadequate treatment or be secondary to CKD.
Strengths of this study include the large number of patients, inclusion of women with all hypertensive disorders of pregnancy rather than just those with pre-eclampsia and the use of clearly defined protocols for the assessment of blood pressure and proteinuria. Limitations include the use of two different methodologies to assess proteinuria due to changes in national guidelines in the study period, which may have affected the proportion of women with values above abnormal thresholds, and the lack of long-term renal outcome data.
Pregnancy affords a unique opportunity for the assessment of future maternal health and enables early identification of reduced eGFR even in those who do not meet KDIGO criteria for CKD. In general populations, cardiovascular mortality increases with a reduction in eGFR below 90 ml/min/1.73 m2.[30] Longitudinal studies assessing the role of postpartum assessment of ACR and reduced eGFR to predict future CKD and cardiovascular disease are needed to determine the clinical value of this assessment.
Availability of data and material
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L (2014) Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2(6):e323–e333. https://doi.org/10.1016/s2214-109x(14)70227-x
Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A (2017) All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension 70(4):798–803. https://doi.org/10.1161/HYPERTENSIONAHA.117.09246
Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM (2008) Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359(8):800–809. https://doi.org/10.1056/NEJMoa0706790
Covella B, Vinturache AE, Cabiddu G, Attini R, Gesualdo L, Versino E, Piccoli GB (2019) A systematic review and meta-analysis indicates long-term risk of chronic and end-stage kidney disease after preeclampsia. Kidney Int 96(3):711–727. https://doi.org/10.1016/j.kint.2019.03.033
Wu C-C, Chen S-H, Ho C-H, Liang F-W, Chu C-C, Wang H-Y, Lu Y-H (2014) End-stage renal disease after hypertensive disorders in pregnancy. Am J Obstet Gynecol 210(2):147.e141-147.e148. https://doi.org/10.1016/j.ajog.2013.09.027
Barrett PM, McCarthy FP, Kublickiene K, Cormican S, Judge C, Evans M, Kublickas M, Perry IJ, Stenvinkel P, Khashan AS (2020) Adverse pregnancy outcomes and long-term maternal kidney disease. JAMA Netw Open 3(2):e1920964. https://doi.org/10.1001/jamanetworkopen.2019.20964
McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ (2010) Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis 55(6):1026–1039. https://doi.org/10.1053/j.ajkd.2009.12.036
National Institute for Health and Care Excellence (2019) Hypertension in pregnancy: diagnosis and management (NICE guideline 133)
Khan N, Andrade W, De Castro H, Wright A, Wright D, Nicolaides KH (2020) Impact of new definitions of pre-eclampsia on incidence and performance of first-trimester screening. Ultrasound Obstet Gynecol 55(1):50–57. https://doi.org/10.1002/uog.21867
Nzelu D, Dumitrascu-Biris D, Hunt KF, Cordina M, Kametas NA (2018) Pregnancy outcomes in women with previous gestational hypertension: a cohort study to guide counselling and management. Pregnancy Hypertens 12:194–200. https://doi.org/10.1016/j.preghy.2017.10.011
Clark K, Snowball O, Nzelu D, Kay P, Kametas NA (2018) Validation of the Microlife WatchBP Home blood pressure device in pregnancy for medium and large arm circumferences. Blood Press Monit 23(3):171–174. https://doi.org/10.1097/MBP.0000000000000315
Mitchell RJ (1973) Improved method for specific determination of creatinine in serum and urine. Clin Chem 19(4):408–410
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
National Collaborating Centre for Chronic Conditions (NCC-CC) (2008) Chronic Kidney Disease: National clinical guideline for early identification and management in adults in primary and secondary care
National Institute for health and Care Excellence (NICE) (2014) Chronic kidney disease in adults: assessment and management (clinical guideline 182)
Burtis CA, Bruns DE (2014) Tietz fundamentals of clinical chemistry and molecular diagnostics-e-book. Elsevier Health Sciences, Berlin
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S, International Society for the Study of Hypertension in P (2018) The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 13:291–310. https://doi.org/10.1016/j.preghy.2018.05.004
Nicolaides KH, Wright D, Syngelaki A, Wright A, Akolekar R (2018) Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet Gynecol 52(1):44–51. https://doi.org/10.1002/uog.19073
Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco ALM, De Jong PE, Griffith KE, Hemmelgarn BR, Iseki K, Lamb EJ, Levey AS, Riella MC, Shlipak MG, Wang H, White CT, Winearls CG (2013) Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3(1):1–150. https://doi.org/10.1038/kisup.2012.73
National Institute for Health and Care Excellence (NICE) (2011) Caesarean section, Clinical guideline [CG132]
Berks D, Steegers EA, Molas M, Visser W (2009) Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 114(6):1307–1314. https://doi.org/10.1097/AOG.0b013e3181c14e3e
Lopes van Balen VA, Spaan JJ, Cornelis T, Spaanderman MEA (2017) Prevalence of chronic kidney disease after preeclampsia. J Nephrol 30(3):403–409. https://doi.org/10.1007/s40620-016-0342-1
Escouto DC, Green A, Kurlak L, Walker K, Loughna P, Chappell L, Broughton Pipkin F, Bramham K (2018) Postpartum evaluation of cardiovascular disease risk for women with pregnancies complicated by hypertension. Pregnancy Hypertens 13:218–224. https://doi.org/10.1016/j.preghy.2018.06.019
Correa ME, Côté A-M, De Silva DA, Wang L, Packianathan P, von Dadelszen P, Magee LA (2017) Visual or automated dipstick testing for proteinuria in pregnancy? Pregnancy Hypertens Int J Women’s Cardiovasc Health 7:50–53. https://doi.org/10.1016/j.preghy.2017.01.005
Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE (2011) The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79(12):1361–1369. https://doi.org/10.1038/ki.2011.42
Tangren JS, Powe CE, Ankers E, Ecker J, Bramham K, Hladunewich MA, Karumanchi SA, Thadhani R (2017) Pregnancy outcomes after clinical recovery from AKI. J Am Soc Nephrol 28(5):1566–1574. https://doi.org/10.1681/asn.2016070806
Peralta CA, Shlipak MG, Fan D, Ordoñez J, Lash JP, Chertow GM, Go AS (2006) Risks for End-Stage Renal Disease, Cardiovascular Events, And Death In Hispanic Versus Non-Hispanic White Adults With Chronic Kidney Disease. J Am Soc Nephrol 17(10):2892–2899. https://doi.org/10.1681/asn.2005101122
Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, Klag MJ (2001) Prevalence of high blood pressure and elevated serum creatinine level in the United States. Arch Intern Med 161(9):1207. https://doi.org/10.1001/archinte.161.9.1207
Piccoli GB, Cabiddu G, Castellino S, Gernone G, Santoro D, Moroni G, Spotti D, Giacchino F, Attini R, Limardo M, Maxia S, Fois A, Gammaro L, Todros T (2017) A best practice position statement on the role of the nephrologist in the prevention and follow-up of preeclampsia: the Italian study group on kidney and pregnancy. J Nephrol 30(3):307–317. https://doi.org/10.1007/s40620-017-0390-1
Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR, Chronic Kidney Disease Prognosis C (2013) Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ 346:f324. https://doi.org/10.1136/bmj.f324
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
EK: data collection, statistical analysis, manuscript preparation. CK: data collection, manuscript preparation. PK: data collection, manuscript preparation. NB: data collection. KB: participated in the design of the study, manuscript preparation and final review. NK: conceived and designed the study, data collection, statistical analysis, manuscript preparation and final review. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
For the current study, the advice of our Local Research and Development Committee and the Local Research Ethics Committee (London‐Dulwich NRES Committee) was sought, and we were advised that formal consideration would not be required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kountouris, E., Clark, K., Kay, P. et al. Postnatal assessment for renal dysfunction in women with hypertensive disorders of pregnancy. J Nephrol 34, 1641–1649 (2021). https://doi.org/10.1007/s40620-021-01134-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-021-01134-7