Abstract
Purpose
Differentiated thyroid cancer (DTC) presents a complex clinical challenge, especially in patients with distant metastases and resistance to standard treatments. This study aimed to investigate the influence of specific genes and their germline single nucleotide polymorphisms (SNPs) linked to both inflammatory processes and other neoplasms on the clinical and pathological characteristics of DTC, particularly their potential impact on radioiodine (RAI) treatment efficacy.
Methods
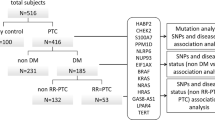
This retrospective analysis involved a cohort of 646 patients diagnosed with DTC after thyroidectomy. Study covering 1998–2014, updated in 2023, included 567 women and 79 men (median age: 49; range: 7–83). SNP selection targeted functional significance, while mutational status was assessed by pyrosequencing for comprehensive characterization. Patient genetic profiles were assessed for associations with disease characteristics, RAI response, and cancer pathology.
Results
Significant correlations emerged between certain SNPs and DTC features. Notably, the NOD2 c.802 T > C variant (rs2066842) was identified as a marker distinguishing between papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC). Moreover, the c.802 T allele was associated with an enhanced response to RAI treatment, indicating a more substantial decrease in posttreatment stimulated thyroglobulin (sTg) concentrations. The NFKB1A allele c.126A (rs696) exhibited connections with lower FTC stages and a reduced probability of multifocality.
Conclusion
This study explored the molecular mechanisms of particular SNPs, highlighting the role of NOD2 in innate immunity and the stress response, and its potential impact on RAI efficacy. This research underscores the clinical promise of SNP analysis and contributes to personalized treatment strategies for DTC, emphasizing the relevance of genetic factors in cancer progression and treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differentiated thyroid cancer (DTC) is the prevailing endocrine malignancy [1] and is predominantly treated with surgery and radioiodine [2]. Despite its generally promising clinical trajectory, patients presenting with distant metastases encounter a significant challenge, with a five-year cancer-specific survival rate of merely 40% [3, 4] This population often develops resistance to radioiodine, posing a substantial hurdle in managing their condition [5].
Differentiated thyroid cancer (DTC) has been shown to potentially be associated with inflammation, particularly chronic lymphocytic thyroiditis (CLT) [5]. Several studies have indicated a potential link between the presence of CLT and an increased risk of developing DTC [6,7,8]. However, they also showed a protective role of CLT in preventing the spread of DTC by limiting tumor growth to the primary site [9], possibly mediated by the cancer microenvironment [10, 11] and cellular cytotoxic and humoral immune reactions [6, 12].
Inflammation within the thyroid gland, as observed in CLT, may create an environment conducive to the initiation or progression of thyroid cancer [5, 13, 14]. However, the exact relationship between inflammation and the development of DTC is complex and requires further investigation to determine the mechanisms involved and the extent of their influence on cancer progression [15, 16]. Moreover, data on the impact of the CLT on the clinical and pathological parameters of DTC are ambiguous [15,16,17,18,19,20,21].
Advancements in our understanding of differentiated thyroid cancer (DTC) have been propelled by innovative genome-analyzing techniques such as high-throughput next-generation sequencing (NGS) [22, 23]. These methods have revealed shared molecular pathways between DTC and various other malignancies [24]. However, the interconnections among differentiated thyroid cancer, inflammation, and the pathogenesis of other malignancies remain unclear and warrant further investigation [25, 26].
Although strides have been made in understanding how tumor-specific genetic changes can predict outcomes, the accuracy of forecasting outcomes for DTC patients remains significantly constrained by its low specificity [27]. This limitation predominantly affects the care of individuals with nonadvanced disease, encompassing a diverse group of patients with DTC, many of whom typically experience remission after thyroid ablation (surgery with or without radioiodine) but might encounter recurrence later [2, 28]. The progression of cancer could hinge more on a patient’s genetic background than on their environmental influences, hinting at the possible involvement of inherited traits in the progression of cancer [29, 30]. Furthermore, for ascertaining germline mutational status, the acquisition of a patient’s blood sample is notably more convenient and accessible than obtaining tumor samples for determining somatic mutational status. Therefore, germline mutational status may help in describing the prognosis of this disease and the best method of treatment.
Consequently, this study primarily aimed to assess the influence of specific genes and their germline single nucleotide polymorphisms (SNPs) linked to both inflammatory processes and other neoplasms on the clinical and pathological characteristics of DTC in a homogenous patient population. The secondary objective was to describe the potential impact of these agents on the efficacy of radioiodine treatment in managing DTC.
Patients and methods
Patient characteristics
We retrospectively analyzed 646 patients diagnosed with thyroid cancer at the single endocrinological center in Poznan according to the fifth edition of the Classification of Endocrine and Neuroendocrine Tumors released by the World Health Organization (WHO). The group consisted of 567 women and 79 men who were Caucasian, with a median age at diagnosis of 49 years and ranging from 7 to 83 years. Patients diagnosed with differentiated thyroid cancer after thyroidectomy were enrolled in the study. The exclusion criterion was the lack of a patient's histopathological description of DTC. The date of diagnosis was set as the date of thyroidectomy.
The analysis covers the data collected between 1998 and 2014 and updated in March 2023. The Bioethical Committee of Poznan University of Medical Sciences approved the study (approval no. 629/07 from June 2007), which was conducted in accordance with the Declaration of Helsinki.
Histopathological, laboratory and clinical data
CLT diagnosis was confirmed through a detailed examination of tissue obtained from thyroidectomy at the time of DTC diagnosis. This examination revealed the widespread infiltration of lymphocytes and oxyphilic cells and the development of both primary and secondary lymphoid follicles [31, 32]. We considered Hashimoto’s thyroiditis and the expansion of individual lymphoid follicles to be CLTs [33]. To avoid biases, we defined CLT considering the histopathological examination (infiltration of lymphocytes) without the autoimmune pattern (antibody positivity).
Tumors were categorized as multifocal when two or more distinct areas were identified. In the case of multifocality, the tumor size was determined by the largest focus. The staging procedures adhered to the American Joint Committee on Cancer TNM staging system (8th Edition) [34].
The molecular information was integrated into the clinical context by correlating it with the histopathological analysis of specimens obtained from thyroidectomy, along with the available clinical data. These included details about the patients and samples, such as age at diagnosis, sex, tumor size, the presence of multifocality (when two or more distinct areas were detected), extrathyroidal extension, evidence of chronic lymphocytic thyroiditis in histopathological assessments, histopathological staging (pTNM) following the 8th edition of the tumor-node-metastasis (TNM) classification [2, 34], concentrations of stimulated thyroglobulin (sTg), antithyroglobulin antibodies (aTg), anti-thyroid peroxidase antibodies (aTPO), and thyrotropin (TSH), and the difference between sTg at first qualification to radioiodine (RAI) treatment and sTg at one year after RAI administration (so-called delta sTg). All sample information was anonymized to maintain patient confidentiality and privacy.
The stimulated thyroglobulin concentration was assessed by an immunoradiometric assay with the use of Brahms diagnostic kits and was determined to be credible for TSH stimulation [35] when the concentration was above 30 μIU/ml (assessed by the electrochemiluminescence method with the use of Roche diagnostic kits and the COBAS e601 analyzer from Hitachi).
All individuals examined in the present study agreed to undergo genetic testing in accordance with the requirements of the Ethical Committee of Poznań University of Medical Science (acceptance no. 629/07).
SNP selection
The choice of candidate SNPs (refer to Table 1) was guided by their documented functional significance, if available, and their established connections with radiosensitivity or the risk of cancer, especially thyroid cancer.
The first step was to define the list of SNP, which already been linked to increased risk of developing cancer, especially DTC and/or to radioiodine sensitivity. Our search strategy included Medical Subject Headings terms and keywords: “Single Nucleotide Polymorphism” AND “thyroid cancer” OR “cancer” OR “radioiodine”. Reference lists of all the selected articles, previous meta‑analyses, and reviews were hand‑searched for any additional articles. We included studies, regardless of their sample size, that investigated the association between SNP and DTC occurrence or cancerogenesis or radioiodine sensitivity. We carried out a systematic review following the guidelines formulated in the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta‑Analyses (PRISMA) guidelines. We searched the following databases: PubMed, MEDLINE, Academic Search Complete, CINAHL Complete, CINAHL, Scopus, Cochrane, Health Source: Nursing/Academic Edition, Web of Knowledge, MasterFILE Premier, Health Source‑Consumer Edition, Agricola, Dentistry, and Oral Science Source from January 2000 up to January 2023 to find all relevant, full‑ text journal articles written in English.
Three authors (MB, MKR, SH) independently selected the publications that met the inclusion criteria specified above and extracted data for the outcomes using a standardized data extraction form. Abstracts/papers focusing on medullary thyroid cancer were excluded. The final list of SNPs was identified.
This selection process did not involve seeking tag SNPs or considering the genetic diversity within the regions where these SNPs are located. All the SNPs listed are cataloged in the public database dbSNP (Single Nucleotide Polymorphism Database) and have been validated across diverse ethnic populations. To ensure robustness in our calculations, only SNPs with a minor allele frequency (MAF) exceeding 1% were incorporated into the analysis.
Molecular studies
Assessment of mutational status was conducted with pyrosequencing. DNA was extracted from whole peripheral blood leukocytes with guanidine isothiocyanate and phenol‒chloroform. The primers used were designed with PyroMark Assay Design Software 2.0 (Qiagen, Venlo, Limburg, Netherlands). The mutational status was assessed from the patients’ initial blood samples, acquired at the time of thyroidectomy or within a period of maximum six months from the time of diagnosis. Reactions were performed on a PSQ96 device (Pyrosequencing AB, Uppsala, Sweden) using the PyroMark Gold Q96 reagent kit (Qiagen, Venlo, Limburg, Netherlands).
To identify the genes within the CNVs, we used the UCSC database [36] and Ensemble [37]. Gene annotation and gene overlap were assigned using the human genome build 19 (hg19) and NetAffx [38]. In addition, the identified alterations were compared with data deposited in the COSMIC database [39] to look for overlaps with up-to-date known genomic cancer regions and genes.
Statistical analysis
To estimate the effect of the variants on the thyroid cancer type (PTC vs FTC) and stage (pT1b-pT4 vs pT1a), we used a logistic regression model defined as follows:
where y is an \(ln = \frac{P}{1-P}\) with P being the probability of developing FTC and the probability of diagnosing the pT1a stage of FTC. X and Z are design matrices corresponding to β, a vector of nongenetic effects of age at the time of diagnosis and sex, respectively. u corresponds to the genetic effect of the analyzed variant, where this effect is assumed to be dominant where both homozygous and heterozygous mutations contribute equally or as additive where the variant effect increases with the number of mutant alleles.
Variant effects on the delta sTg were estimated with a linear regression model, where y was defined as the average change in the delta sTg ng/ml. Nongenetic, and genetic effects were modeled in the same way as in the logistic regression models. Due to the large portion of missing data and the different number of samples per variant, we decided to analyze each variant separately instead of via joint analysis. All analyses were performed using a generalized linear model implemented in R, and all the visualizations were performed with the ggplot2 package [40, 41]. For easier interpretation, we estimated marginal effects for each analyzed variant [42], i.e., change in the phenotype (probability or mean) when the allele count was changed by one unit (Figs. 1, 2).
To compare differences between groups, we used the chi-square test or Fisher’s exact test (2 × 2 contingency table), as appropriate, for categorical variables. Interval data were compared with the use of the Mann‒Whitney U test with post hoc Dunn’s test since the data did not follow a normal distribution. Odds ratios (ORs) and 95% confidence intervals (95%CIs) were calculated. To compare differences between the groups for categorical variables, the chi-square test was used if the Cochrane assumptions were met; otherwise, Fisher’s exact test was used. Interval data were compared with the use of the Mann–Whitney U test, as the data were not normally distributed [43]. A p value of less than 0.05 was regarded as significant. Statistical analyses were performed with StatSoft Statistica v13.0, PQStat v1.6.8, and R v4.3.2 software.
Results
A total of 634 patients with DTC were ultimately included in the study. Among them, 549 patients were diagnosed with papillary thyroid cancer (PTC), and 85 were diagnosed with follicular thyroid cancer (FTC). There was no significant difference in sex distribution or age at diagnosis between patients with PTC and those with FTC. Patient characteristics are presented in Table 2.
A total of 236 patients were diagnosed with DTC and CLT. The mean concentration of sTg was 20.74 ng/ml ± SE 59.97, the mean concentration of aTG was 53.27 IU/mL ± SE 176.94, and the mean concentration of aTPO was 150.70 IU/ml ± 295.45. The expression of any of the analyzed SNPs differed between patients with and without CLT (Table 2).
Among the identified NOD2 rs2066842 SNPs, differentiated PTC from FTC and the c.802 T allele were more common in patients with follicular thyroid cancer than in patients with papillary thyroid cancer (additive model: p = 0.02, OR = 1.65 ± SE 0.40–2.89; dominant model: p = 0.031, OR = 2.00 ± SE 0.62–3.38).
All patients who had both FTC and CLT were NOD2 rs2066842 positive.
sTg at first hospitalization was greater in patients with CLT (mean 5.35 vs. 2.17, p = 0.003). In all patients with DTC, the NOD2 rs2066842 allele c.802 T was associated with a greater decrease in thyroglobulin after the first radioiodine administration (delta in sTg at first qualification to RAI and sTg one year after the first administration of RAI), with 0.03 and a mean ΔTg 22.54 ± SE 12.32–32.76 ng/ml per mutant allele (c.802 T) in the additive model and a mean delta ΔTg 31.78 ± SE 17.07–46,49 ng/ml for carriers in the dominant model.
The NFKB1A rs696 allele c.126A was associated with a lower stage of FTC (pT1b-pT4 vs pT1a), with an additive OR = 1.02 ± SE 0.01–2.025 and a dominant OR = 1.8971 ± SE (0.631.12–3.152.61); p = 0.00613. The NFKB1A rs696 allele c.126A was also associated with a lower probability of multifocality (OR = 1.74 [1.02–2.97, p = 0.04]).
Discussion
The results of our study, performed in the largest reported homogenous cohort of subjects to date, confirmed that SNPs may be involved in the clinical course of DTC. Among these polymorphisms, the NOD2 rs2066842 polymorphism may distinguish PTC from FTC. It may also be a marker of better response to RAI in DTC patients, as it was linked with a greater decrease in sTg before and after the first RAI administration. Moreover, NFKB1A rs696 may predict a lower stage of FTC and a lower probability of PTC multifocality.
Critical factors influencing thyroid tumorigenesis are genetic. A major role is attributed to the activation of proto-oncogenes, wherein the basal expression level of oncogenes regulates cell growth and differentiation by participating in signal transduction to the cell nucleus. These include growth factors, receptors for growth factors, tyrosine kinases, and transcription factors.
Like the activation of oncogenes, the inactivation of tumor suppressor genes, such as TP53, whose function is to prevent tumorigenesis, is of great importance. Loss or inactivation of both alleles of a tumor suppressor gene leads to tumor development. Mutation of the TP53 gene is the most known genetic alteration involved in human cancer formation. On the other hand, mutations in the TP53 suppressor gene are associated with mutations in the CHEK2 gene and are associated with the Li-Fraumeni syndrome (LFS) phenotype. Mutation in mutator genes that can repair mutations leading to oncogene activation or suppressor gene inactivation may also be a cause of the tumorigenesis process.
Other genetic factors may include abnormalities in growth factors and their receptors, such as tumor necrosis factor (TNF) and other cytokines. Because single nucleotide changes may result in increased protein expression, a variant of the TNF gene has also been of interest to us.
The nucleotide-binding oligomerization domain containing 2 (NOD2) gene, also known as CARD15, is located at chromosomal region 16q21 [44]. NOD2 is a member of the evolutionarily conserved Nod-like receptor (NLR) family and plays a crucial role in detecting elements within microbial cell walls [45, 46]. The gene is a NOD1/APAF-1 family member that encodes proteins with two caspase recruitment domains and six leucine-rich repeats (LRRs) [47]. Studies suggest its involvement in controlling both programmed cell death (apoptosis) and long-term inflammatory disorders. The protein NOD2 is primarily expressed in humans in peripheral blood leukocytes [47]. It plays a vital role in the immune response, especially involving intracellular bacterial lipopolysaccharides (LPSs), by recognizing muramyl dipeptide (MDP), which is derived from bacteria and, in this way, activating the NFκB protein [47]. Interestingly, it has been suggested that the NOD2 protein acts as a regulator of appetite by sensing MDP molecules in a subset of brain neurons: microbial MDP may reach the brain and bind and activate the NOD2 protein in inhibitory hypothalamic neurons, leading to a decrease in neuronal activity, thus regulating satiety perception and probably influencing body temperature [48].
The role of NOD2 has yet to be determined, but it seems that it might be involved in many immunological pathways. It plays an essential role as a regulator of autophagy, especially in dendritic cells, via its interaction with the ATG16L1 protein, possibly through recruiting ATG16L1 at the site of bacterial entry [49]. Studies have also shown that NOD2 activation in the small intestine crypt contributes to intestinal stem cell survival and proper function. Hence, NOD2 may act by promoting mitophagy via its association with ATG16L1, as mentioned above [50, 51]. In addition to its important role in innate immunity, NOD2 controls the adaptive immune system by regulating helper T cells and regulatory T cells (Tregs) [50, 51]. In addition to being involved in pathogen recognition, NOD2 is involved in the endoplasmic reticulum stress response. It may act by sensing and binding to sphingosine-1-phosphate (S1P), a cytosolic metabolite generated in human cells in response to endoplasmic reticulum stress. This event usually initiates an inflammatory process leading to the activation of NF-kappa-B and MAP kinase signaling, which is very often dysregulated in cancer [50, 51].
The rs2066842 missense mutation is one of the most studied polymorphisms. It was initially found to be associated with an increased risk of Blau syndrome, as well as Crohn’s disease and ulcerative colitis [52]. It is located at coding regions and might affect the expression and function of NOD2 by altering amino acids. For the first time, NOD2 polymorphisms were linked to the risk of colorectal cancer [53]. Subsequently, further studies revealed an association between NOD2 polymorphisms and the risk of various cancers, including breast cancer and ovarian, endometrial, gastric, and laryngeal cancers [54]. However, the findings across individual studies varied and lacked consistency. A meta-analysis investigating overall cancer risk in relation to NOD2 polymorphisms showed that the NOD2 rs2066844 C/T, rs2066845 C/G, and rs2066847 (3020insC) polymorphisms might be associated with increased cancer risk. However, no significant association was observed between the NOD2 rs2066842 C/T polymorphism and cancer risk. However, thyroid cancer was not included in the analysis [54]. Only one group has studied the role of rs2066842 in thyroid cancer, and no increase in the occurrence of this gene was detected [55].
Our study showed a difference between FTC and PTC and was even more clinically relevant. Our study showed that the NOD2 rs2066842 polymorphism was linked to a better response to the RAI. NOD2 rs2066842 was linked to a greater decrease in the sTg marker after the first RAI.
The sTg measurement is the cornerstone of modern management of differentiated thyroid cancer, and clinical decisions on treatment and follow-up are based on the results of such measurements [35]. Tg production occurs primarily within normal and well-differentiated malignant thyrocytes, rendering Tg an ideal “tumor marker” after elimination of both healthy and pathological tissue. The introduction of highly sensitive thyroglobulin assays has significantly enhanced analytical sensitivity and stability in measuring Tg within the lower detection range. This has substantial implications for interpreting results in contemporary clinical practice [35]. A significant correlation existed between decreasing concentrations of sTg and the disappearance of thyroid tissue and between decreasing concentrations of sTg and RAI treatment efficacy.
RAI ablation success is the main predictor of DTC overall prognosis. Therefore, markers of RAI efficacy are highly needed. According to our study, NOD2 rs2066842 may be an easy and stable marker for the prediction of RAI efficacy.
It is not clear which mechanism, NOD2 rs2066842, may facilitate RAI action. One possible hypothesis is that the inflammatory process in which the NOD2 pathway participates may be involved in RAI action or may be protective against the progression of DTC. RAI activity is determined by host genetic background rather than the environment [56]. Therefore, SNPs may demonstrate prognostic value in DTC. Based on our data, the SNP NOD2 rs2066842 seems to be the most feasible candidate for determining the efficacy of RAI in DTC patients, thus affecting prognosis. Increased expression of NOD2 has been documented in various human metabolic disorders and chronic diseases linked to mitochondrial dysfunction [57]. It may manifest through direct signaling pathways, indirect modulation of cellular stress responses, or exacerbation of inflammatory processes [57]. RAI therapy in patients with DTC may increase proliferative lymphocyte responses and interferon-γ levels, demonstrating its proinflammatory function [58, 59].
The ongoing discourse revolves around the pivotal role of the cancer microenvironment. Cunha et al. proposed that the protective effect of CLT against the spread of DTC could be attributed to the involvement of various immune cells, such as CD4 + , CD8 + , CD201 + , Th17, and regulatory T cells (Tregs) [60]. Additionally, the interleukin-1 (IL-1) secreted by infiltrating lymphocytes in the CLT might exert an antitumorigenic effect by influencing the differentiation and replication of DTC cells [56, 61, 62]. Immune-mediated destruction of thyroid tissue may involve the NOD2 pathway, and our findings revealed the possible role of NOD2 rs2066842.
The same NOD2 polymorphism was found to be protective against multiple sclerosis and ameliorate the response to interferon-beta therapy, most likely as a part of a complex multidirectional system of genetic, immunological and environmental factors [63]. The NOD2 pathway may facilitate the action of pattern recognition receptors, which are germline-encoded host sensors that detect pathogens and play a crucial role in the proper function of the innate immune system [44].
According to the results of our study, NOD2 rs2066842 may serve as a marker for response to RAI, complementing the established role of Tg.
We suggest the additional use of NOD2 rs2066842 as a marker for response to RAI in patients with CLT and high concentration of aTg, where we suspect possible interference of Tg and aTg [35], potentially confounding and biasing the role of Tg as a marker of DTC. NOD2 rs2066842 may offer superior prognostication for response to RAI compared to Tg alone and facilitate better planning of RAI treatment. In patients who are NOD2 rs2066842-negative, it should be considered whether administering a higher activity of RAI would achieve the same effect as in NOD2 rs2066842-positive patients. The inflammatory process mediated through the NOD2 pathway and the activation of NF-kappa-B and MAP kinase signaling may enhance RAI efficacy. However, this hypothesis requires further investigation. In the text of our publication, we have clarified the suggested clinical role of NOD2 rs2066842 and its relationship with Tg.
Among the analyzed SNPs, NFKB1A rs696 may predict a lower stage of FTC and a lower probability of PTC multifocality. In inflammation, nuclear factor kappa B (NFKB) potentially exerts an oncogenic influence by fostering the proliferation and survival of numerous solid tumors [64]. Its protective role in DTC is consistent with the finding from the meta-analysis of Zhang et al. that NFKB1A rs696 seems to be associated with decreased susceptibility to cancer, especially Hodgkin lymphoma [65].
The nuclear factor-κB (NF-κB) gene, NFKB1A, resides on chromosome 14q13. The polymorphic variants within this gene rs2233406, rs3138053, and rs696 are positioned in regions that bind to CCAAT/enhancer binding protein and GATA binding protein 2. These variations potentially modulate the expression of IκBα, consequently impacting the activation of NF-κB. Moreover, these polymorphisms (rs2233406, rs3138053, and rs696) are directly associated with processes such as apoptosis, irregular immune cell maturation, and slowed cell growth [65, 66]. NF-κB belongs to a family of transcription factors that play crucial roles in inflammatory and immune reactions [67]. The protective effect of NFKB1A rs696 against higher stages of FTC and multifocality may be involved in these reactions.
Limitations of the study
Our findings are affected by a factor that restricts their interpretation: selection bias. It is crucial to acknowledge this influence before drawing any conclusions. To reduce bias in selection and control for external factors, our study group primarily comprises a homogenous population (with just 4.7% nonethnic Poles within Poland). This places our group in the seventh position among 159 countries worldwide and third in Europe in terms of homogeneity [68].
Another limitation was the difference in methods used for sTg measurement (as different assays have been used in recent years [35]); however, every patient was a control for themselves, and only the difference in sTg concentration before and after RAI was considered significant in our study.
Additionally, there was a notable difference in the prevalence of patients diagnosed with PTC compared to those diagnosed with FTC in the study group. However, this disproportion is reflective of the respective ratios of these diagnoses within the general population [28].
There were missing data in our study, including genetic analysis of SNPs, particular parameters of tumor histopathology, and particular measurements of thyroglobulin. To address these issues, we excluded missing data from the analysis by pairwise deletion (we used available data for each specific analysis).
Acknowledging the limitations of this study, it is pertinent to note that SNP analysis may be considered somewhat outdated in the context of the multiomics era. Consequently, conducting research involving genome sequencing is a more advanced and beneficial approach. However, we decided to analyze SNPs because of the ease of their performance in laboratories and the relatively low price of these methods, which make it possible to perform these tests on all patients in many countries; additionally, these methods are reimbursed by national insurance systems, which is still not true for multiomics. Due to its clinical utility and cost-effectiveness, SNP analysis has also been used to study thyroid cancer [55, 69, 70].
Conclusions
Our research identified possible stable and easily accessible prognostic markers in DTC patients. Moreover, SNPs represent a promising tool for empowering prognostic stratification of DTC and assessing the probability of RAI efficacy.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
van Houten P, Netea-Maier RT, Smit JW (2023) Differentiated thyroid carcinoma: an update. Best Pract Res Clin Endocrinol Metab 37:101687. https://doi.org/10.1016/j.beem.2022.101687
Jarząb B, Dedecjus M, Lewiński A et al (2022) Diagnosis and treatment of thyroid cancer in adult patients—recommendations of polish scientific societies and the national oncological strategy. 2022 update [diagnostyka i leczenie raka tarczycy u chorych dorosłych - rekomendacje polskich towarzystw naukowych oraz narodowej strategii onkologicznej. aktualizacja na rok 2022]. Endokrynol Pol 73:173–300. https://doi.org/10.5603/EP.a2022.0028
Yu L, Huang Z, Xiao Z et al (2024) Unveiling the best predictive models for early-onset metastatic cancer: insights and innovations (Review). Oncol Rep 51:60. https://doi.org/10.3892/or.2024.8719
Mo S, Zhou Z, Dai W et al (2020) Development and external validation of a predictive scoring system associated with metastasis of T1–2 colorectal tumors to lymph nodes. Clin Transl Med 10:275–287. https://doi.org/10.1002/ctm2.30
Vargas-Uricoechea H (2024) Autoimmune thyroid disease and differentiated thyroid carcinoma: a review of the mechanisms that explain an intriguing and exciting relationship. World J Oncol. https://doi.org/10.14740/wjon1728
Heo DB, Won H-R, Tae K et al (2024) Clinical impact of coexistent chronic lymphocytic thyroiditis on central lymph node metastasis in low- to intermediate-risk papillary thyroid carcinoma: the MASTER study. Surgery 175:1049–1054. https://doi.org/10.1016/j.surg.2023.12.023
Yang I, Yu JM, Chung HS et al (2024) Hashimoto thyroiditis and mortality in patients with differentiated thyroid cancer: the national epidemiologic survey of thyroid cancer in korea and meta-analysis. Endocrinol Metab (Seoul) 39:140–151. https://doi.org/10.3803/EnM.2023.1748
Park SK, Ryoo J-H, Kim M-H et al (2024) Association between eight autoimmune diseases and thyroid cancer: a nationwide cohort study. Thyroid 34:206–214. https://doi.org/10.1089/thy.2023.0353
Borowczyk M, Janicki A, Dworacki G et al (2019) Decreased staging of differentiated thyroid cancer in patients with chronic lymphocytic thyroiditis. J Endocrinol Invest 42:45–52. https://doi.org/10.1007/s40618-018-0882-4
Santana VB, Krüger VM, Abrahão MCY et al (2024) Chronic lymphocytic thyroiditis with oncocytic metaplasia influences pd-l1 expression in papillary thyroid carcinoma. Head Neck Pathol 18:14. https://doi.org/10.1007/s12105-024-01618-5
Pan J, Ye F, Yu C et al (2021) Papillary thyroid carcinoma landscape and its immunological link with hashimoto thyroiditis at single-cell resolution. Front Cell Dev Biol 9:758339. https://doi.org/10.3389/fcell.2021.758339
Dos Santos Valsecchi VA, Betoni FR, Ward LS, Cunha LL (2024) Clinical and molecular impact of concurrent thyroid autoimmune disease and thyroid cancer: from the bench to bedside. Rev Endocr Metab Disord 25:5–17. https://doi.org/10.1007/s11154-023-09846-w
Pani F, Caria P, Yasuda Y et al (2022) The immune landscape of papillary thyroid cancer in the context of autoimmune thyroiditis. Cancers (Basel) 14:4287. https://doi.org/10.3390/cancers14174287
Liu T-T, Yin D-T, Wang N et al (2023) Identifying and analyzing the key genes shared by papillary thyroid carcinoma and Hashimoto’s thyroiditis using bioinformatics methods. Front Endocrinol (Lausanne) 14:1140094. https://doi.org/10.3389/fendo.2023.1140094
Ma B, Chen X, Zhao Z et al (2022) Coexisting CLT in PTC is an independent predictor of tumor aggressiveness for patients aged under 55: a retrospective analysis of 635 patients. BMC Endocr Disord 22:55. https://doi.org/10.1186/s12902-022-00945-4
Sakiz D, Sencar ME, Calapkulu M et al (2021) The effects of chronic lymphocytic thyroiditis on clinicopathologic factors in papillary thyroid cancer. Endocr Pract 27:1199–1204. https://doi.org/10.1016/j.eprac.2021.07.011
Cho YY, Chung YJ, Kim HS (2021) Malignancy rate of bethesda class iii thyroid nodules based on the presence of chronic lymphocytic thyroiditis in surgical patients. Front Endocrinol (Lausanne) 12:745395. https://doi.org/10.3389/fendo.2021.745395
Aydoğan Bİ, Mutlu ABB, Yüksel S et al (2021) The association of histologically proven chronic lymphocytic thyroiditis with clinicopathological features, lymph node metastasis, and recurrence rates of differentiated thyroid cancer. Endocr Pathol 32:280–287. https://doi.org/10.1007/s12022-020-09653-y
Ryu YJ, Yoon JH (2020) Chronic lymphocytic thyroiditis protects against recurrence in patients with cN0 papillary thyroid cancer. Surg Oncol 34:67–73. https://doi.org/10.1016/j.suronc.2020.03.008
Lee I, Kim HK, Soh EY, Lee J (2020) The association between chronic lymphocytic thyroiditis and the progress of papillary thyroid cancer. World J Surg 44:1506–1513. https://doi.org/10.1007/s00268-019-05337-9
Osorio C, Ibarra S, Arrieta J et al (2020) Association between chronic lymphocytic thyroiditis and papillary thyroid carcinoma: a retrospective study in surgical specimens. Rev Esp Patol 53:149–157. https://doi.org/10.1016/j.patol.2019.07.004
Borowczyk M, Szczepanek-Parulska E, Dębicki S et al (2019) Differences in mutational profile between follicular thyroid carcinoma and follicular thyroid adenoma identified using next generation sequencing. Int J Mol Sci 20:E3126. https://doi.org/10.3390/ijms20133126
Borowczyk M, Szczepanek-Parulska E, Dębicki S et al (2020) High incidence of FLT3 mutations in follicular thyroid cancer: potential therapeutic target in patients with advanced disease stage. Ther Adv Med Oncol 12:1758835920907534. https://doi.org/10.1177/1758835920907534
Agrawal N, Akbani R, Aksoy BA et al (2014) Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690. https://doi.org/10.1016/j.cell.2014.09.050
Fagin JA, Nikiforov YE (2023) Progress in thyroid cancer genomics: a 40-year journey. Thyroid 33:1271–1286. https://doi.org/10.1089/thy.2023.0045
Liu JB, Ramonell KM, Carty SE et al (2023) Association of comprehensive thyroid cancer molecular profiling with tumor phenotype and cancer-specific outcomes. Surgery 173:252–259. https://doi.org/10.1016/j.surg.2022.05.048
Borowczyk M, Szczepanek-Parulska E, Olejarz M et al (2019) Evaluation of 167 gene expression classifier (gec) and thyroseq v2 diagnostic accuracy in the preoperative assessment of indeterminate thyroid nodules: bivariate/hroc meta-analysis. Endocr Pathol 30:8–15. https://doi.org/10.1007/s12022-018-9560-5
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133. https://doi.org/10.1089/thy.2015.0020
Romei C, Elisei R (2021) A narrative review of genetic alterations in primary thyroid epithelial cancer. Int J Mol Sci 22:1726. https://doi.org/10.3390/ijms22041726
Kalarani IB, Sivamani G, Veerabathiran R (2023) Identification of crucial genes involved in thyroid cancer development. J Egypt Natl Canc Inst 35:15. https://doi.org/10.1186/s43046-023-00177-0
Gejoe G, Yadev IP, Kumaran A et al (2022) Coexistence of histologically proven chronic lymphocytic thyroiditis with other thyroid disorders: a retrospective study. Surg J (N Y) 08:e131–e135. https://doi.org/10.1055/s-0041-1740626
Mincer DL, Jialal I (2024) Hashimoto Thyroiditis. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
Ralli M, Angeletti D, Fiore M et al (2020) Hashimoto’s thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev 19:102649. https://doi.org/10.1016/j.autrev.2020.102649
Liao X, Zhang D (2021) The 8th edition american joint committee on cancer staging for hepato-pancreato-biliary cancer: a review and update. Arch Pathol Lab Med 145:543–553. https://doi.org/10.5858/arpa.2020-0032-RA
Giovanella L, D’Aurizio F, Algeciras-Schimnich A et al (2023) Thyroglobulin and thyroglobulin antibody: an updated clinical and laboratory expert consensus. Eur J Endocrinol 189:R11–R27. https://doi.org/10.1093/ejendo/lvad109
Raney BJ, Barber GP, Benet-Pagès A et al (2024) The UCSC genome browser database: 2024 update. Nucleic Acids Res 52:D1082–D1088. https://doi.org/10.1093/nar/gkad987
Martin FJ, Amode MR, Aneja A et al (2023) Ensembl 2023. Nucleic Acids Res 51:D933–D941. https://doi.org/10.1093/nar/gkac958
Liu G, Loraine AE, Shigeta R et al (2003) NetAffx: affymetrix probesets and annotations. Nucleic Acids Res 31:82–86
Sondka Z, Dhir NB, Carvalho-Silva D et al (2024) COSMIC: a curated database of somatic variants and clinical data for cancer. Nucleic Acids Res 52:D1210–D1217. https://doi.org/10.1093/nar/gkad986
Wickham H, Averick M, Bryan J et al (2019) Welcome to the tidyverse. J Open Source Softw. https://doi.org/10.21105/joss.01686
Dessau RB, Pipper CB (2008) ’’R"–project for statistical computing. Ugeskr Laeger 170:328–330
Lüdecke D (2018) ggeffects: tidy data frames of marginal effects from regression models. J Open Source Softw. https://doi.org/10.21105/joss.00772
Guzik P, Więckowska B (2023) Data distribution analysis – a preliminary approach to quantitative data in biomedical research. J Med Sci 92:e869–e869. https://doi.org/10.20883/medical.e869
Almeida-da-Silva CLC, Savio LEB, Coutinho-Silva R, Ojcius DM (2023) The role of NOD-like receptors in innate immunity. Front Immunol. https://doi.org/10.3389/fimmu.2023.1122586
Babamale AO, Chen S-T (2021) Nod-like receptors: critical intracellular sensors for host protection and cell death in microbial and parasitic infections. Int J Mol Sci 22:11398. https://doi.org/10.3390/ijms222111398
Mukherjee T, Hovingh ES, Foerster EG et al (2019) NOD1 and NOD2 in inflammation, immunity and disease. Arch Biochem Biophys 670:69–81. https://doi.org/10.1016/j.abb.2018.12.022
Safran M, Rosen N, Twik M et al (2021) The GeneCards Suite. In: Abugessaisa I, Kasukawa T (eds) Practical Guide to Life Science Databases. Springer Nature, Singapore
Gabanyi I, Lepousez G, Wheeler R et al (2022) Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science. https://doi.org/10.1126/science.abj3986
Homer CR, Richmond AL, Rebert NA et al (2010) ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in crohn’s disease pathogenesis. Gastroenterology 139(1630–1641):1641.e1–2. https://doi.org/10.1053/j.gastro.2010.07.006
Keestra-Gounder AM, Byndloss MX, Seyffert N et al (2016) NOD1 and NOD2 signalling links ER stress with inflammation. Nature 532:394–397. https://doi.org/10.1038/nature17631
Pei G, Zyla J, He L et al (2021) Cellular stress promotes NOD1/2-dependent inflammation via the endogenous metabolite sphingosine-1-phosphate. EMBO J. https://doi.org/10.15252/embj.2020106272
Kaczmarek-Ryś M, Hryhorowicz ST, Lis E et al (2021) Crohn’s disease susceptibility and onset are strongly related to three nod2 gene haplotypes. J Clin Med 10:3777. https://doi.org/10.3390/jcm10173777
Tian Y, Li Y, Hu Z et al (2010) Differential effects of NOD2 polymorphisms on colorectal cancer risk: a meta-analysis. Int J Colorectal Dis 25:161–168. https://doi.org/10.1007/s00384-009-0809-9
Liu J, He C, Xu Q et al (2014) NOD2 polymorphisms associated with cancer risk: a meta-analysis. PLoS ONE 9:e89340. https://doi.org/10.1371/journal.pone.0089340
Kaczmarek-Ryś M, Ziemnicka K, Hryhorowicz ST et al (2015) The c.470 T > C CHEK2 missense variant increases the risk of differentiated thyroid carcinoma in the Great Poland population. Hered Cancer Clin Pract. https://doi.org/10.1186/s13053-015-0030-5
Mu Z, Zhang X, Sun D et al (2024) Characterizing genetic alterations related to radioiodine avidity in metastatic thyroid cancer. J Clin Endocrinol Metab 109:1231–1240. https://doi.org/10.1210/clinem/dgad697
Zangara MT, Johnston I, Johnson EE, McDonald C (2021) Mediators of metabolism: an unconventional role for NOD1 and NOD2. Int J Mol Sci 22:1156. https://doi.org/10.3390/ijms22031156
Stanciu AE, Hurduc A, Stanciu MM et al (2023) Portrait of the inflammatory response to radioiodine therapy in female patients with differentiated thyroid cancer with/without type 2 diabetes mellitus. Cancers (Basel) 15:3793. https://doi.org/10.3390/cancers15153793
De la Vieja A, Riesco-Eizaguirre G (2021) Radio-iodide treatment: from molecular aspects to the clinical view. Cancers (Basel) 13:995. https://doi.org/10.3390/cancers13050995
Cunha LL, Morari EC, Guihen ACT et al (2012) Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol 77:918–925. https://doi.org/10.1111/j.1365-2265.2012.04482.x
Shin E, Koo JS (2022) Cell component and function of tumor microenvironment in thyroid cancer. Int J Mol Sci 23:12578. https://doi.org/10.3390/ijms232012578
Xi C, Zhang G-Q, Sun Z-K et al (2020) Interleukins in thyroid cancer: from basic researches to applications in clinical practice. Front Immunol 11:1124. https://doi.org/10.3389/fimmu.2020.01124
Zečkanović A, Maver A, Ristić S et al (2021) Potential protective role of a NOD2 polymorphism in the susceptibility to multiple sclerosis is not associated with interferon therapy. Biomed Rep 15:100. https://doi.org/10.3892/br.2021.1476
Zhang T, Ma C, Zhang Z et al (2020) (2021) NF-κB signaling in inflammation and cancer. MedComm 2:618–653. https://doi.org/10.1002/mco2.104
Zhang M, Huang J, Tan X et al (2015) Common polymorphisms in the nfkbia gene and cancer susceptibility: a meta-analysis. Med Sci Monit 21:3186–3196. https://doi.org/10.12659/msm.895257
Li L, Zhang Z-T (2019) Genetic association between nfkbia and nfkb1 gene polymorphisms and the susceptibility to head and neck cancer: a meta-analysis. Dis Markers 2019:6523837. https://doi.org/10.1155/2019/6523837
Barnabei L, Laplantine E, Mbongo W et al (2021) NF-κB: At the borders of autoimmunity and inflammation. Front Immunol. https://doi.org/10.3389/fimmu.2021.716469
Kilfoy BA, Zheng T, Holford TR et al (2009) International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 20:525–531. https://doi.org/10.1007/s10552-008-9260-4
Hryhorowicz S, Ziemnicka K, Kaczmarek-Rys M et al (2015) Cyclin D1 and CHEK2 polymorphic variants as low risk susceptibility alleles in DTC patients. Hered Cancer in Clin Pract 13:A15. https://doi.org/10.1186/1897-4287-13-S1-A15
Hryhorowicz S, Ziemnicka K, Kaczmarek-Ryś M et al (2015) CCND1 gene polymorphic variants in patients with differentiated thyroid carcinoma. Oncol Lett 9:442–448. https://doi.org/10.3892/ol.2014.2617
Funding
This research was funded by the Polish National Science Center (grant no. NN402287436) (awarded to Marta Kaczmarek-Ryś) and a doctoral scholarship (awarded to Szymon Hryhorowicz) within the project ‘Scholarship support for PhD students specializing in majors strategic for Wielkopolska's development’, Sub‑measure 8.2.2 Human Capital Operational Program, co‑financed by the European Union under the European Social Fund. The APC was funded by Poznan University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Conception or design: MB, MKR, SH, MS, and KZ. Methodology: MB, MKR, SH, MS, and KZ. Investigation: MB, MKR, SH, MS and KZ. Data curation: DF, MO. Statistical analysis: MB, MS. Visualization: MS. Writing—original draft preparation: MB. Writing—review and editing: MKR, SH, MS, DF, PD, MO, MR, and KZ. Supervision: KZ. Project administration: MB, MKR, SH, and KZ. Funding acquisition: MKR, SH, MR. All the authors revised and approved the final manuscript and are accountable for the accuracy and integrity of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no potential conflicts of interest.
Ethical approval
This study was performed in accordance with the principles of the Declaration of Helsinki. Approval was granted by the Bioethical Committee of Poznan University of Medicine (approval no. 629/07 from June 2007).
Informed consent
All participants provided informed consent prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borowczyk, M., Kaczmarek-Ryś, M., Hryhorowicz, S. et al. Germline polymorphisms of the NOD2 pathway may predict the effectiveness of radioiodine in differentiated thyroid cancer treatment. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-024-02389-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40618-024-02389-0