Abstract
Purpose
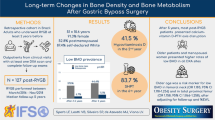
Potential negative effects of metabolic surgery on skeletal integrity remain a concern, since long-term data of different surgical approaches are poor. This study aimed to describe changes in bone metabolism in subjects with obesity undergoing both Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG).
Methods
A single center, retrospective, observational clinical study on real-world data was performed enrolling subjects undergoing metabolic surgery.
Results
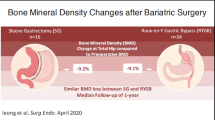
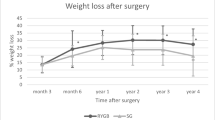
123 subjects were enrolled (males 31: females 92; ages 48.2 ± 7.9 years). All patients were evaluated until 16.9 ± 8.1 months after surgery, while a small group was evaluated up to 4.5 years. All patients were treated after surgery with calcium and vitamin D integration. Both calcium and phosphate serum levels significantly increased after metabolic surgery and remained stable during follow-up. These trends did not differ between RYGB and SG (p = 0.245). Ca/P ratio decreased after surgery compared to baseline (p < 0.001) and this decrease remained among follow-up visits. While 24-h urinary calcium remained stable across all visits, 24-h urinary phosphate showed lower levels after surgery (p = 0.014), also according to surgery technique. Parathyroid hormone decreased (p < 0.001) and both vitamin D (p < 0.001) and C-terminal telopeptide of type I collagen (p = 0.001) increased after surgery.
Conclusion
We demonstrated that calcium and phosphorous metabolism shows slight modification even after several years since metabolic surgery, irrespective of calcium and vitamin D supplementation. This different set point is characterized by a phosphate serum levels increase, together with a persistent bone loss, suggesting that supplementation alone may not ensure the maintenance of bone health in these patients.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Phillips BT, Shikora SA (2018) The history of metabolic and bariatric surgery: development of standards for patient safety and efficacy. Metab Clin Exp 79:97–107. https://doi.org/10.1016/j.metabol.2017.12.010
Arterburn DE, Telem DA, Kushner RF, Courcoulas AP (2020) Benefits and risks of bariatric surgery in adults: a review. JAMA 324:879–887. https://doi.org/10.1001/jama.2020.12567
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737. https://doi.org/10.1001/jama.292.14.1724
Rubino F (2013) From bariatric to metabolic surgery: definition of a new discipline and implications for clinical practice. Curr Atheroscler Rep 15:369. https://doi.org/10.1007/s11883-013-0369-x
Colquitt JL, Pickett K, Loveman E, Frampton GK (2014) Surgery for weight loss in adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003641.pub4
Melissas J, Stavroulakis K, Tzikoulis V, Peristeri A, Papadakis JA, Pazouki A, Khalaj A, Kabir A (2017) Sleeve gastrectomy vs Roux-en-Y gastric bypass. Data from IFSO-European chapter center of excellence program. Obes Surg 27:847–855. https://doi.org/10.1007/s11695-016-2395-6
Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, Schultes B, Beglinger C, Drewe J, Schiesser M et al (2018) Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA 319:255–265. https://doi.org/10.1001/jama.2017.20897
Grönroos S, Helmiö M, Juuti A, Tiusanen R, Hurme S, Löyttyniemi E, Ovaska J, Leivonen M, Peromaa-Haavisto P, Mäklin S et al (2020) Effect of laparoscopic sleeve gastrectomy vs Roux-en-Y gastric bypass on weight loss and quality of life at 7 years in patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. https://doi.org/10.1001/jamasurg.2020.5666
Casimiro I, Sam S, Brady MJ (2019) Endocrine implications of bariatric surgery: a review on the intersection between incretins, bone, and sex hormones. Physiol Rep 7:e14111. https://doi.org/10.14814/phy2.14111
Saad RK, Ghezzawi M, Habli D, Alami RS, Chakhtoura M (2022) Fracture risk following bariatric surgery: a systematic review and meta-analysis. Osteoporos Int 33:511–526. https://doi.org/10.1007/s00198-021-06206-9
Thorell A, MacCormick AD, Awad S, Reynolds N, Roulin D, Demartines N, Vignaud M, Alvarez A, Singh PM, Lobo DN (2016) Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg 40:2065–2083. https://doi.org/10.1007/s00268-016-3492-3
Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, Kushner RF, Lindquist R, Pessah-Pollack R, Seger J et al (2019) Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American association of clinical endocrinologists/American college of endocrinology, the obesity society, American society for metabolic & bariatric surgery, obesity medicine association, and American society of anesthesiologists. Endocr Pract 25:1346–1359. https://doi.org/10.4158/GL-2019-0406
O’Kane M, Parretti HM, Pinkney J, Welbourn R, Hughes CA, Mok J, Walker N, Thomas D, Devin J, Coulman KD et al (2020) British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery-2020 update. Obes Rev 21:e13087. https://doi.org/10.1111/obr.13087
Di Lorenzo N, Antoniou SA, Batterham RL, Busetto L, Godoroja D, Iossa A, Carrano FM, Agresta F, Alarçon I, Azran C et al (2020) Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg Endosc 34:2332–2358. https://doi.org/10.1007/s00464-020-07555-y
Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, Hjelmesæth J, Kinzl J, Leitner DR, Makaronidis JM et al (2017) Practical recommendations of the obesity management task force of the European association for the study of obesity for the post-bariatric surgery medical management. Obes Facts 10:597–632. https://doi.org/10.1159/000481825
Pereira FA, de Castro JA, dos Santos JE, Foss MC, Paula FJ (2007) Impact of marked weight loss induced by bariatric surgery on bone mineral density and remodeling. Braz J Med Biol Res 40:509–517. https://doi.org/10.1590/s0100-879x2007000400009
Costa TL, Paganotto M, Radominski RB, Kulak CM, Borba VC (2015) Calcium metabolism, vitamin D and bone mineral density after bariatric surgery. Osteoporos Int 26:757–764. https://doi.org/10.1007/s00198-014-2962-4
Alencar MAVS, Araújo IM, Parreiras-E-Silva LT, Nogueira-Barbosa MH, Salgado W, Elias J, Salmon CEG, Paula FJA (2021) Hashtag bone: detrimental effects on bone contrast with metabolic benefits one and five years after Roux-en-Y gastric bypass. Braz J Med Biol Res 54:e11499. https://doi.org/10.1590/1414-431X2021e11499
Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ (2008) The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab 93:3735–3740. https://doi.org/10.1210/jc.2008-0481
Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA (2006) True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 14:1940–1948. https://doi.org/10.1038/oby.2006.226
Tian Z, Fan XT, Li SZ, Zhai T, Dong J (2020) Changes in bone metabolism after sleeve gastrectomy versus gastric bypass: a meta-analysis. Obes Surg 30:77–86. https://doi.org/10.1007/s11695-019-04119-5
Ou X, Chen M, Xu L, Lin W, Huang H, Chen G, Wen J (2022) Changes in bone mineral density after bariatric surgery in patients of different ages or patients with different postoperative periods: a systematic review and meta-analysis. Eur J Med Res 27:144. https://doi.org/10.1186/s40001-022-00774-0
Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, Ballem N, Kligman M, Kothari S, ACI Committee (2015) Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis 11:489–506. https://doi.org/10.1016/j.soard.2015.02.003
Pedrazzoni M, Girasole G, Bertoldo F, Bianchi G, Cepollaro C, Del Puente A, Giannini S, Gonnelli S, Maggio D, Marcocci C et al (2003) Definition of a population-specific DXA reference standard in Italian women: the Densitometric Italian Normative Study (DINS). Osteoporos Int 14:978–982. https://doi.org/10.1007/s00198-003-1521-1
Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, Krause KR, Schram JL, Kole KL, Finks JF, Birkmeyer JD et al (2013) The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg 257:791–797. https://doi.org/10.1097/SLA.0b013e3182879ded
Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N (2017) Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg 27:2279–2289. https://doi.org/10.1007/s11695-017-2666-x
Madeo B, De Vincentis S, Kara E, Vescini F, Trenti T, Guaraldi G, Rochira V (2019) Reliability of calcium-phosphorus (Ca/P) ratio as a new, accurate and inexpensive tool in the diagnosis of some Ca-P disorders. J Endocrinol Invest 42:1041–1049. https://doi.org/10.1007/s40618-019-01025-6
Greco C, Passerini F, Coluccia S, Bondi M, Mecheri F, Trapani V, Volpe A, Toschi P, Carubbi F, Simoni M et al (2022) Effects of bariatric and metabolic surgical procedures on dyslipidemia: a retrospective, observational analysis. Metab Target Organ Dam. https://doi.org/10.20517/mtod.2022.22
Madeo B, De Vincentis S, Repaci A, Altieri P, Vicennati V, Kara E, Vescini F, Amadori P, Balestrieri A, Pagotto U et al (2020) The calcium-to-phosphorous (Ca/P) ratio in the diagnosis of primary hyperparathyroidism and hypoparathyroidism: a multicentric study. Endocrine 68:679–687. https://doi.org/10.1007/s12020-020-02276-7
Wu KC, Cao S, Weaver CM, King NJ, Patel S, Kim TY, Black DM, Kingman H, Shafer MM, Rogers SJ et al (2022) Intestinal calcium absorption decreases after laparoscopic sleeve gastrectomy despite optimization of vitamin D status. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgac579
Billington EO, Murphy R, Gamble GD, Callon K, Davies N, Plank LD, Booth M, Reid IR (2019) Fibroblast growth factor 23 levels decline following sleeve gastrectomy. Clin Endocrinol (Oxf) 91:87–93. https://doi.org/10.1111/cen.13981
Peacock M (2010) Calcium metabolism in health and disease. Clin J Am Soc Nephrol 5(Suppl 1):S23-30. https://doi.org/10.2215/CJN.05910809
Schafer AL (2017) Vitamin D and intestinal calcium transport after bariatric surgery. J Steroid Biochem Mol Biol 173:202–210. https://doi.org/10.1016/j.jsbmb.2016.12.012
Wongdee K, Chanpaisaeng K, Teerapornpuntakit J, Charoenphandhu N (2021) Intestinal calcium absorption. Compr Physiol 11:2047–2073. https://doi.org/10.1002/cphy.c200014
Wasserman RH (2004) Vitamin D and the dual processes of intestinal calcium absorption. J Nutr 134:3137–3139. https://doi.org/10.1093/jn/134.11.3137
García Martín A, Varsavsky M, Cortés Berdonces M, Ávila Rubio V, Alhambra Expósito MR, Novo Rodríguez C, Rozas Moreno P, Romero Muñoz M, Jódar Gimeno E, Rodríguez Ortega P et al (2020) Phosphate disorders and clinical management of hypophosphatemia and hyperphosphatemia. Endocrinol Diabetes Nutr (Engl Ed) 67:205–215. https://doi.org/10.1016/j.endinu.2019.06.004
Liu C, Wu D, Zhang JF, Xu D, Xu WF, Chen Y, Liu BY, Li P, Li L (2016) Changes in bone metabolism in morbidly obese patients after bariatric surgery: a meta-analysis. Obes Surg 26:91–97. https://doi.org/10.1007/s11695-015-1724-5
Barzin M, Ebadinejad A, Vahidi F, Khalaj A, Mahdavi M, Valizadeh M, Hosseinpanah F (2022) The mediating role of bariatric surgery in the metabolic relationship between parathyroid hormone and 25-hydroxyvitamin D. Osteoporos Int 33:2585–2594. https://doi.org/10.1007/s00198-022-06533-5
Ko BJ, Myung SK, Cho KH, Park YG, Kim SG, Kim DH, Kim SM (2016) Relationship between bariatric surgery and bone mineral density: a meta-analysis. Obes Surg 26:1414–1421. https://doi.org/10.1007/s11695-015-1928-8
Paccou J, Caiazzo R, Lespessailles E, Cortet B (2022) Bariatric surgery and osteoporosis. Calcif Tissue Int 110:576–591. https://doi.org/10.1007/s00223-020-00798-w
Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Shapses SA, Nguyen TV, Sainsbury A (2015) Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J Bone Miner Res 30:2168–2178. https://doi.org/10.1002/jbmr.2564
Muschitz C, Kocijan R, Haschka J, Zendeli A, Pirker T, Geiger C, Müller A, Tschinder B, Kocijan A, Marterer C et al (2016) The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: the BABS study. J Bone Miner Res 31:672–682. https://doi.org/10.1002/jbmr.2707
Pead MJ, Skerry TM, Lanyon LE (1988) Direct transformation from quiescence to bone formation in the adult periosteum following a single brief period of bone loading. J Bone Miner Res 3:647–656. https://doi.org/10.1002/jbmr.5650030610
Jagger CJ, Chambers TJ, Chow JW (1995) Stimulation of bone formation by dynamic mechanical loading of rat caudal vertebrae is not suppressed by 3-amino-1-hydroxypropylidene-1-bisphosphonate (AHPrBP). Bone 16:309–313. https://doi.org/10.1016/8756-3282(94)00043-3
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
All the authors included in this article made substantial contributions to the data included, as well as assisted with critical revisions of the writing, and approved the final version for submission for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The study approval was granted by the Ethics Committee of Modena (Prot. Azienda Ospedaliero-Universitaria 0022400/21, approved on July 21, 2021).
Informed consent
According to the Ethics Committee recommendations and considering the retrospective study design, informed consent was waived since data were collected anonymously.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Greco, C., Passerini, F., Coluccia, S. et al. Long-term trajectories of bone metabolism parameters and bone mineral density (BMD) in obese patients treated with metabolic surgery: a real-world, retrospective study. J Endocrinol Invest 46, 2133–2146 (2023). https://doi.org/10.1007/s40618-023-02066-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02066-8