Abstract
Aims
SIRT1 deficiency has been associated with diabetes, and a variant of the SIRT1 gene has been found to be involved in human autoimmune diabetes; however, it is unclear whether this genetic variation exists in Han Chinese with type 1 diabetes (T1D) and whether it contributes to development of T1D. Therefore, we aimed to explore the association of the SIRT1 gene single-nucleotide polymorphisms (SNPs) rs10997866 and rs3818292 in a Han Chinese population with T1D.
Methods
This study recruited 2653 unrelated Han Chinese individuals, of whom 1289 had T1D and 1364 were healthy controls. Allelic and genotypic distributions of SIRT1 polymorphisms (rs10997866 and rs3818292) were determined by MassARRAY. Basic characteristics, genotype and allele frequencies of selected SNPs were compared between the T1D patients and healthy controls. Further genotype–phenotype association analysis of the SNPs was performed on the T1D patients divided into three groups according to genotype. Statistical analyses included the chi-square test, Mann‒Whitney U test, Kruskal‒Wallis H test and logistic regression.
Results
The allelic (G vs. A) and genotypic (GA vs. AA) distributions of SIRT1 rs10997866 were significantly different in T1D patients and healthy controls (P = 0.039, P = 0.027), and rs10997866 was associated with T1D susceptibility under dominant, overdominant and additive models (P = 0.026, P = 0.030 and P = 0.027, respectively). Moreover, genotype–phenotype association analysis showed the GG genotype of rs10997866 and the GG genotype of rs3818292 to be associated with higher titers of IA-2A (P = 0.013 and P = 0.038, respectively).
Conclusion
SIRT1 rs10997866 is significantly associated with T1D susceptibility, with the minor allele G conferring a higher risk of T1D. Moreover, SIRT1 gene rs10997866 and rs3818292 correlate with the titer of IA-2A in Han Chinese individuals with T1D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes (T1D) is an organ-specific chronic autoimmune disease characterized by insulin deficiency and hyperglycemia. Although the mechanisms of T1D development and progression are not completely clear, it is currently believed to be a complex genetic disease resulting from interaction of a series of genetic and environmental factors [1, 2]. Although most (approximately 70%) T1D patients do not have a positive family history, the family genetic tendency of T1D, a polygenic disease, is obvious [3]. In addition, the comorbidity risk of T1D between siblings is higher than that between parents and offspring among first-degree relatives, indicating that T1D has strong genetic susceptibility [4].
Previous studies have shown that the human leukocyte antigen (HLA) region contains the main susceptibility genes for human T1D, with HLA II genes contributing approximately 50% of genetic risk [5]. Genome-wide association studies (GWASs) have identified over 60 non-HLA genes related to risk of T1D, and these genes contribute to inheritance of the disease through small genetic effects resulting from their various combinations [5]. In general, understanding the association of non-HLA genes with T1D is helpful for identifying potential targets for treatment and for developing specific immune-related interventions.
SIRT1 is a kind of NAD+-dependent histone deacetylase that plays an important role in energy metabolism, apoptosis and aging [6]. A variety of studies have shown that SIRT1 regulates inflammation, gluconeogenesis, lipolysis, β cell survival and glucose-dependent insulin secretion by interacting with many histone and nonhistone substrates, directly or indirectly affecting the occurrence and development of diabetes, especially type 2 diabetes (T2D) [7, 8]. A series of studies have highlighted the influence of single-nucleotide polymorphisms (SNPs) of the SIRT1 gene on various aspects of T2D, including its risk, insulin resistance, diabetes-related traits and associated complications [9], for example, T2DM-related coronary heart disease (rs16924934 and rs3818291 in the Han Chinese population [10] and rs7896005 [10, 11]) and diabetic kidney disease (rs10823108, rs3818292 and rs7069102) [12,13,14]. Furthermore, rs7895833 and rs1467568 of SIRT1 are involved in fetal programming during malnutrition, thus affecting T2D risk later in life [15].
Importantly, SIRT1 plays a role in the disease susceptibility of T2D and its associated complications, and some studies have suggested that SIRT1 gene variants influence autoimmune diseases. SIRT1 promoter rs3758391 was shown to modify the morbidity risk due to systemic lupus erythematosus (SLE), and the T allele acts as a risk factor for progression of nephritis and a higher SLE disease activity index [16]. Recently, researchers have found that a novel rare variant of the SIRT1 gene (Leu107Pro) is responsible for the autoimmune diabetes phenotype, providing a novel idea and direction for further exploration of SIRT1 single-gene variants related to the pathogenesis of T1D [17].
The present study extends the only report thus far in T1D by investigating the association between SIRT1 gene polymorphisms and the clinical characteristics of T1D patients in a cross-sectional analysis. The basic characteristics and allele and genotype frequencies of selected SNPs in T1D patients and healthy controls in the Han Chinese population were analyzed to confirm whether there is a correlation between SIRT1 variants and risk of T1D.
Methods
Patients and healthy subjects
The participants in this study consisted of 1289 T1D patients from the endocrine inpatient and outpatient clinic of the Second Xiangya Hospital of Central South University (Changsha, China) and 1364 healthy controls recruited from the same hospital physical examination center and epidemiological investigations. All participants were from the Han Chinese population and provided signed informed consent, and the ethics committee of the Second Xiangya Hospital of Central South University approved this study.
A standard diagnosis of T1D was first made in accordance with the 1999 WHO diagnostic criteria for diabetes and with acute onset, and insulin injections were needed within half a year of diagnosis to achieve adequate glucose control. In addition, we tested for islet self-antibodies, including glutamic acid decarboxylase autoantibody (GADA), protein tyrosine phosphatase autoantibody (IA-2A) and zinc transporter eight antibody (ZnT8A). Patients with serum positivity for at least one were included in the study. We excluded patients with T2D, gestational diabetes, specific types of diabetes and other autoimmune diseases.
The healthy controls with a fasting plasma glucose (FPG) level < 5.6 mmol/L and a 2-h postprandial plasma glucose (PPG) level < 7.8 mmol/L according to the 75 g oral glucose tolerance test (OGTT) were recruited through normal clinical examination results. To reduce bias, we strictly followed the admission rules of the control group, which not only included the nondiabetes Han Chinese population but also excluded people with chronic diseases and endocrine diseases, other types of autoimmune diseases, family history of diabetes, and malignant tumors.
Information collection
Information for all subjects, including age, sex, diagnosis and treatment process, current medical history, past and family history, height and weight, was measured and collected by trained physicians. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Levels of FBG, PPG and other biochemical indicators were determined via automated liquid chromatography and chemiluminescence methods; levels of GADA, IA-2A and ZnT8A were detected via radioligand binding assays. Fasting C-peptide (FCP) and 2-h postprandial C-peptide (2hCP) levels were assessed among individuals diagnosed with T1D after ameliorating glucotoxicity. The cutoff point for deficient beta-cell function was defined as FCP or 2hCP < 16.5 pmol/L. Impaired beta-cell function was indicated as FCP or 2hCP between 16.5 and 200 pmol/L, and patients with both FCP and 2hCP exceeding 200 pmol/L were defined as having preserved beta-cell function, as previously reported. All biochemical indicators were determined in the Department of Metabolism and Endocrinology, The Second Xiangya Hospital of Central South University.
DNA preparation and genotyping assay
Approximately 4 mL of peripheral blood from all participants was collected through venipuncture and placed in tubes containing sodium ethylenediaminetetraacetic acid. Genomic DNA was extracted from the blood samples using a GeneNode Genomic DNA Extraction kit (Genenode Biotech Co., Ltd., Beijing), and the quality of the DNA was assessed using agarose gel electrophoresis (Bio-Rad Company, USA). The DNA samples were stored at -80 °C before being analyzed for SNP genotype using MassARRAY technology (Agena, MassARRAY® Analyzer 4) at BGI Genomics (Beijing Genomics Institute, Shenzhen, China).
Single-nucleotide polymorphism selection
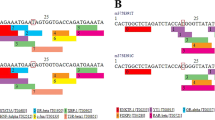
We chose two tagSNPs, rs10997866 and rs3818292, which both satisfy minor allele frequency (MAF) > 0.05 in the Asian population and are not located in the same linkage region (Fig. 1). The r2 between rs10997866 and rs3818292 is 0.084 in the East Asian population (https://ldlink.nci.nih.gov/?tab=home). In addition, these two SNPs of the SIRT1 gene were selected mainly depending on the loci related to some autoimmune diseases reported in recent years [13, 18].
Statistical analysis
All statistical analyses were performed using SPSS 26.0 software (IBM Corporation, Armonk, NY, USA). Measurement data conforming to a normal distribution are expressed as the mean ± standard deviation (SD). Continuous variables not normally distributed are presented as the median and interquartile range (IQR), and categorical data are presented as frequencies (percentages). Hardy–Weinberg equilibrium for each SNP in the control population was assessed for further analysis. Student's t test or the Mann–Whitney U test was used to compare differences between groups, and the chi-square test or Fisher's exact test was used to identify differences in proportions between groups. Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained using logistic regression analysis to assess differences in alleles/genotypes between the T1D patients and healthy controls. The Kruskal‒Wallis H test and chi-square test were used to compare the characteristics of different genotypes in T1D patients. A two-tailed P < 0.05 was considered statistically significant.
Results
Basic characteristics
The basic characteristics of the T1D patients and healthy controls are shown in Table 1. The results demonstrated that the sex ratio did not differ significantly between the two groups (P = 0.932). However, significant differences were observed in other basic characteristics, including age, BMI, FBG, and PBG, (all, P < 0.05), but not for sex. The healthy controls had higher age and BMI values than the T1D patients (P < 0.001 and P < 0.001), and the T1D patients had higher levels of FBG and PBG (P < 0.001 and P < 0.001). Our data also indicated that the enrolled T1D patients had FCP and PCP levels of 88.01 (33.42, 170.00) pmol/L and 173.00 (63.73, 351.60) pmol/L, respectively, and HbA1c levels of 9.7 (7.3–12.7)%.
Associations between alleles and genotypes of single-nucleotide polymorphisms in the SIRT1 gene and T1D
All the genotype frequency distributions of the two SNPs (rs10997866 and rs3818292) in our healthy controls were consistent with Hardy–Weinberg equilibrium (HWE) proportions (P = 0.335 and P = 0.656, respectively), which indicated that the recruited subjects are representative of the population.
The results indicated that SIRT1 rs10997866 is linked to an increased risk of developing T1D, as the G allele of rs10997866 conferred a higher risk of T1D (OR = 1.18, 95% CI = 1.01–1.37, P = 0.039). Moreover, we compared three different genotypes of this SNP locus in pairs. The AA genotype was taken as the reference, with an OR value of 1. The OR value and 95% CI of homozygous and heterozygous minor alleles were calculated. The heterozygous GA genotype of rs10997866 showed a significant association with T1D risk (OR = 1.22, 95% CI = 1.02–1.46, P = 0.027), whereas no significant association was found between the homozygous AA genotype and T1D risk (P > 0.05). There was no significant difference in the distribution of alleles and genotypes of rs3818292 between the T1D patients and healthy controls (Table 2). Furthermore, the association between the two SNPs of SIRT1 and T1D susceptibility was examined under various genetic models, including dominant, recessive, overdominant, and additive. The results suggested that only SIRT1 rs10997866 has a statistically significant association with T1D susceptibility under dominant, overdominant, and additive genetic models (P = 0.026, P = 0.030 and P = 0.027, respectively). Conversely, no statistically significant association was found between SIRT1 rs3818292 and T1D susceptibility under the four genetic inheritance models (Table 3).
Associations of the two SNPs and the clinical characteristics of the T1D patients
We further evaluated whether a genotype–phenotype relationship for the SIRT1 gene polymorphisms with the clinical characteristics of the T1D patients exists. The characteristics assessed included basic information (sex, age of onset, course of the disease, and BMI), biochemical measurements (FCP, PCP and HbA1c), and antibody results (GADA positivity rate and titer, IA-2A positivity rate and titer, and ZnT8A positivity rate). The results showed that both SIRT1 rs10997866 and rs3818292 were significantly associated with the titer of IA-2A in T1D patients. Furthermore, the T1D patients with the GG genotype had higher IA-2A titers than those with the GA and AA genotypes for these two SNPs (P = 0.013 and P = 0.038, respectively, Tables 4 and 5).
Discussion
There is growing evidence supporting the cytoprotective role of SIRT1 in diabetes and its complications [6, 19,20,21]. As an antioxidant, SIRT1 reduces oxidative stress (OS) in coronary arterial endothelial cells exposed to elevated glucose levels and then regulates insulin signaling [22]. A recent study demonstrated that varying expression levels of SIRT1 in diabetes may be partly attributed to OS [20]; this is consistent with early-stage diabetic rats showing decreased SIRT1 expression, with expression levels returning to normal after treatment with the antioxidant glucagon-like peptide 1 analog exendin-4 (EX4) [23]. In addition, downregulated SIRT1 has been reported to be associated with gestational diabetes and to play a possible role in reducing hypervascularization early in pregnancy and protecting against subsequent pregnancy complications caused by impaired placental growth [19].
Studies to date have mainly focused on the effects of altered SIRT1 protein expression on diabetes and related symptoms; nevertheless, as a complex autoimmune disease for which both genetic and environmental factors influence susceptibility [24], it has been widely accepted that genetic factors play an important role in the etiology of T1D [2, 24]. Indeed, a series of studies exploring the association between SIRT1 gene polymorphisms in inflammation and autoimmune destruction have been carried out [17]. However, there is still minimal information about the role of SIRT1 gene polymorphisms in the pathogenesis of T1D. Therefore, deeper exploration of the effect of SIRT1 on T1D susceptibility from a genetic perspective is needed.
This case‒control study aimed to detect two selected SIRT1 SNPs (rs10997866 and rs3818292) in the Han Chinese population with T1D to provide clues for elucidating the relationship between the SIRT1 gene and T1D. Overall, our results showed that the SIRT1 SNP rs10997866 is associated with an increased risk for T1D, with the G allele conferring higher risk. However, the association between T1D susceptibility and rs10997866 was observed for the GA genotype only, whereas no significant difference was detected for the GG genotype, possibly due to the relatively small sample size of GG genotype patients. Expanding the sample size may lead to statistically significant differences. Then, we found an association between rs10997866 and T1D susceptibility in genetic inheritance models (under dominant, overdominant and additive models). We also identified a link between these SIRT1 SNPs and islet autoantibody titers in T1D patients, whereby T1D patients with the GG genotype of rs10997866 and rs3818292 had higher IA-2A titers than those with the GA and AA genotypes, suggesting an association between SIRT1 risk alleles and IA-2A titers. These findings suggest that SIRT1 risk variants might play a role in regulating autoimmunity and have the potential to be used as biomarkers for T1D progression.
To date, islet autoantibodies are still the most valuable biomarkers of islet autoimmunity, which indicates disease progression and diagnosis [25]. IA-2A has been identified as an independent predictor of more rapid progression to diabetes, especially when combined with GADA antibodies. Its longer prevalence time compared to other autoantibodies makes it a valuable tool in diagnosing latent autoimmune diabetes [26]. Individuals with IA-2A tend to be younger, have lower fasting and stimulated C-peptide levels, and experience a shorter duration of symptoms. Some immune-associated variants, such as in IL27, IFIH1, and CTLA4, have been linked to the presence of IA-2A [27,28,29]. However, there is significant variability in the rate of pancreatic β cell destruction, which may be caused by genetic susceptibility [26]. As it is believed that an individual's genotype remains constant from birth and is not influenced by age, any age discrepancies between cases and controls are not expected to impact results. Additionally, we conducted logistic regression analysis to assess whether differences in BMI influence the distribution of genotype and allele frequencies of the 2 SNPs. Our findings revealed no significant difference in genotype or allele frequency between the two groups (P > 0.05) after adjusting for BMI. Thus, we excluded the possibility that the observed differences were not due to age or BMI discrepancies.
Our study is the first to identify a correlation between SIRT1 gene variants and increased T1D risk in a large sample of the Han Chinese population. However, there are some limitations. Importantly, in addition to genetic polymorphisms, the relationship between the SIRT1 gene and T1D is complex and influenced by various factors; despite a reported positive association with this SNP and T2D or other autoimmune diseases, a correlation between SIRT1 rs3818292 and T1D was not found in our study. We also analyzed the relationship between these SIRT1 gene polymorphisms and clinical characteristics of T1D patients, but certain measurements assessing liver and renal function, such as blood lipids, liver and kidney function, and 24 h urinary albumin, were not obtained for all enrolled individuals. Furthermore, owing to the extensive temporal span encompassing our sample collection period, a subset of early-collected samples lacked the contemporaneous recording of insulin dosage administration. Consequently, we encountered obstacles in delineating insulin dosage utilization among individuals harboring distinct SIRT1 genotypes and its potential interplay with C-peptide levels within the purview of this investigation. Although our results suggested an association between SIRT1 SNPs and T1D susceptibility, it is unclear whether these selected SNPs affect expression levels of SIRT1 and the functional role they play in the pathogenesis of T1D due to the lack of experimental data. Therefore, further research is required to investigate the biological mechanisms involved in the development of T1D.
Conclusion
SIRT1 rs10997866 is significantly associated with T1D susceptibility, and the minor allele G confers higher risk of T1D. Moreover, rs10997866 and rs3818292 in the SIRT1 gene correlate with the rate of IA-2A positivity in Han Chinese individuals with T1D.
Data availability
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Xie Z, Chang C, Zhou Z (2014) Molecular mechanisms in autoimmune type 1 diabetes: a critical review. Clin Rev Allergy Immunol 47(2):174–192. https://doi.org/10.1007/s12016-014-8422-2
Wang Z, Xie Z, Lu Q et al (2017) Beyond Genetics: What Causes Type 1 Diabetes. Clin Rev Allergy Immunol 52(2):273–286. https://doi.org/10.1007/s12016-016-8592-1
Redondo MJ, Jeffrey J, Fain PR et al (2008) Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 359(26):2849–2850. https://doi.org/10.1056/NEJMc0805398
Pociot F, Lernmark A (2016) Genetic risk factors for type 1 diabetes. Lancet 387(10035):2331–2339. https://doi.org/10.1016/S0140-6736(16)30582-7
Noble JA (2015) Immunogenetics of type 1 diabetes: A comprehensive review. J Autoimmun 64:101–112. https://doi.org/10.1016/j.jaut.2015.07.014
Nogueiras R, Habegger KM, Chaudhary N et al (2012) Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 92(3):1479–1514. https://doi.org/10.1152/physrev.00022.2011
Haigis MC, Guarente LP (2006) Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev 20(21):2913–2921. https://doi.org/10.1101/gad.1467506
Schwitzgebel VM (2014) Many faces of monogenic diabetes. J Diabetes Investig 5(2):121–133. https://doi.org/10.1111/jdi.12197
Song J, Yang B, Jia X et al (2018) Distinctive Roles of Sirtuins on Diabetes, Protective or Detrimental? Front Endocrinol (Lausanne) 9:724. https://doi.org/10.3389/fendo.2018.00724
Wang Y, Tong L, Gu N et al (2022) Association of Sirtuin 1 Gene Polymorphisms with the Risk of Coronary Heart Disease in Chinese Han Patients with Type 2 Diabetes Mellitus. J Diabetes Res 2022:8494502. https://doi.org/10.1155/2022/8494502
Dardano A, Lucchesi D, Garofolo M et al (2022) SIRT1 rs7896005 polymorphism affects major vascular outcomes, not all-cause mortality, in Caucasians with type 2 diabetes: A 13-year observational study. Diabetes Metab Res Rev 38(4):e3523. https://doi.org/10.1002/dmrr.3523
Zhao Y, Wei J, Hou X et al (2017) SIRT1 rs10823108 and FOXO1 rs17446614 responsible for genetic susceptibility to diabetic nephropathy. Sci Rep 7(1):10285. https://doi.org/10.1038/s41598-017-10612-7
Yue XG, Yang ZG, Zhang Y et al (2018) Correlations between SIRT1 gene polymorphisms and diabetic kidney disease. R Soc Open Sci 5(6):171871. https://doi.org/10.1098/rsos.171871
Letonja J, Zavrsnik M, Makuc J et al (2021) Sirtuin 1 rs7069102 polymorphism is associated with diabetic nephropathy in patients with type 2 diabetes mellitus. Bosn J Basic Med Sci 21(5):642–646. https://doi.org/10.17305/bjbms.2020.5368
Botden IP, Zillikens MC, de Rooij SR et al (2012) Variants in the SIRT1 gene may affect diabetes risk in interaction with prenatal exposure to famine. Diabetes Care 35(2):424–426. https://doi.org/10.2337/dc11-1203
Consiglio CR, Juliana da Silveira S, Monticielo OA et al (2014) SIRT1 promoter polymorphisms as clinical modifiers on systemic lupus erythematosus. Mol Biol Rep 41(7):4233–4239. https://doi.org/10.1007/s11033-014-3294-3
Biason-Lauber A, Boni-Schnetzler M, Hubbard BP et al (2013) Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab 17(3):448–455. https://doi.org/10.1016/j.cmet.2013.02.001
Maszlag-Torok R, Boros FA, Vecsei L et al (2021) Gene variants and expression changes of SIRT1 and SIRT6 in peripheral blood are associated with Parkinson’s disease. Sci Rep 11(1):10677. https://doi.org/10.1038/s41598-021-90059-z
Alqudah A, Eastwood KA, Jerotic D et al (2021) FKBPL and SIRT-1 Are Downregulated by Diabetes in Pregnancy Impacting on Angiogenesis and Endothelial Function. Front Endocrinol (Lausanne) 12:650328. https://doi.org/10.3389/fendo.2021.650328
Al-Khaldi A, Sultan S (2019) The expression of sirtuins, superoxide dismutase, and lipid peroxidation status in peripheral blood from patients with diabetes and hypothyroidism. BMC Endocr Disord 19(1):19. https://doi.org/10.1186/s12902-019-0350-y
Dong Y, Guo T, Traurig M et al (2011) SIRT1 is associated with a decrease in acute insulin secretion and a sex specific increase in risk for type 2 diabetes in Pima Indians. Mol Genet Metab 104(4):661–665. https://doi.org/10.1016/j.ymgme.2011.08.001
Ungvari Z, Labinskyy N, Mukhopadhyay P et al (2009) Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297(5):H1876–H1881. https://doi.org/10.1152/ajpheart.00375.2009
Zeng Y, Yang K, Wang F et al (2016) The glucagon like peptide 1 analogue, exendin-4, attenuates oxidative stress-induced retinal cell death in early diabetic rats through promoting Sirt1 and Sirt3 expression. Exp Eye Res 151:203–211. https://doi.org/10.1016/j.exer.2016.05.002
DiMeglio LA, Evans-Molina C, Oram RA (2018) Type 1 diabetes. Lancet 391(10138):2449–2462. https://doi.org/10.1016/S0140-6736(18)31320-5
Lempainen J, Laine AP, Hammais A et al (2015) Non-HLA gene effects on the disease process of type 1 diabetes: From HLA susceptibility to overt disease. J Autoimmun 61:45–53. https://doi.org/10.1016/j.jaut.2015.05.005
Long AE, George G, Williams CL (2021) Persistence of islet autoantibodies after diagnosis in type 1 diabetes. Diabet Med 38(12):e14712. https://doi.org/10.1111/dme.14712
Chen Y, Chen S, Gu Y et al (2018) CTLA-4 +49 G/A, a functional T1D risk SNP, affects CTLA-4 level in Treg subsets and IA-2A positivity, but not beta-cell function. Sci Rep 8(1):10074. https://doi.org/10.1038/s41598-018-28423-9
Plagnol V, Howson JM, Smyth DJ et al (2011) Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genet 7(8):e1002216. https://doi.org/10.1371/journal.pgen.1002216
Li J, Sun X, Luo S et al (2021) The Positivity Rate of IA-2A and ZnT8A in the Chinese Han Population With Type 1 Diabetes Mellitus: Association With rs1143627 and rs1143643 Polymorphisms in the IL1B Gene. Front Pharmacol 12:729890. https://doi.org/10.3389/fphar.2021.729890
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 82070813, 81873634) and Hunan Province Natural Science Foundation of China (Grant No. 2018JJ2573, 2020JJ2053).
Author information
Authors and Affiliations
Contributions
J.L. contributed to formal analysis and writing the original draft of the manuscript. J.L. and Y.Y. were responsible for the software and data curation, methodology and investigation. J.L., Y.X., S.L., J.L., Y.X., X.L., G.H., L.Y., Z.X. and Z.Z. contributed to the discussion and edited and revised the manuscript. Z.X. designed and supervised the project. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Statement of human and animal rights
All procedures were approved by the ethics committee of the Second Xiangya Hospital of Central South University.
Informed consent
All participants provided informed consent prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Yang, Y., Xia, Y. et al. Effect of SIRT1 gene single-nucleotide polymorphisms on susceptibility to type 1 diabetes in a Han Chinese population. J Endocrinol Invest 47, 819–826 (2024). https://doi.org/10.1007/s40618-023-02190-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02190-5