Abstract
Background
Klotho is a pleotropic hormone involved in a multitude of biological processes necessary for healthy aging, and affords protection from adverse events such as cardiovascular disease, inflammation, and various cancers. Emerging evidence suggests that klotho is also an important component of biochemical pathways that regulate hormone balance, which may include those pathways governing testosterone production and men’s sexual health, though data are limited and results are mixed.
Objective
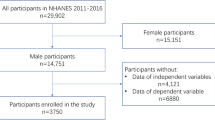
Using a cohort of 767 men from the NHANES 2015–2016 survey cycle, we set out to quantify the association between serum klotho levels and serum testosterone levels, as well as clinical markers of men’s sexual health (e.g., testosterone:estrogen ratio, bioavailable testosterone, and free testosterone).
Methods
Multivariable linear and logistic regression models while controlling for potential confounders were constructed to quantify the relationship between serum klotho and testosterone, as well as between serum klotho and odds of low testosterone (serum testosterone < 300 ng/dL).
Results
A positive association was observed between serum klotho and testosterone (β = 0.18, p = 0.04). Serum klotho levels were also stratified into quartiles, and we observed statistically significant increases in testosterone for increasing quartile level of klotho using the first quartile as the reference group (β = 90.51, p = 0.001, β = 106.93, p = 0.002, β = 95.33, p = 0.03 for quartiles 2, 3, and 4, respectively). The average testosterone values by quartiles of klotho were 306.9 ng/dL, 390 ng/dL, 409.3 ng/dL, and 436.6 ng/dL, respectively. We modeled important proxies for sexual health including bioavailable and free testosterone, the testosterone:estradiol ratio, and C-reactive protein. Men in the second quartile of klotho had a significantly lower odds of an abnormal testosterone:estradiol ratio compared to the first quartile [OR = 0.18, 95% CI = (0.03, 0.98)].We observed null associations between continuous serum klotho and odds of low testosterone [OR = 1.0, 95% CI = (1.0, 1.0)], and when stratified by quartile, we observed a significant decrease in the odds of low testosterone for individuals in the second quartile of klotho compared to the first quartile [OR = 0.21, 95% CI = (0.05, 0.91)]. In addition, C-reactive protein was inversely associated with testosterone in men (β = − 4.65, p = 0.001), and inversely associated with quartiles of klotho (β = − 2.28, p = 0.04, β = − 2.22, p = 0.04, β = − 2.28, p = 0.03, for quartiles 2, 3, and 4, respectively).
Conclusion
Our findings support previous studies suggesting a role for klotho in testosterone levels and sexual function among men. Future studies are warranted to corroborate these findings, determine clinical significance, and elucidate potential mechanisms underlying these associations.


Similar content being viewed by others
Availability of data and materials
A full list of data sets supporting the results in this research article can be found at: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013.
References
Köhn FM (2006) Testosterone and body functions. Aging Male 9(4):183–188. https://doi.org/10.1080/13685530601060396. (Epub 2006/12/21. PubMed PMID: 17178552)
Shea JL, Wong PY, Chen Y (2014) Free testosterone: clinical utility and important analytical aspects of measurement. Adv Clin Chem 63:59–84. https://doi.org/10.1016/b978-0-12-800094-6.00002-9. (Epub 2014/05/03. PubMed PMID: 24783351)
Abhyankar N, Shoshany O, Niederberger C (2016) Testosterone to estradiol ratio correlates with sperm concentration improvement in hypogonadal oligozoosermic patients treated with anastrozole. Fertil Steril 106(3):e239–e240. https://doi.org/10.1016/j.fertnstert.2016.07.690
Kusters CD, Paul KC, Lu AT, Ferruci L, Ritz BR, Binder AM, Horvath S (2023) Higher testosterone and testosterone/estradiol ratio in men are associated with better epigenetic estimators of mortality risk. medRxiv. https://doi.org/10.1101/2023.02.16.23285997
Belladelli F, Del Giudice F, Kasman A, Salonia A, Eisenberg ML (2021) The association between testosterone, estradiol and their ratio and mortality among US men. Andrologia 53(4):e13993. https://doi.org/10.1111/and.13993
Schulster M, Bernie AM, Ramasamy R (2016) The role of estradiol in male reproductive function. Asian J Androl 18(3):435–440. https://doi.org/10.4103/1008-682x.173932. (Epub 2016/02/26. PubMed PMID: 26908066; PMCID: PMC4854098)
Jia H, Sullivan CT, McCoy SC, Yarrow JF, Morrow M, Borst SE (2015) Review of health risks of low testosterone and testosterone administration. World J Clin Cases 3(4):338–344. https://doi.org/10.12998/wjcc.v3.i4.338. (PubMed PMID: 25879005)
Ross A, Bhasin S (2016) Hypogonadism: its prevalence and diagnosis. Urol Clin North Am 43(2):163–176. https://doi.org/10.1016/j.ucl.2016.01.002. (Epub 2016/05/03. PubMed PMID: 27132573)
Glover FE, Caudle WM, Del Giudice F, Belladelli F, Mulloy E, Lawal E, Eisenberg ML (2022) The association between caffeine intake and testosterone: NHANES 2013–2014. Nutr J 21(1):33. https://doi.org/10.1186/s12937-022-00783-z
Glover FE, Del Giudice F, Belladelli F, Ryan PB, Chen T, Eisenberg ML, Caudle WM (2021) The association between 2,4-D and serum testosterone levels: NHANES 2013–2014. J Endocrinol Invest. https://doi.org/10.1007/s40618-021-01709-y
Holt SK, Lopushnyan N, Hotaling J, Sarma AV, Dunn RL, Cleary PA, Braffett BH, Gatcomb P, Martin C, Herman WH, Wessells H (2014) Prevalence of low testosterone and predisposing risk factors in men with type 1 diabetes mellitus: findings from the DCCT/EDIC. J Clin Endocrinol Metab 99(9):E1655–E1660. https://doi.org/10.1210/jc.2014-1317. (Epub 2014/07/12. PubMed PMID: 25013994; PMCID: PMC4154094)
Del Giudice F, Glover F, Belladelli F, De Berardinis E, Sciarra A, Salciccia S, Kasman AM, Chen T, Eisenberg ML (2021) Association of daily step count and serum testosterone among men in the United States. Endocrine 72(3):874–881. https://doi.org/10.1007/s12020-021-02631-2
John GB, Cheng CY, Kuro-o M (2011) Role of Klotho in aging, phosphate metabolism, and CKD. Am J Kidney Dis 58(1):127–134. https://doi.org/10.1053/j.ajkd.2010.12.027. (Epub 2011/04/19. PubMed PMID: 21496980; PMCID: PMC3191324)
Chen Y-Y, Chen W-L (2022) The relationship between polycyclic aromatic hydrocarbons exposure and serum klotho among adult population. BMC Geriatr 22(1):198. https://doi.org/10.1186/s12877-022-02924-9
Mostafidi E, Moeen A, Nasri H, Ghorbani Hagjo A, Ardalan M (2016) Serum klotho levels in trained athletes. Nephrourol Mon. 8(1):e30245. https://doi.org/10.5812/numonthly.30245. (PubMed PMID: 26981496)
Kuwahara N, Sasaki S, Kobara M, Nakata T, Tatsumi T, Irie H, Narumiya H, Hatta T, Takeda K, Matsubara H, Hushiki S (2008) HMG-CoA reductase inhibition improves anti-aging klotho protein expression and arteriosclerosis in rats with chronic inhibition of nitric oxide synthesis. Int J Cardiol 123(2):84–90. https://doi.org/10.1016/j.ijcard.2007.02.029. (Epub 2007/04/17. PubMed PMID: 17434618)
Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, James S, Wilkinson IB, Ting S, Hsiao L-L, Hiemstra TF, Zehnder D (2015) α-klotho expression in human tissues. J Clin Endocrinol Metab 100(10):E1308–E1318. https://doi.org/10.1210/jc.2015-1800. (Epub 2015/08/17. PubMed PMID: 26280509)
Hsu SC, Huang SM, Lin SH, Ka SM, Chen A, Shih MF, Hsu YJ (2014) Testosterone increases renal anti-aging klotho gene expression via the androgen receptor-mediated pathway. Biochem J 464(2):221–229. https://doi.org/10.1042/bj20140739. (Epub 2014/08/28. PubMed PMID: 25163025)
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390(6655):45–51. https://doi.org/10.1038/36285. (Epub 1997/11/18. PubMed PMID: 9363890)
Dote-Montero M, Amaro-Gahete FJ, De-la OA, Jurado-Fasoli L, Gutierrez A, Castillo MJ (2019) Study of the association of DHEAS, testosterone and cortisol with S-klotho plasma levels in healthy sedentary middle-aged adults. Exp Gerontol 121:55–61. https://doi.org/10.1016/j.exger.2019.03.010. (Epub 2019/04/01. PubMed PMID: 30928678)
Ekström L, Knutsson JE, Stephanou C, Hirschberg AL (2022) Klotho polymorphism in association with serum testosterone and knee strength in women after testosterone administration. Front Physiol. https://doi.org/10.3389/fphys.2022.844133
Zhang Z, Qiu S, Huang X, Jin K, Zhou X, Lin T, Zou X, Yang Q, Yang L, Wei Q (2022) Association between testosterone and serum soluble α-klotho in US males: a cross-sectional study. BMC Geriatr 22(1):570. https://doi.org/10.1186/s12877-022-03265-3. (Epub 2022/07/13. PubMed PMID: 35820842; PMCID: PMC9275159)
Prevention CfDCa. NHANES survey methods and analytic guidelines 2020. https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx. Cited 26 Oct 2020
Prevention CfDCa. NHANES 2013–2014 Questionnaire Data Overview 2020. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/OverviewQuex.aspx?BeginYear=2013. Updated 24 Aug 2020
Patrick ME, Azar B (2018) High-intensity drinking. Alcohol Res 39(1):49–55 (Epub 2018/12/18. PubMed PMID: 30557148; PMCID: PMC6104968 interests)
Abel EL, Kruger ML, Friedl J (1998) How do physicians define “light,” “moderate,” and “heavy” drinking? Alcohol Clin Exp Res 22(5):979–984. https://doi.org/10.1111/j.1530-0277.1998.tb03692.x. (Epub 1998/09/03. PubMed PMID: 9726266)
Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH (2016) Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol 45(6):1887–1894. https://doi.org/10.1093/ije/dyw341
Rivas AM, Mulkey Z, Lado-Abeal J, Yarbrough S (2014) Diagnosing and managing low serum testosterone. Proc (Bayl Univ Med Cent). 27(4):321–324. https://doi.org/10.1080/08998280.2014.11929145. (Epub 2014/12/09. PubMed PMID: 25484498; PMCID: PMC4255853)
Zhu A, Andino J, Daignault-Newton S, Chopra Z, Sarma A, Dupree JM (2022) What is a normal testosterone level for young men? Rethinking the 300 ng/dL cutoff for testosterone deficiency in men 20–44 years old. J Urol 208(6):1295–1302. https://doi.org/10.1097/ju.0000000000002928. (Epub 2022/10/26. PubMed PMID: 36282060)
Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH (2007) The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol 156(5):595–602. https://doi.org/10.1530/EJE-06-0737
Di Bona D, Accardi G, Virruso C, Candore G, Caruso C (2014) Association of klotho polymorphisms with healthy aging: a systematic review and meta-analysis. Rejuvenation Res 17(2):212–216. https://doi.org/10.1089/rej.2013.1523. (Epub 2013/10/30. PubMed PMID: 24164579)
Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y (1998) Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242(3):626–630. https://doi.org/10.1006/bbrc.1997.8019. (Epub 1998/02/17. PubMed PMID: 9464267)
Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, Maekawa Y, Kida I, Miyazaki J, Ogihara T (2007) Anti-oxidative effect of klotho on endothelial cells through cAMP activation. Endocrine 31(1):82–87. https://doi.org/10.1007/s12020-007-0016-9. (Epub 2007/08/22. PubMed PMID: 17709902)
Dote-Montero M, De-la OA, Castillo MJ, Amaro-Gahete FJ (2020) Predictors of sexual desire and sexual function in sedentary middle-aged adults: the role of lean mass index and S-klotho plasma levels. The FIT-AGEING study. J Sex Med 17(4):665–677. https://doi.org/10.1016/j.jsxm.2020.01.016. (Epub 2020/02/25. PubMed PMID: 32089483)
Drüeke TB, Massy ZA (2013) Circulating klotho levels: clinical relevance and relationship with tissue klotho expression. Kidney Int 83(1):13–15. https://doi.org/10.1038/ki.2012.370
Bøllehuus Hansen L, Kaludjerovic J, Nielsen JE, Rehfeld A, Poulsen NN, Ide N, Skakkebaek NE, Frederiksen H, Juul A, Lanske B, Blomberg JM (2020) Influence of FGF23 and Klotho on male reproduction: systemic vs direct effects. FASEB J 34(9):12436–12449. https://doi.org/10.1096/fj.202000061RR
Muraleedharan V, Jones TH (2010) Testosterone and the metabolic syndrome. Ther Adv Endocrinol Metab. 1(5):207–223. https://doi.org/10.1177/2042018810390258. (Epub 2010/10/01. PubMed PMID: 23148165; PMCID: PMC3474619)
Zhang C, Bian H, Chen Z, Tian B, Wang H, Tu X, Cai B, Jin K, Zheng X, Yang L, Qiu S (2021) The association between dietary inflammatory index and sex hormones among men in the United States. J Urol 206(1):97–103. https://doi.org/10.1097/JU.0000000000001703
Jin M, Lou J, Yu H, Miao M, Wang G, Ai H, Huang Y, Han S, Han D, Yu G (2018) Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin promotes inflammation in mouse testes: the critical role of klotho in sertoli cells. Toxicol Lett 295:134–143. https://doi.org/10.1016/j.toxlet.2018.06.001. (Epub 2018/06/10. PubMed PMID: 29885354)
Funding
The authors received no external funding for this research study.
Author information
Authors and Affiliations
Contributions
FG is the primary author who drafted the manuscript, obtained references, performed most of the data analysis, and helped with the conceptualization of the study. FB and FDG both performed data analysis with the regression models, obtained background information for references, and proof read the manuscript drafts. ES and EM also performed extensive background research, as well as proofed all drafts of the manuscript and helped created figures and tables. MLE provided guidance at the conceptualization stage, proofed all drafts of the manuscript, and helped perform regression analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing financial interests.
Ethical approval and consent to participate
Health information collected in the NHANES is kept in strictest confidence. During the informed consent process, survey participants were assured that data collected will be used only for stated purposes and will not be disclosed or released to others without the consent of the individual or the establishment in accordance with Section 308 (d) of the Public Health Service Act (42 U.S.C. 242m).
Consent for publication
Participants in this study agreed to consent for publication in accordance with Section 308 (d) of the Public Health Service Act (42 U.S.C. 242m).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Glover, F., Sullivan, E., Mulloy, E. et al. The relationship between klotho, testosterone, and sexual health parameters among US adult men. J Endocrinol Invest 47, 523–533 (2024). https://doi.org/10.1007/s40618-023-02163-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02163-8