Abstract
Background
We aimed to develop a nomogram model of overall survival (OS) and cancer-specific survival (CSS) in patients with differentiated thyroid cancer with distant metastases, and to evaluate and validate the nomogram. Also, its prognostic value was compared with that of the 8th edition of the American Joint Committee on Cancer tumor–node–metastasis staging system (AJCC8SS).
Methods
Patients with distant metastatic differentiated thyroid cancer (DMDTC) from 2004 to 2015 were selected from the Surveillance, Epidemiology, and End Results (SEER) Program to extract the clinical variables used for analysis. A total of 906 patients were divided into a training set (n = 634) and validation set (n = 272). OS and CSS were selected as the primary end point and secondary end point. LASSO regression analysis and multivariate Cox regression analysis were applied to screen variables for constructing OS and CSS nomograms for survival probability at 3, 5, and 10 years. Nomograms were evaluated and validated using the consistency index (C-index), time-dependent receiver operator characteristic (ROC) curves, area under the ROC curve, calibration curves, and decision curve analysis (DCA). The predictive survival of the nomogram was compared with that of AJCC8SS. Kaplan–Meier curves and log-rank tests were used to evaluate the risk-stratification ability OS and CSS nomograms.
Results
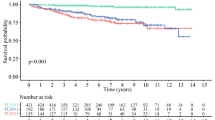
CS and CSS nomograms included six independent predictors: age, marital status, type of surgical procedure, lymphadenectomy, radiotherapy, and T stage. The C-index for the OS nomogram was 0.7474 (95% CI = 0.7199–0.775), and that for the CSS nomogram was 0.7572 (0.7281–0.7862). The nomogram showed good agreement with the “ideal” calibration curve in the training set and validation sets. DCA confirmed that the survival probability predicted by the nomogram had high clinical predictive value. The nomogram could stratify patients more accurately, and showed more robust accuracy and predictive power, than AJCC8SS.
Conclusions
We established and validated prognostic nomograms for patients with DMDTC, which had significant clinical value compared with AJCC8SS.








Similar content being viewed by others
Data availability
The analyzed datasets for this study can be found in the [Surveillance, Epidemiology, and End Results (SEER) Program] [https://seer.cancer.gov/]. Alternatively, these datasets can be obtained from the corresponding authors whenever reasonably requested.
Code availability
Not applicable.
References
Cabanillas ME, McFadden DG, Durante C (2016) Thyroid cancer. Lancet 388(10061):2783–2795
Leite AK, Kulcsar MA, de Godoi Cavalheiro B, de Mello ES, Alves VA, Cernea CR, et al. death related to pulmonary metastasis in patients with differentiated thyroid cancer. Endocr Pract. 2017;23(1):72–8.
Zunino A, Pitoia F, Faure E, Reyes A, Sala M, Sklate R et al (2019) Unusual metastases from differentiated thyroid carcinoma: analysis of 36 cases. Endocrine 65(3):630–636
Vuong HG, Le MK, Hassell L, Kondo T, Kakudo K (2022) The differences in distant metastatic patterns and their corresponding survival between thyroid cancer subtypes. Head Neck 44(4):926–932
Mihailovic J, Stefanovic L, Malesevic M, Markoski B (2009) The importance of age over radioiodine avidity as a prognostic factor in differentiated thyroid carcinoma with distant metastases. Thyroid 19(3):227–232
Benbassat CA, Mechlis-Frish S, Hirsch D (2006) Clinicopathological characteristics and long-term outcome in patients with distant metastases from differentiated thyroid cancer. World J Surg 30(6):1088–1095
Lee J, Soh EY (2010) Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann Surg 251(1):114–119
Haq M, Harmer C (2005) Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf) 63(1):87–93
Shaha AR, Migliacci JC, Nixon IJ, Wang LY, Wong RJ, Morris LGT, et al. Stage migration with the new American Joint Committee on Cancer (AJCC) staging system (8th edition) for differentiated thyroid cancer. Surgery. 2019;165(1):6–11.
van Velsen EFS, Stegenga MT, van Kemenade FJ, Kam BLR, van Ginhoven TM, Visser WE, et al. Comparing the Prognostic Value of the Eighth Edition of the American Joint Committee on Cancer/Tumor Node Metastasis Staging System Between Papillary and Follicular Thyroid Cancer. Thyroid. 2018;28(8):976–81.
Shteinshnaider M, Muallem Kalmovich L, Koren S, Or K, Cantrell D, Benbassat C (2018) Reassessment of differentiated thyroid cancer patients using the eighth TNM/AJCC classification system: a comparative study. Thyroid 28(2):201–209
Pontius LN, Oyekunle TO, Thomas SM, Stang MT, Scheri RP, Roman SA et al (2017) Projecting survival in papillary thyroid cancer: a comparison of the seventh and eighth editions of the american joint commission on cancer/union for international cancer control staging systems in two contemporary national patient cohorts. Thyroid 27(11):1408–1416
Lang BH, Wong KP, Cheung CY, Wan KY, Lo CY (2013) Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 20(4):1329–1335
Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Freedman S, Brennan MF et al (2003) Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg 197(2):191–197
Zhang XY, Sun JW, Qiu ZL, Wang Y, Chen XY, Zhao JH et al (2019) Clinical outcomes and prognostic factors in patients with no less than three distant organ system metastases from differentiated thyroid carcinoma. Endocrine 66(2):254–265
Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP et al (2012) The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid 22(9):884–889
Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP et al (2006) Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91(8):2892–2899
Nunes KS, Matos LL, Cavalheiro BG, Magnabosco FF, Tavares MR, Kulcsar MA et al (2022) Risk factors associated with disease-specific mortality in papillary thyroid cancer patients with distant metastases. Endocrine 75(3):814–822
Jin S, Liu H, Yang J, Zhou J, Peng D, Liu X et al (2022) Development and validation of a nomogram model for cancer-specific survival of patients with poorly differentiated thyroid carcinoma: A SEER database analysis. Front Endocrinol (Lausanne) 13:882279
Wen Q, Yu Y, Yang J, Wang X, Wen J, Wen Y et al (2019) Development and Validation of a Nomogram for Predicting Survival in Patients with Thyroid Cancer. Med Sci Monit 25:5561–5571
Zhang R, Xu M, Liu X, Wang M, Jia Q, Wang S, et al. Establishment and validation of a nomogram model for predicting the survival probability of differentiated thyroid carcinoma patients: a comparison with the eighth edition AJCC cancer staging system. Endocrine. 2021;74(1):108–19.
Wu L, Zhou Y, Guan Y, Xiao R, Cai J, Chen W et al (2021) Seven genes associated with lymphatic metastasis in thyroid cancer that is linked to tumor immune cell infiltration. Front Oncol 11:756246
Wen S, Luo Y, Wu W, Zhang T, Yang Y, Ji Q et al (2021) Identification of lipid metabolism-related genes as prognostic indicators in papillary thyroid cancer. Acta Biochim Biophys Sin (Shanghai) 53(12):1579–1589
Momesso DP, Tuttle RM (2014) Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am 43(2):401–421
Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M (2015) Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the surveillance, epidemiology, and end results database. Thyroid 25(1):125–132
Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A et al (2015) Survival from differentiated thyroid cancer: what has age got to do with It? Thyroid 25(10):1106–1114
Elisei R, Molinaro E, Agate L, Bottici V, Masserini L, Ceccarelli C et al (2010) Are the clinical and pathological features of differentiated thyroid carcinoma really changed over the last 35 years? Study on 4187 patients from a single Italian institution to answer this question. J Clin Endocrinol Metab 95(4):1516–1527
Banerjee M, Muenz DG, Chang JT, Papaleontiou M, Haymart MR (2014) Tree-based model for thyroid cancer prognostication. J Clin Endocrinol Metab 99(10):3737–3745
Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ et al (2013) Marital status and survival in patients with cancer. J Clin Oncol 31(31):3869–3876
Shi RL, Qu N, Lu ZW, Liao T, Gao Y, Ji QH (2016) The impact of marital status at diagnosis on cancer survival in patients with differentiated thyroid cancer. Cancer Med 5(8):2145–2154
Yu J, Deng Y, Liu T, Zhou J, Jia X, Xiao T et al (2020) Lymph node metastasis prediction of papillary thyroid carcinoma based on transfer learning radiomics. Nat Commun 11(1):4807
Zhang J, Cheng X, Shen L, Wang X, Wang L, Sun X et al (2020) The Association Between Lymph Node Stage and Clinical Prognosis in Thyroid Cancer. Front Endocrinol (Lausanne) 11:90
Jeon MJ, Kim WG, Choi YM, Kwon H, Lee YM, Sung TY et al (2016) Features predictive of distant metastasis in papillary thyroid microcarcinomas. Thyroid 26(1):161–168
Ho AS, Luu M, Shafqat I, Mallen-St Clair J, Chen MM, Chen Y et al (2021) Predictive impact of metastatic lymph node burden on distant metastasis across papillary thyroid cancer variants. Thyroid 31(10):1549–1557
Barney BM, Hitchcock YJ, Sharma P, Shrieve DC, Tward JD (2011) Overall and cause-specific survival for patients undergoing lobectomy, near-total, or total thyroidectomy for differentiated thyroid cancer. Head Neck 33(5):645–649
Macedo FI, Mittal VK (2015) Total thyroidectomy versus lobectomy as initial operation for small unilateral papillary thyroid carcinoma: a meta-analysis. Surg Oncol 24(2):117–122
Li C, Wu Q, Sun S (2020) Radioactive iodine therapy in patients with thyroid carcinoma with distant metastases: a SEER-based study. Cancer Control 27(1):1073274820914661
Kwon J, Wu HG, Youn YK, Lee KE, Kim KH, Park DJ (2013) Role of adjuvant postoperative external beam radiotherapy for well differentiated thyroid cancer. Radiat Oncol J 31(3):162–170
Kiess AP, Agrawal N, Brierley JD, Duvvuri U, Ferris RL, Genden E et al (2016) External-beam radiotherapy for differentiated thyroid cancer locoregional control: a statement of the American head and neck society. Head Neck 38(4):493–498
Sun XS, Sun SR, Guevara N, Marcy PY, Peyrottes I, Lassalle S et al (2013) Indications of external beam radiation therapy in non-anaplastic thyroid cancer and impact of innovative radiation techniques. Crit Rev Oncol Hematol 86(1):52–68
Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K et al (2006) Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World J Surg 30(5):780–786
Bellantone R, Lombardi CP, Boscherini M, Ferrante A, Raffaelli M, Rubino F et al (1998) Prognostic factors in differentiated thyroid carcinoma: a multivariate analysis of 234 consecutive patients. J Surg Oncol 68(4):237–241
Andersen PE, Kinsella J, Loree TR, Shaha AR, Shah JP (1995) Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg 170(5):467–470
Tibshirani R (1996) Regression Shrinkage and Selection Via the Lasso. J Roy Stat Soc: Ser B (Methodol) 58(1):267–288
Hernan MA, Hernandez-Diaz S, Robins JM (2004) A structural approach to selection bias. Epidemiology 15(5):615–625
Funding
This work was supported by the National Natural Science Foundation of China (82271205, 81701298). The study was also supported by China Postdoctoral Science Foundation (Grant No. 2019M651970).
Author information
Authors and Affiliations
Contributions
Co-first authors QM and ZC contributed equally to the study. QM and ZC participated in the conception and design of the study and wrote the manuscript. CY and XZ participated in obtaining financial and key revisions, as well as the statistical analysis of data. SL verified the methods and steps of statistical analysis. YF, XW, and NW participated in the preparation of the article. All authors participated in the revision of the manuscript, read and approved the submitted version, and agreed to take responsibility for all aspects of the study to ensure the accuracy of this research. All authors agreed to publish this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Ethical approval
All data used in this study came from publicly available databases, so this study received an exemption from the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Q., Chen, Z., Fang, Y. et al. Development and validation of survival nomograms for patients with differentiated thyroid cancer with distant metastases: a SEER Program-based study. J Endocrinol Invest 47, 115–129 (2024). https://doi.org/10.1007/s40618-023-02129-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02129-w